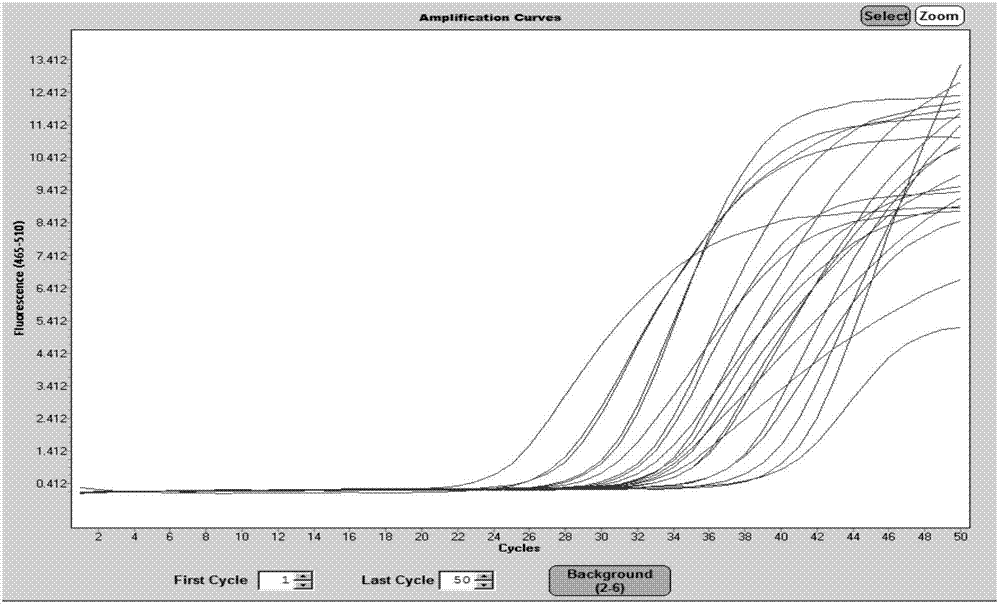
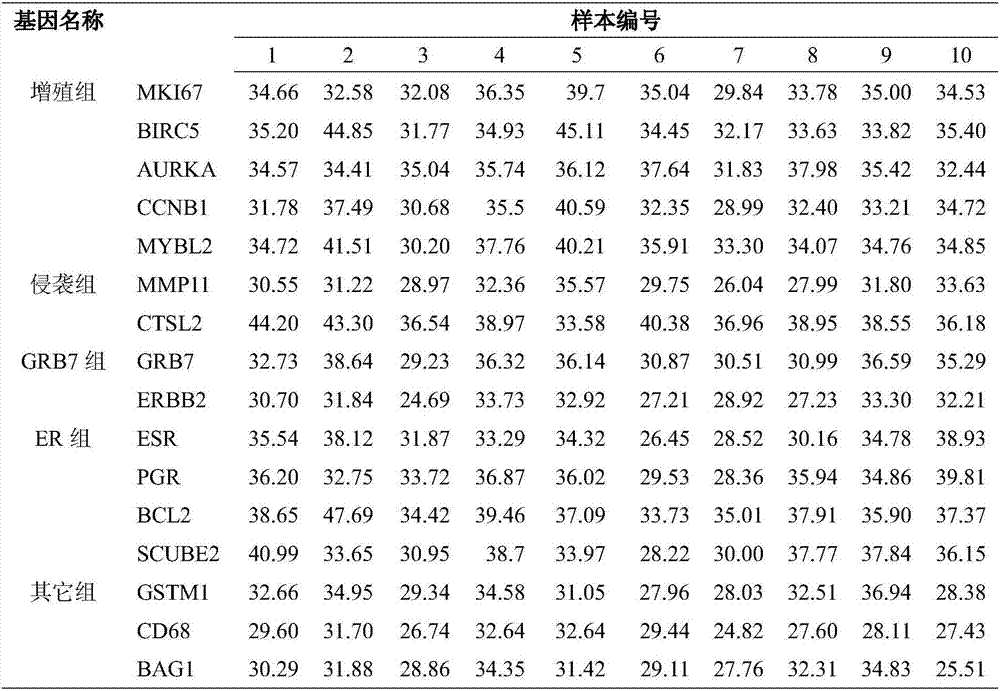
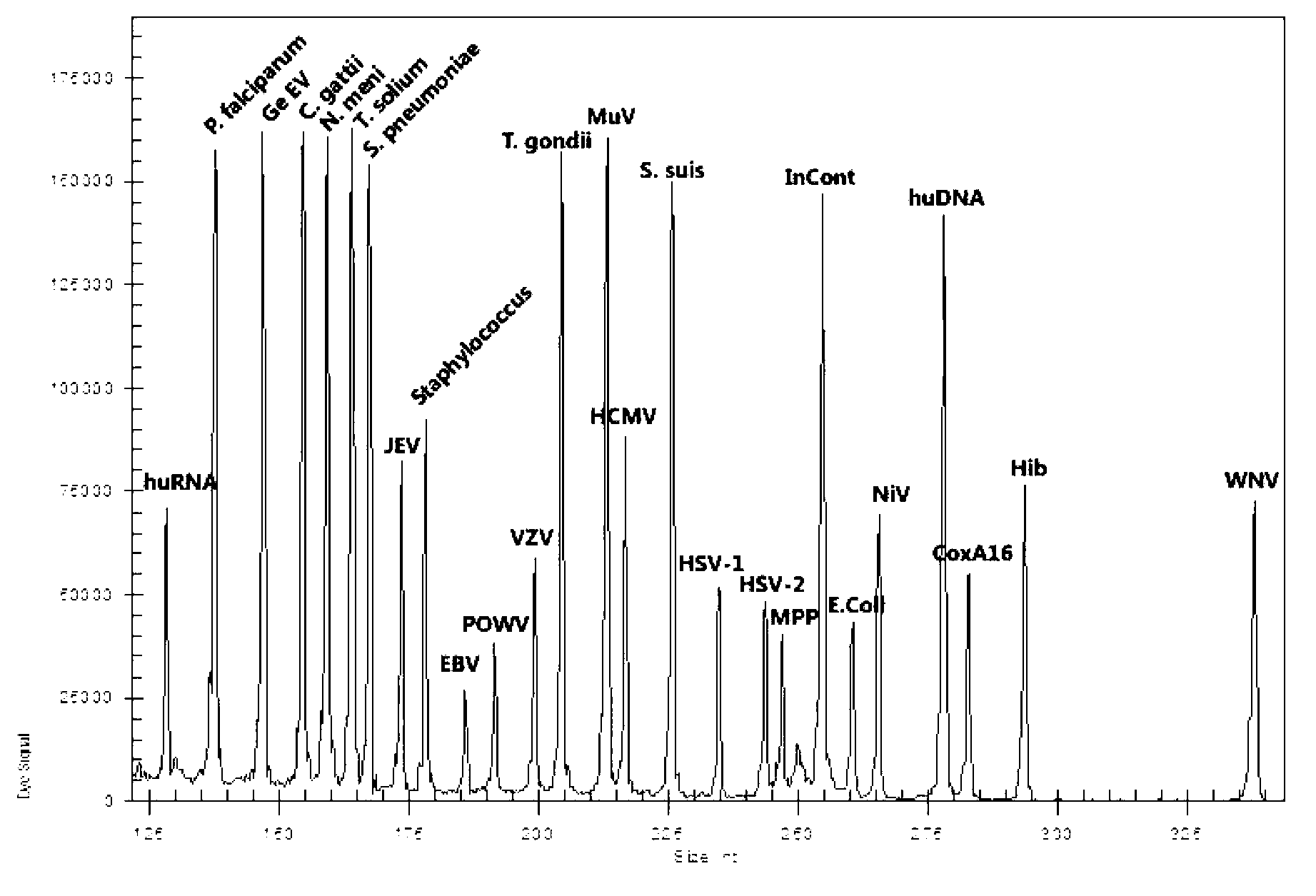
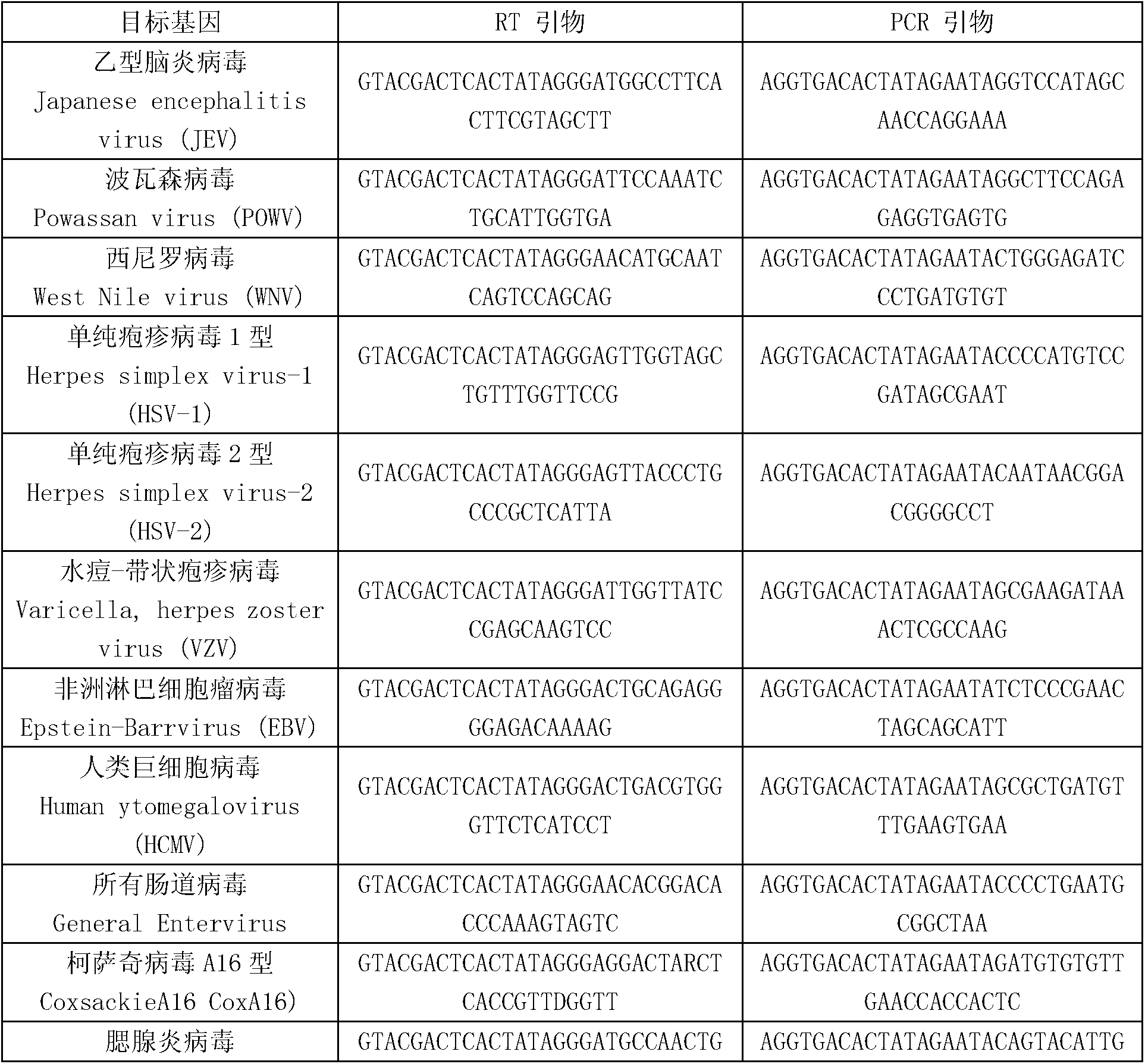
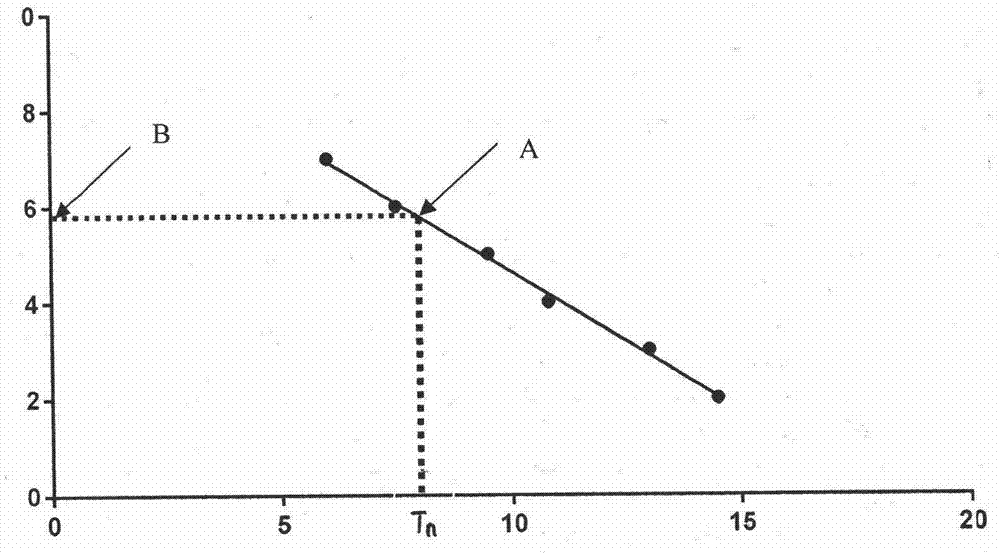
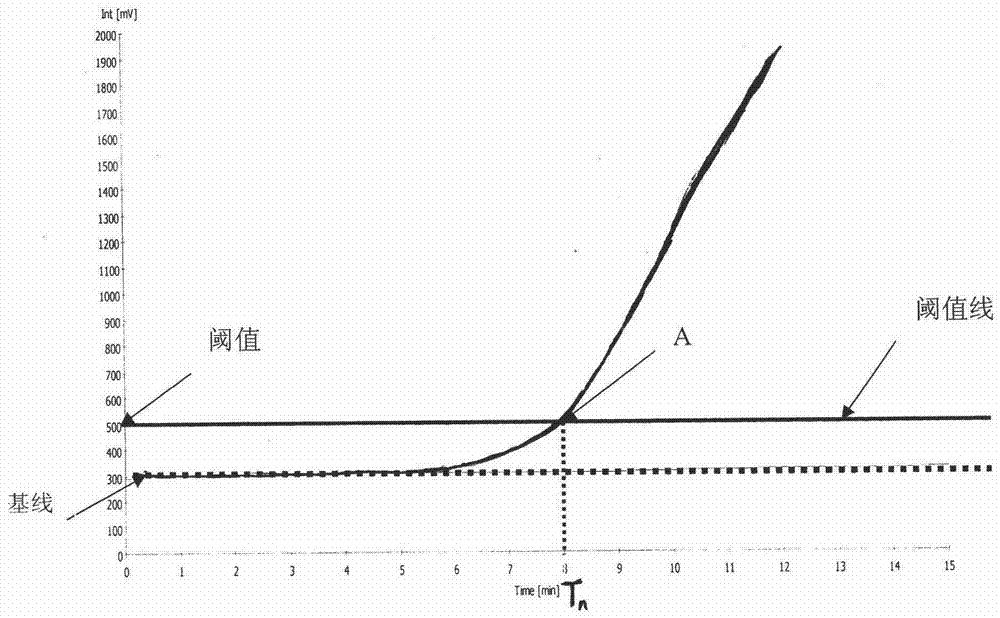
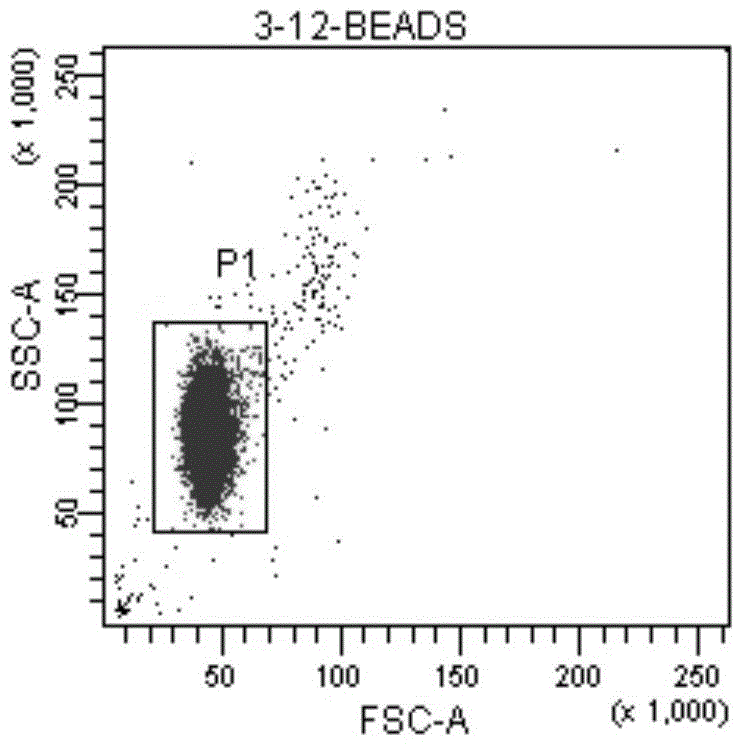
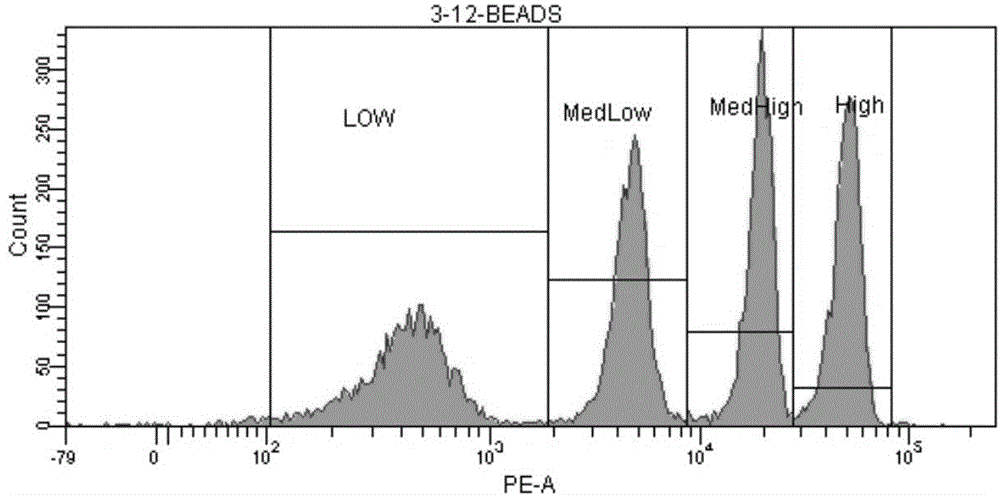
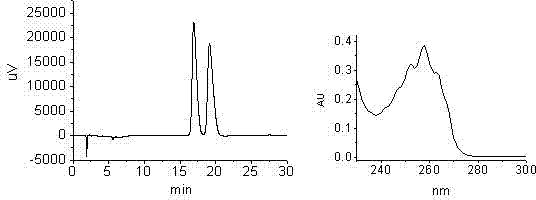
