A fluorescent probe for detecting trace water in 1,4-dioxane and its preparation method
A technology of dioxane and fluorescent probes, applied in the field of detection 1, can solve the problems of inability to use real-time detection, toxicity and the like, and achieve the effect of good practical value and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
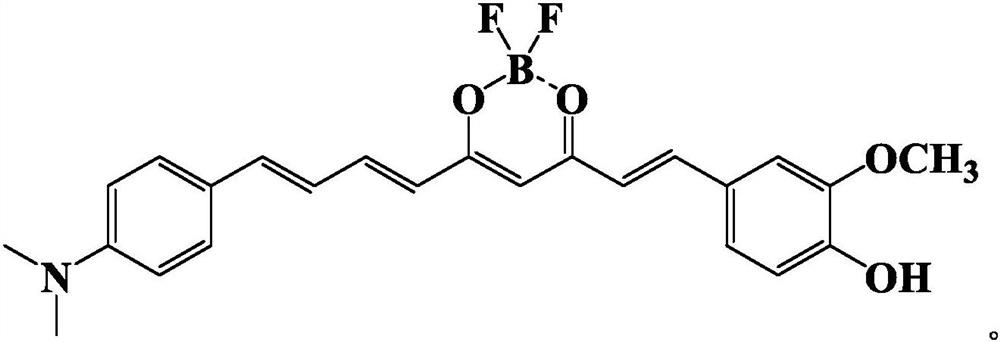
[0031] Preparation of 1-(3-methoxy-4-hydroxyphenyl)-9-(4-dimethylaminophenyl)nonan-1,6,8-triene-3,5 from 4-dimethylaminocinnamaldehyde - diketone boron difluoride complex, the reaction formula is:
[0032]
[0033] Specific steps are as follows:
[0034] 1) Preparation of 2,4-pentanedione boron difluoride complex
[0035] Add 3.02g of 2,4-pentanedione, 10mmol of boron trifluoride diethyl ether and 20mL of dichloromethane into a dry three-necked flask, react at 45°C for 3h, track and detect with TLC, and stop after the reaction is complete. After the reaction was completed, it was cooled to room temperature, and the reaction solution was distilled off under reduced pressure to remove dichloromethane, and then n-hexane was added for cooling and crystallization to obtain 3.13 g of light yellow 2,4-pentanedione boron difluoride complex, yield: 93 %. 1 H NMR (600MHz, DMSO-d6) δ: 6.39(s, 1H), 2.34(s, 6H); 13 C NMR (150MHz, CDCl 3 )δ: 192.48, 101.98, 101.95, 24.10. HRMS (m / z)...
Embodiment 2
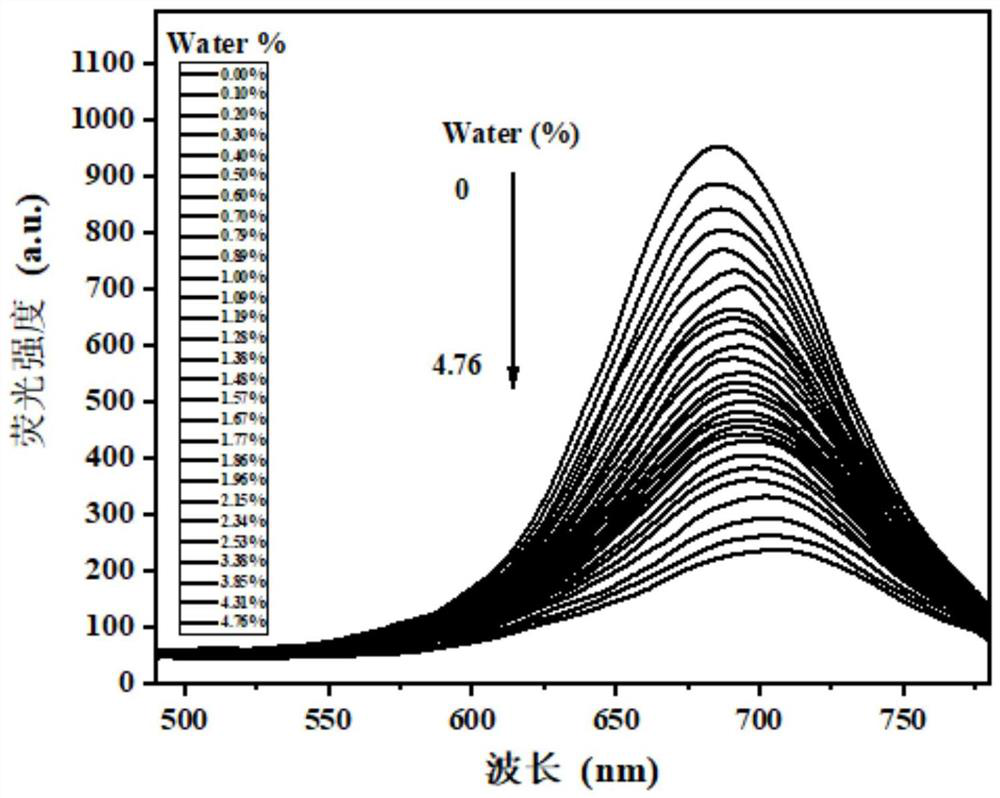
[0041] 1-(3-methoxy-4-hydroxyphenyl)-9-(4-dimethylaminophenyl)nonan-1,6,8-triene-3,5-dioneboron difluoride The compound was dissolved in analytically pure dioxane to prepare 1×10- 5 For the probe solution of M concentration, different amounts of water are added into the probe solution to form a solution with a water content of 0-4.76%. Adopt the standard titration method to measure the water p-1-(3-methoxy-4-hydroxyl phenyl)-9-(4-dimethylaminophenyl) nonyl-1,6 of different content under the fluorescence spectrophotometer, The fluorescence emission spectrum of the 8-triene-3,5-dione boron difluoride complex, as figure 1 shown. The results show that in the range of 0-4.76% water content, with the continuous increase of water content in the system, the red fluorescence of the solution is quenched, which shows that the probe can detect water molecules sensitively and quantitatively.
