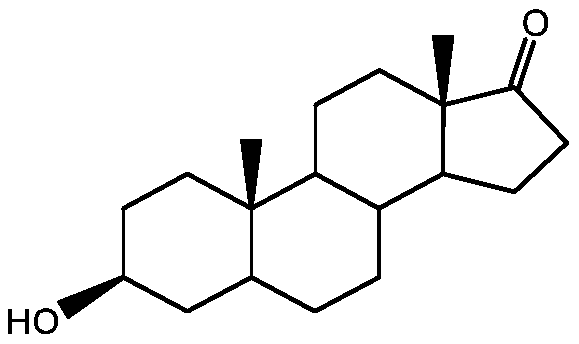
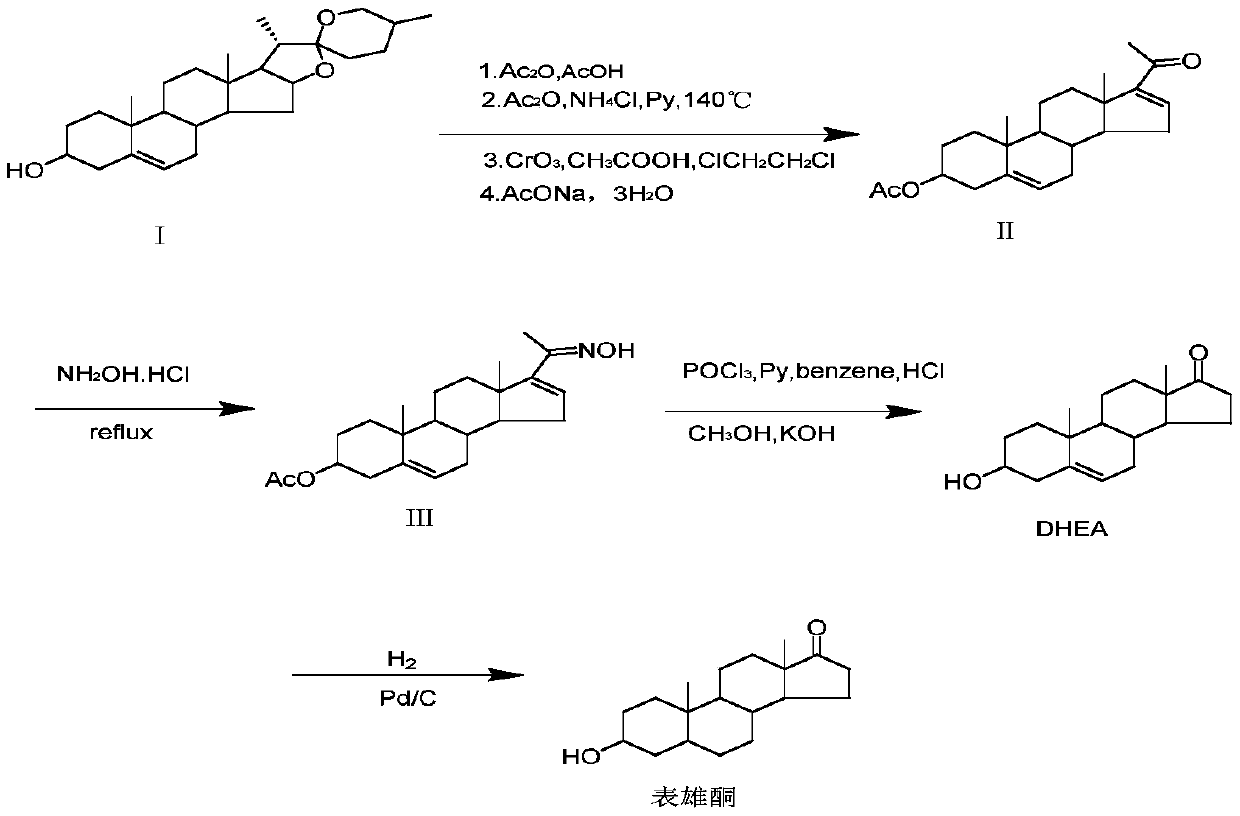
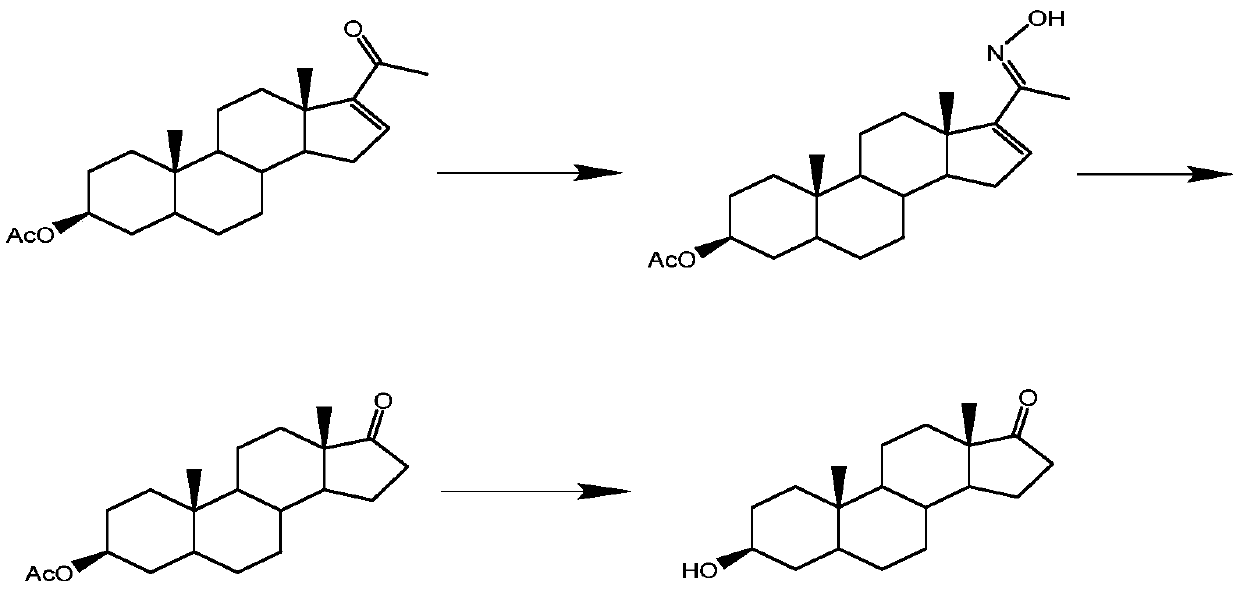
Method for preparing epiandrosterone with androstenedione as raw material
A technology of androstenedione and epiandrosterone, which is applied in the field of preparation of epiandrosterone, can solve the problems of complex reaction process and low product yield, and achieve the effects of high yield and purity, simple recovery method, and pollution reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] In the first step, add 100g (0.349mol) of androstenedione), 200mL (2.12mol) of acetic anhydride, and 200mL of dichloromethane into a 1000mL three-necked flask protected by nitrogen in turn, and the temperature is controlled at about 20°C. Stir until all the materials are dissolved. Then add 4g (0.023mol) p-toluenesulfonic acid, and condense to reflux. The reaction was monitored by TLC, and the reaction was stopped after 20 hours. The reaction solution was poured into ice water for water analysis, and the solid was filtered out and dried in a vacuum oven to obtain 108 g of crude product (2), with a weight yield of 108%.
[0044] In the second step, take 40mL (0.366mol) of trimethyl orthoformate, 80mL (1.44mol) of ethylene glycol, and 8g (0.046mol) of p-toluenesulfonic acid in a three-necked flask, react at 40°C for 2 hours, and then add 100g (0.305mol) ) product (2) crude product, TLC monitoring (V 石油醚 :V 乙酸乙酯 =2:1) Response. After 6 hours, the reaction was basicall...
Embodiment 2