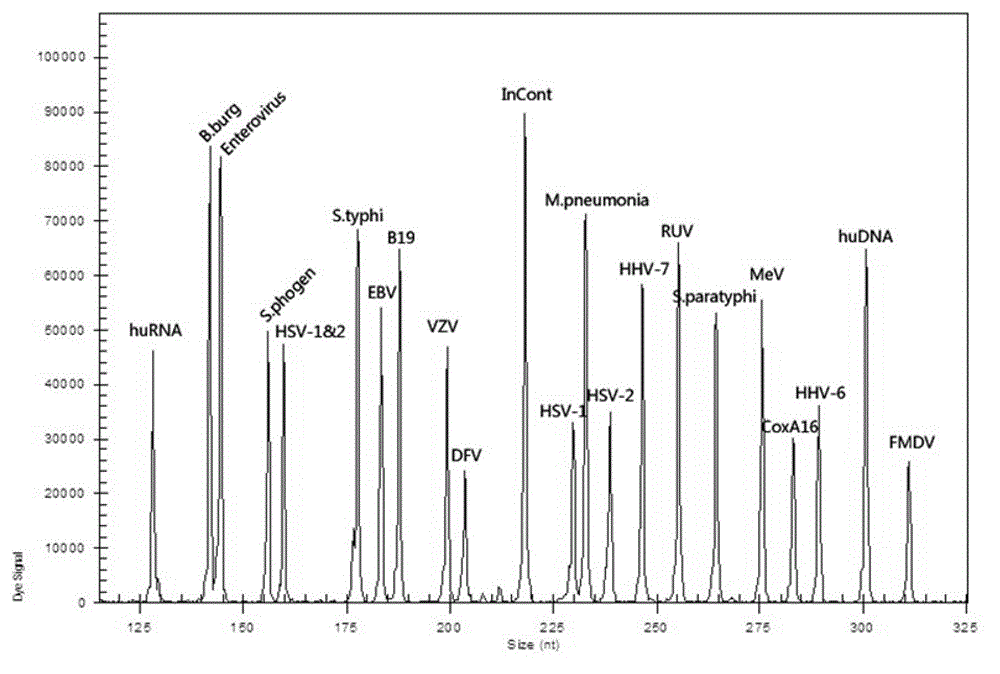
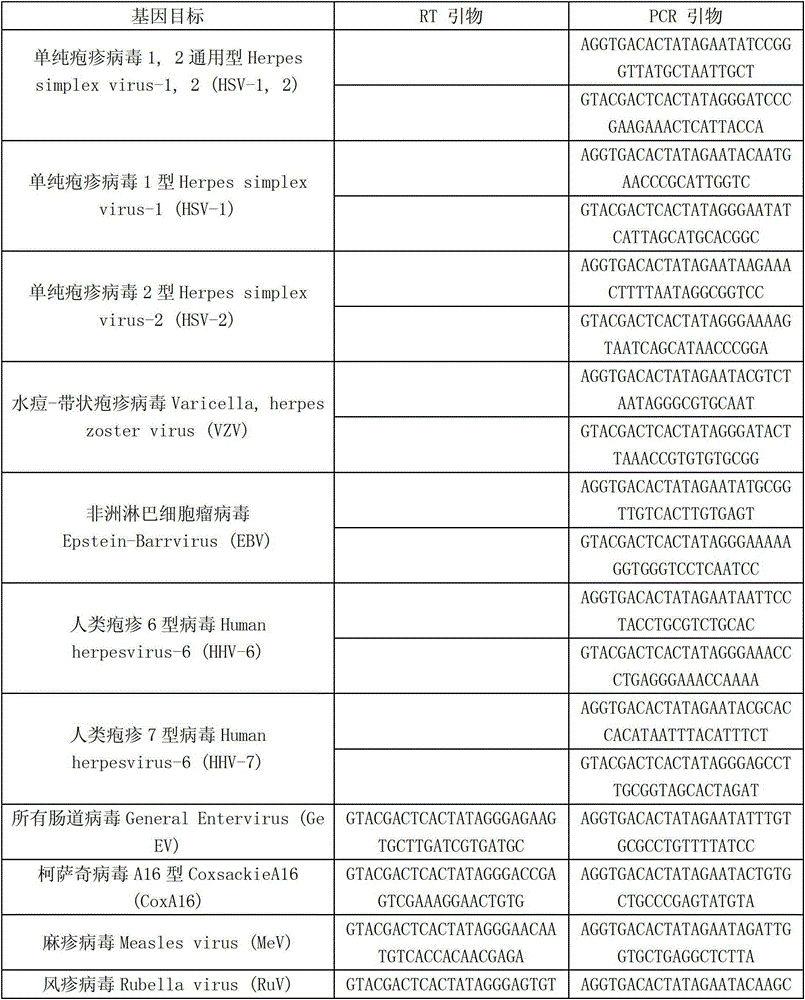
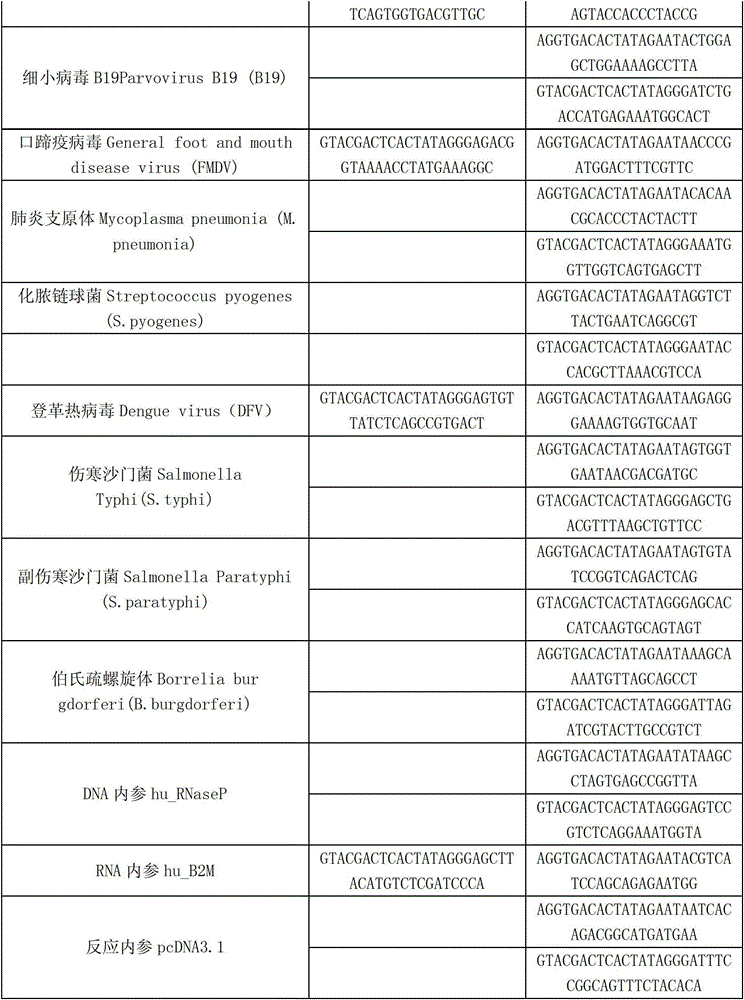
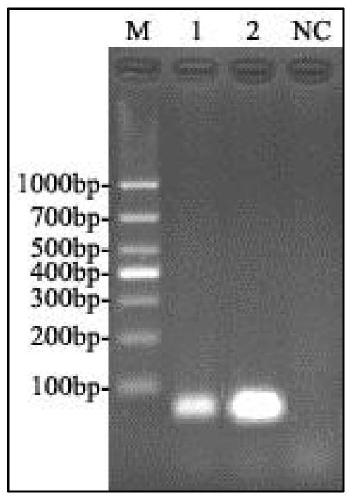
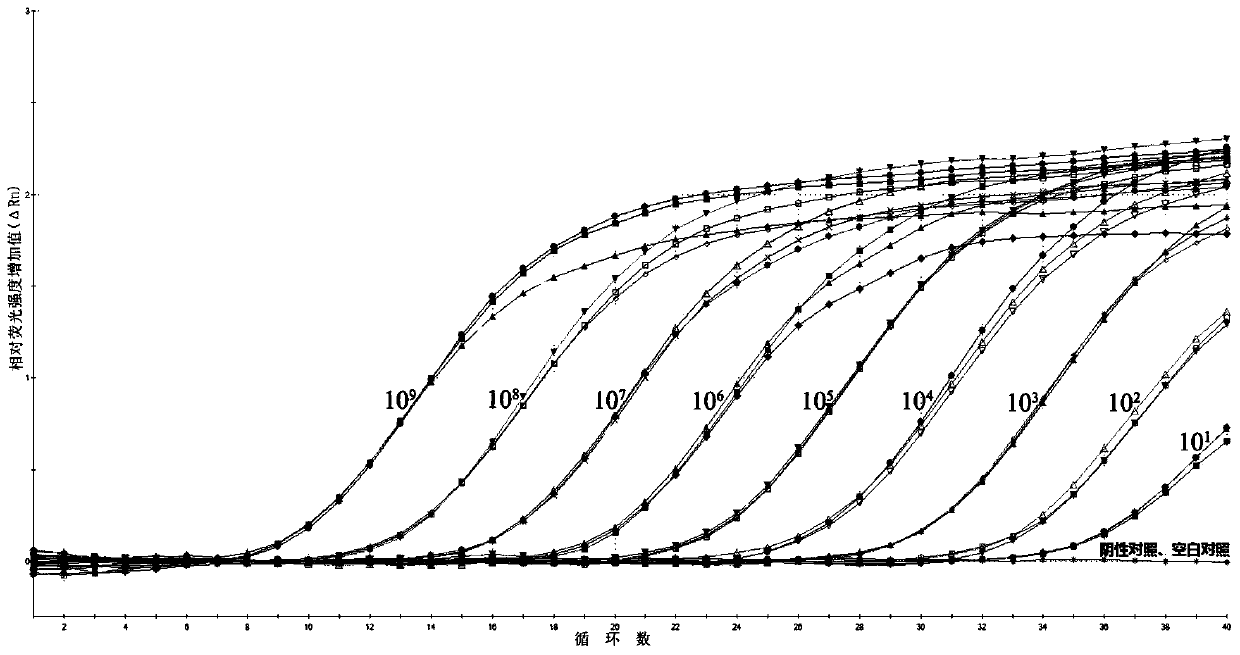
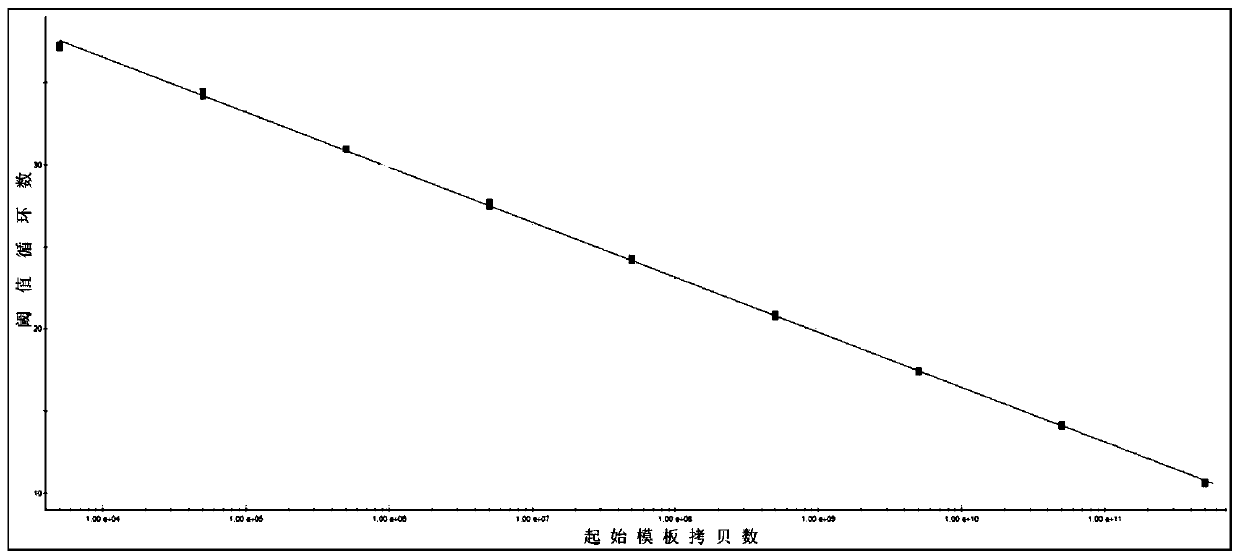
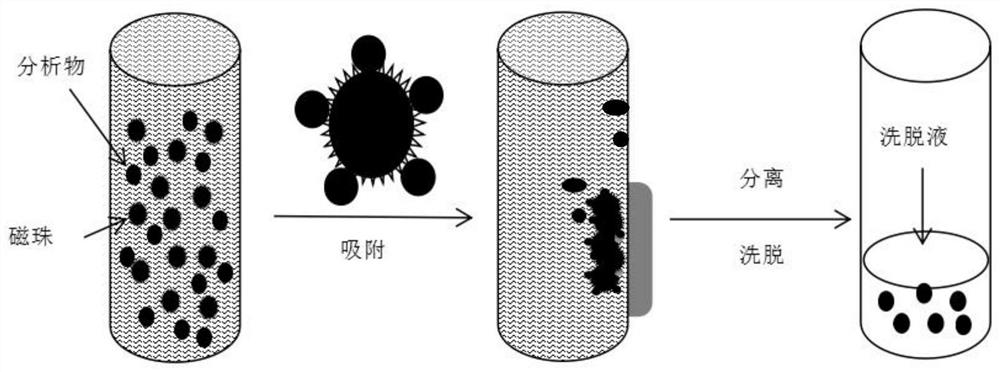
Patents
Literature
86results about How to "Quantitative