Embodiment 3
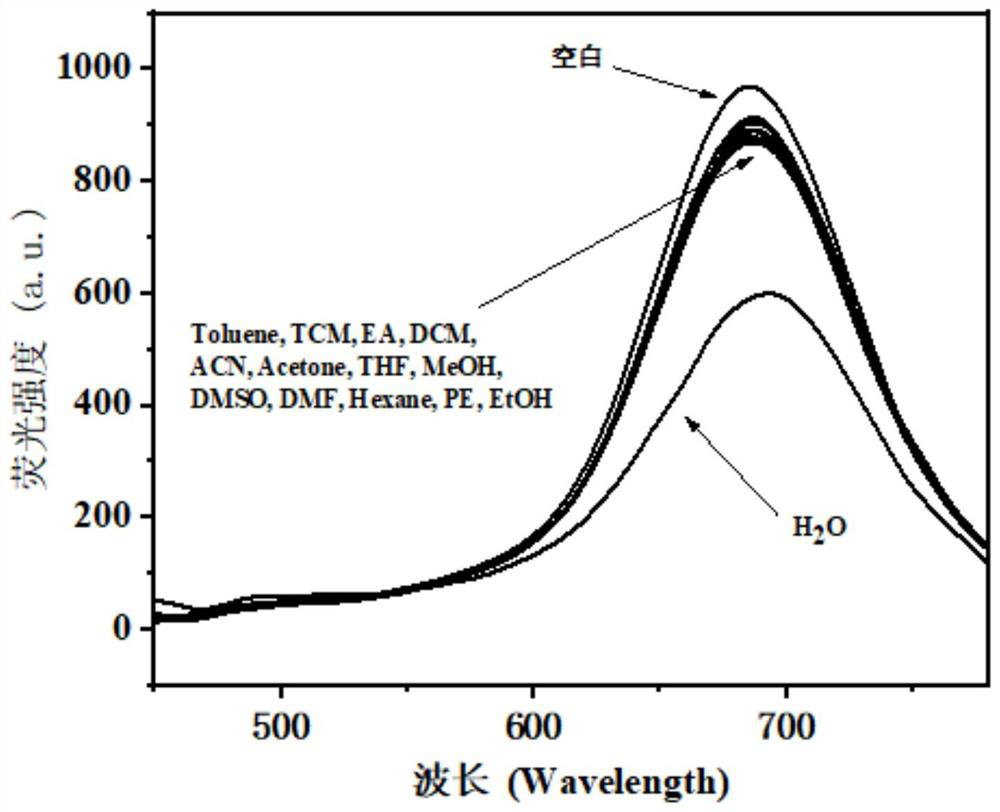
[0043] 1-(3-methoxy-4-hydroxyphenyl)-9-(4-dimethylaminophenyl)nonan-1,6,8-triene-3,5-dioneboron difluoride The compound was dissolved in analytically pure dioxane to prepare 1×10- 5 The probe solution of M concentration, add the various organic solvents of 0.1mL respectively, adopt standard titration method to measure the different organic solvents to 1-(3-methoxy-4-hydroxyphenyl)- The fluorescence emission spectrum of 9-(4-dimethylaminophenyl)nona-1,6,8-triene-3,5-dione boron difluoride complex, as figure 2 shown. The results showed that the fluorescence was obviously quenched after adding water in the probe solution, while adding other solvents such as toluene, chloroform, ethyl acetate, dichloromethane, acetonitrile, acetone, tetrahydrofuran, methanol, dimethyl sulfoxide, N, N-dimethylformamide, n-hexane, petroleum ether, ethanol, etc., the fluorescence spectrum of the solution does not have obvious quenching phenomenon, which shows that the probe can specifically recogn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com