[0048] The first step is the same as in Example 1.
[0049] In the second step, take 30mL (0.275mol) of trimethyl orthoformate, 100mL (1.80mol) of ethylene glycol, and 20g of p-toluenesulfonic acid in a 1L three-necked flask, react at 20°C for 2 hours, and then add 80g (0.244mol) of The crude ene esterification product obtained in one step was monitored by TLC. After 9 hours, the reaction was basically complete. Triethylamine was added to quench the reaction, and the solvent was removed under reduced pressure. The mixture was poured into ice water for water analysis, and the solid was filtered out and dried in vacuo to obtain 89 g of crude product of ketal product (3). Rate 102.5%.
[0050] Subsequent steps are the same as in Example 1.
Embodiment 3
[0052] The first step and the second step are the same as in Example 1.
[0053] The third step is to take 100g (0.269mol) of the crude ketal product obtained in the previous step, add 40mL of methanol solution to dissolve, then add 10g of sodium tert-butoxide, stir at 80°C for 1h until the product (2) completely disappears, and then add 10g of palladium Carbon, reacted in 80℃, 2MPa autoclave for 24 hours. After the reaction was completed, vacuum filtration was performed, palladium carbon was recovered, methanol and water were removed, and 103 g of product (4) after esterification and reduction was obtained, with a weight yield of 103%. The purity of the product determined by HPLC was 96%.
[0054] The fourth step is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com