fast" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Application of near infrared-emission fluorescent probe in quick pesticide residue detection
ActiveCN108318467AFast metabolismRapid responseFluorescence/phosphorescencePesticide residueEnzyme inhibition
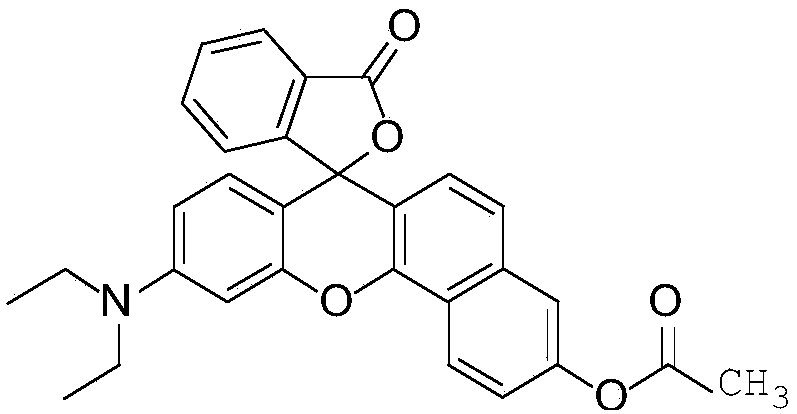
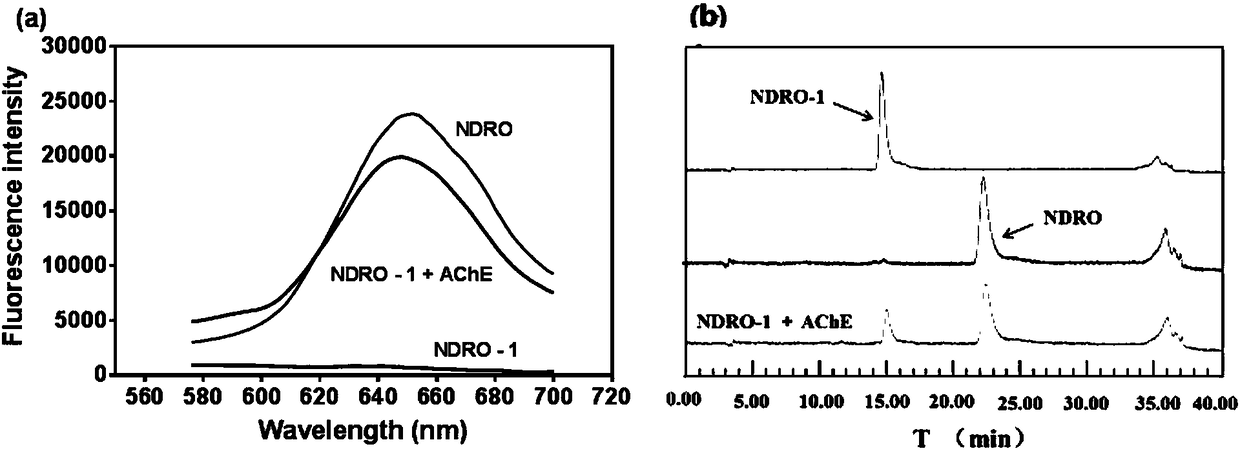
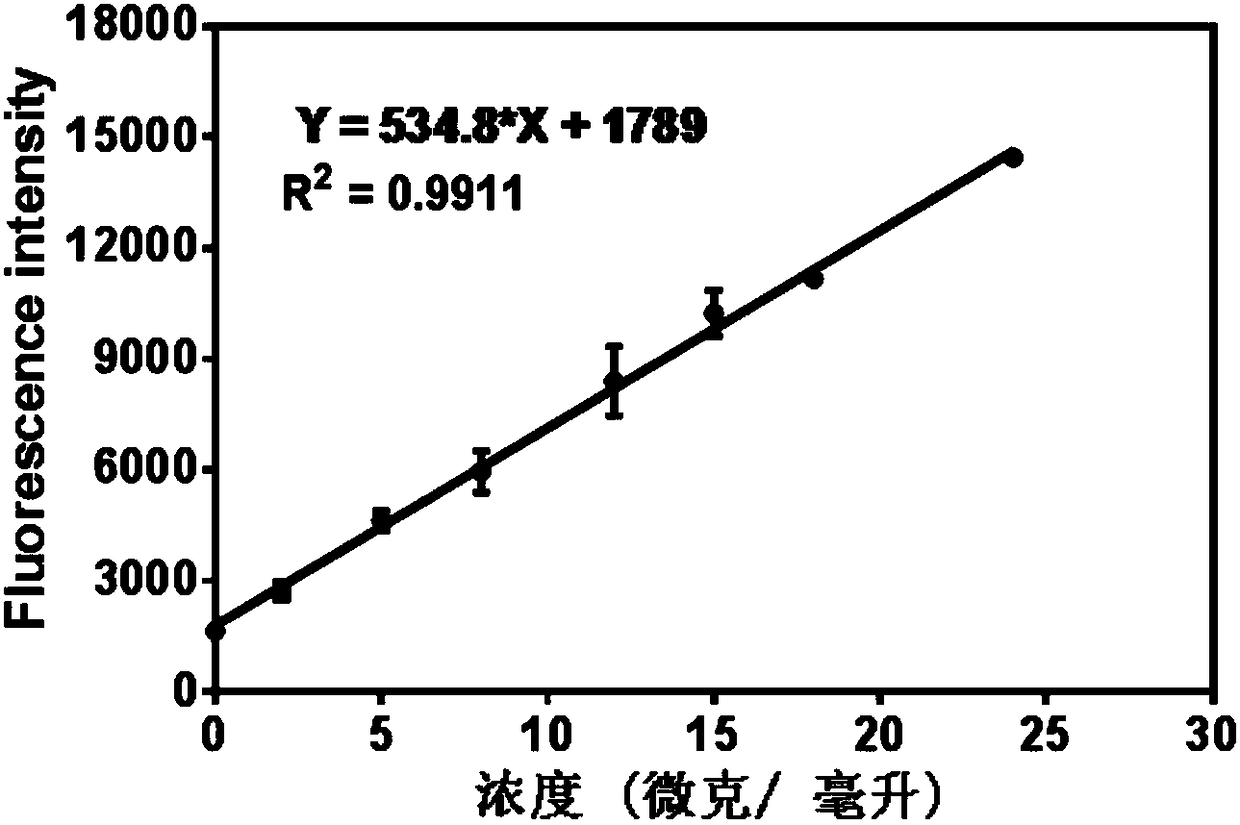
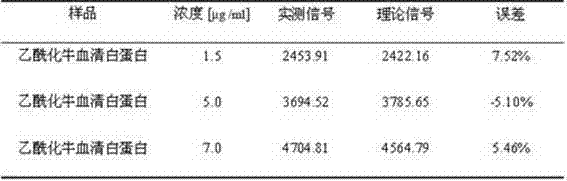
The invention discloses application of a near infrared-emission fluorescent probe in quick pesticide residue detection and belongs to the technical field of quick pesticide detection. The probe is based on rhoda-fluor fluorescence matrix and has specific activity on acetylcholine esterase AChE; by means of an enzyme inhibition detection principle, the probe can be applied to quick detection of organophosphorus pesticide and carbamate pesticide residues in food. The acetylcholine esterase AChE is extracted from duck blood; in an appropriate probe substrate range, enzyme activity and an inhibition rate can be represented by quantitatively detecting fluorescence intensity change of the probe. Four pesticide detection standard curves have small error, and R<2> is larger than 0.98; vegetable pesticide adding standard recovery most reaches 80 to 110%, so that the near infrared-emission fluorescent probe disclosed by the invention can be applied to quantitative, ppb-grade and quick detectionof organophosphorus and carbamate pesticide residues in the food.
Owner:王铮
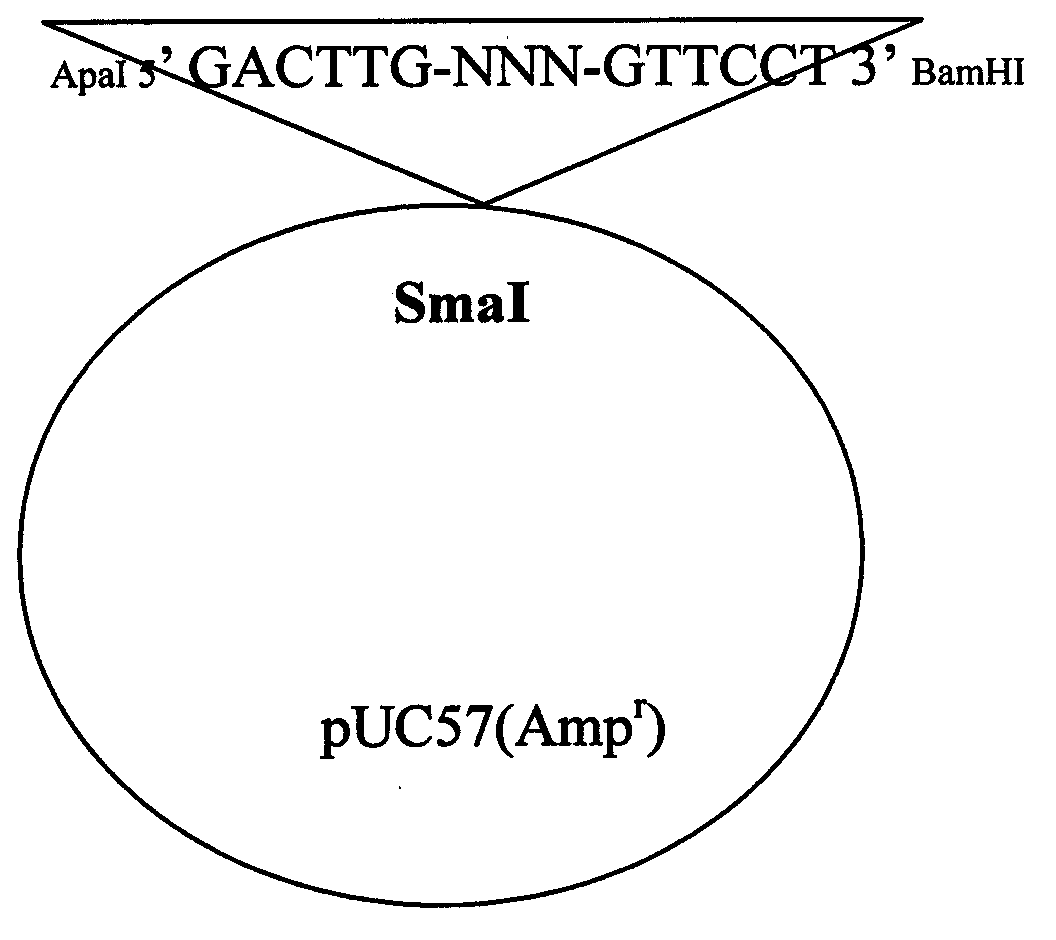
Nocardia seriolae fluorescence quantitative PCR detection kit and detection method
InactiveCN101962678AQuantitatively sensitiveSensitive quantitative PCR detectionMicrobiological testing/measurementFluorescence/phosphorescenceFluorescenceNucleotide sequencing
The invention discloses a nocardia seriolae fluorescence quantitative PCR detection kit and a nocardia seriolae fluorescence quantitative PCR detection method. The detection kit comprises 10.0 microliters of SYBRPremix ExTaqTM special agent, 10 micromol / L upstream primer solution and 0.8 microliters of downstream primer solution, and is characterized in that the nucleotide sequence of the upstream primer is 5,TGCTACAATGGCCGGTACAGAG3; and the nucleotide sequence of the downstream primer is 5,TTCACGAGGTCGAGTTGCAGAC3. The detection method comprises pathogenic bacteria extraction, pathogenic bacteria enzymolysis, DNA coarse extract preparation, DNA coarse extract purification and detection. The detection kit can detect nocardia seriolae in early stage sensitively, quickly, quantificationally and specifically. The sensitivity of the detection kit is up to 10 to 6 microgram / microliter, the quantification can be realized basically on the level of 10 to 5 microgram / microliter.
Owner:NINGBO UNIV
Breast cancer recurrent risk assessment 21 gene detection primer and application thereof
InactiveCN107058523ASimple and fast operationStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationWilms' tumorAdjuvant chemotherapy
The invention relates to a breast cancer recurrent risk assessment 21 gene detection primer and application thereof. The breast cancer recurrent risk assessment 21 gene detection primer comprises a nucleotide sequence as shown in an SEQ ID NO.: 1 to 42, and belongs to the technical field of molecular detection. The detection primer provided by the invention utilizes the advantage of fluorescent quantitation, and adopts fluorescent quantitation polymerase chain reaction to carry out amplification detection on 21 genes for breast cancer recurrent risk assessment, so that whether clinical measurements such as adjuvant chemotherapy are needed or not is considered. A kit provides a PCR (Polymerase Chain Reaction) amplification primer sequence of the 21 genes for breast cancer recurrent risk assessment, and the primer is applicable to detecting ER positive patients, HER2 negative patients, axillary node negative patients, moderately and poorly differentiated patients with the tumor size being 0.6 to 1.0cm and adverse prognostic factors, or patients with the tumor size is larger than 1cm, has the advantages of high sensitivity and specificity, stability, promptness, convenience in operation and the like, can well meet the clinical application of breast cancer recurrent risk assessment, and can be beneficial for reducing invalid medication administration, improving medication administration accuracy, and reducing financial burdens of the patients.
Owner:温州迪安医学检验所有限公司 +1
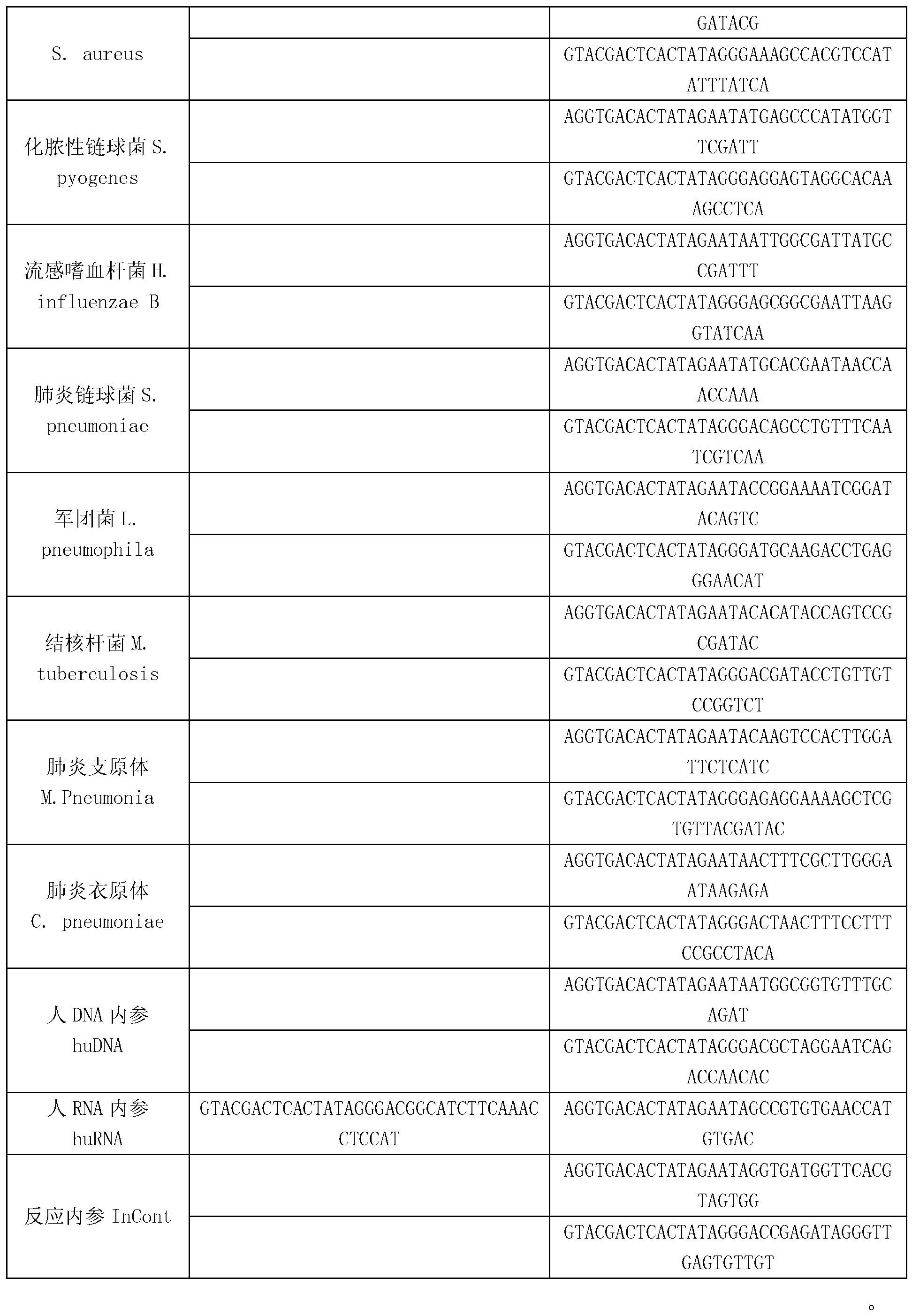
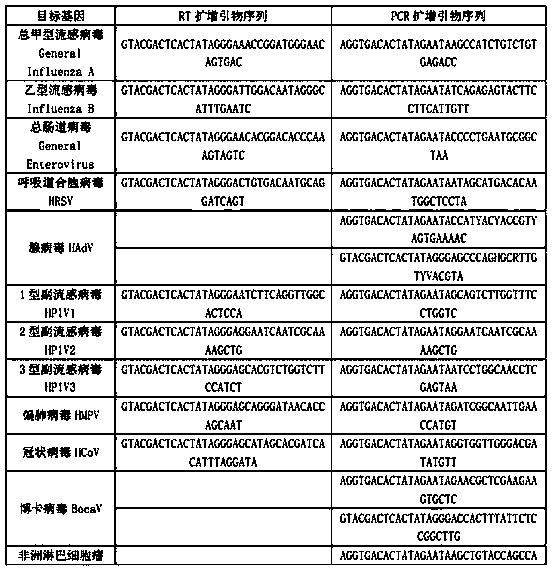
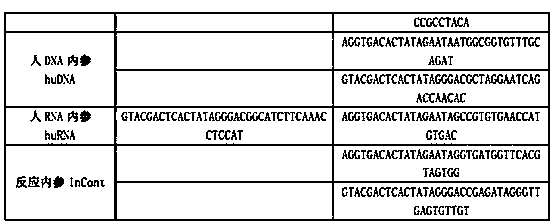
Kit for synchronously detecting twenty-three meningitis pathogens and detection method of kit
ActiveCN103074448AEnsuring Quality JudgmentsStrong specificityMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
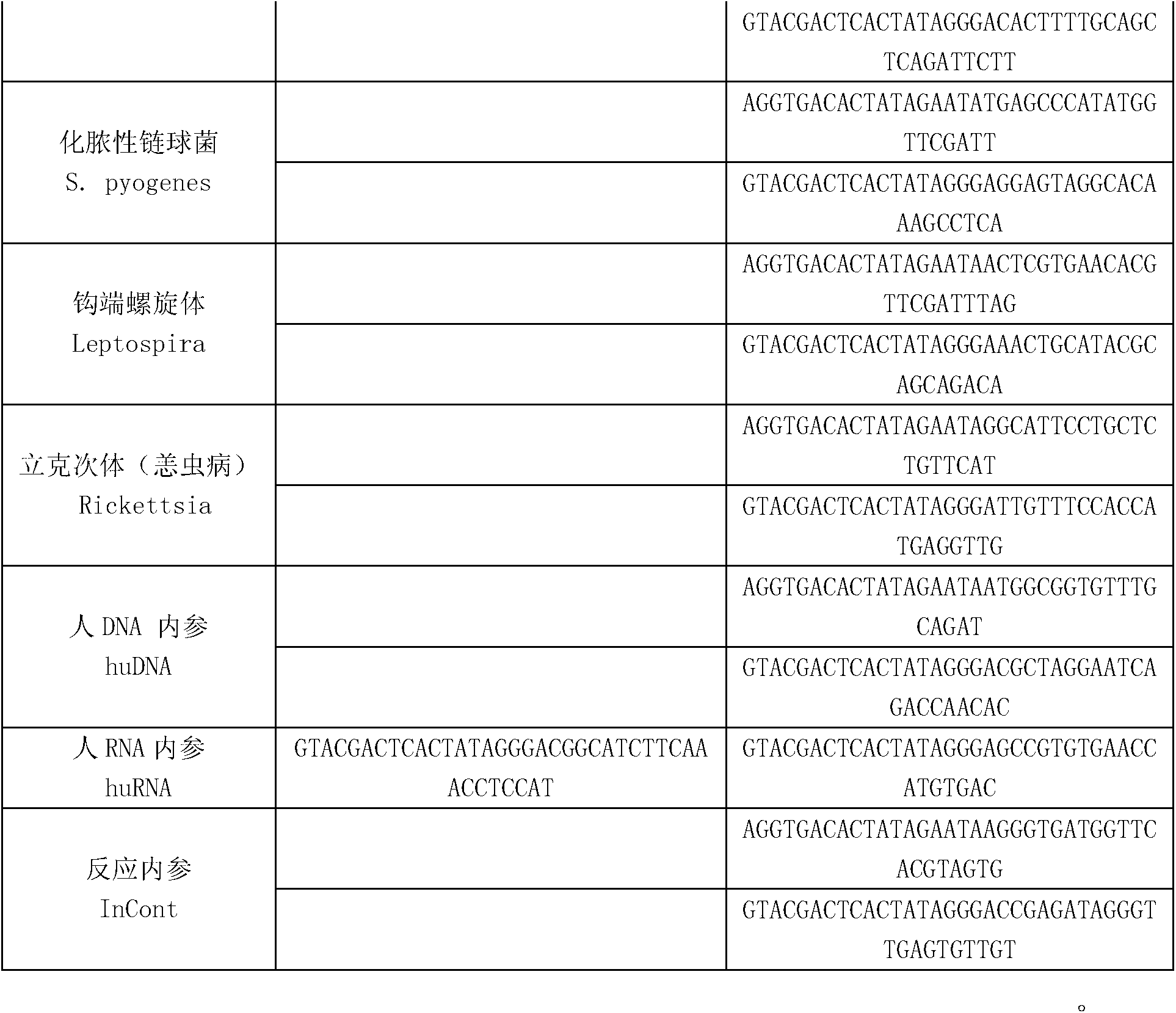
The invention discloses a kit for synchronously detecting twenty-three meningitis pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of the twelve meningitis pathogens and a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-13 (sequence identifier number 1-13), and the PCR primer comprises forward and reverse PCR amplification primers of the rest eleven meningitis pathogens, a human DNA internal reference and a reaction internal reference, and PCR amplification primers of the twelve meningitis pathogens and the human RNA internal reference, and has a gene sequence shown as SEQ ID NO. 14-52. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
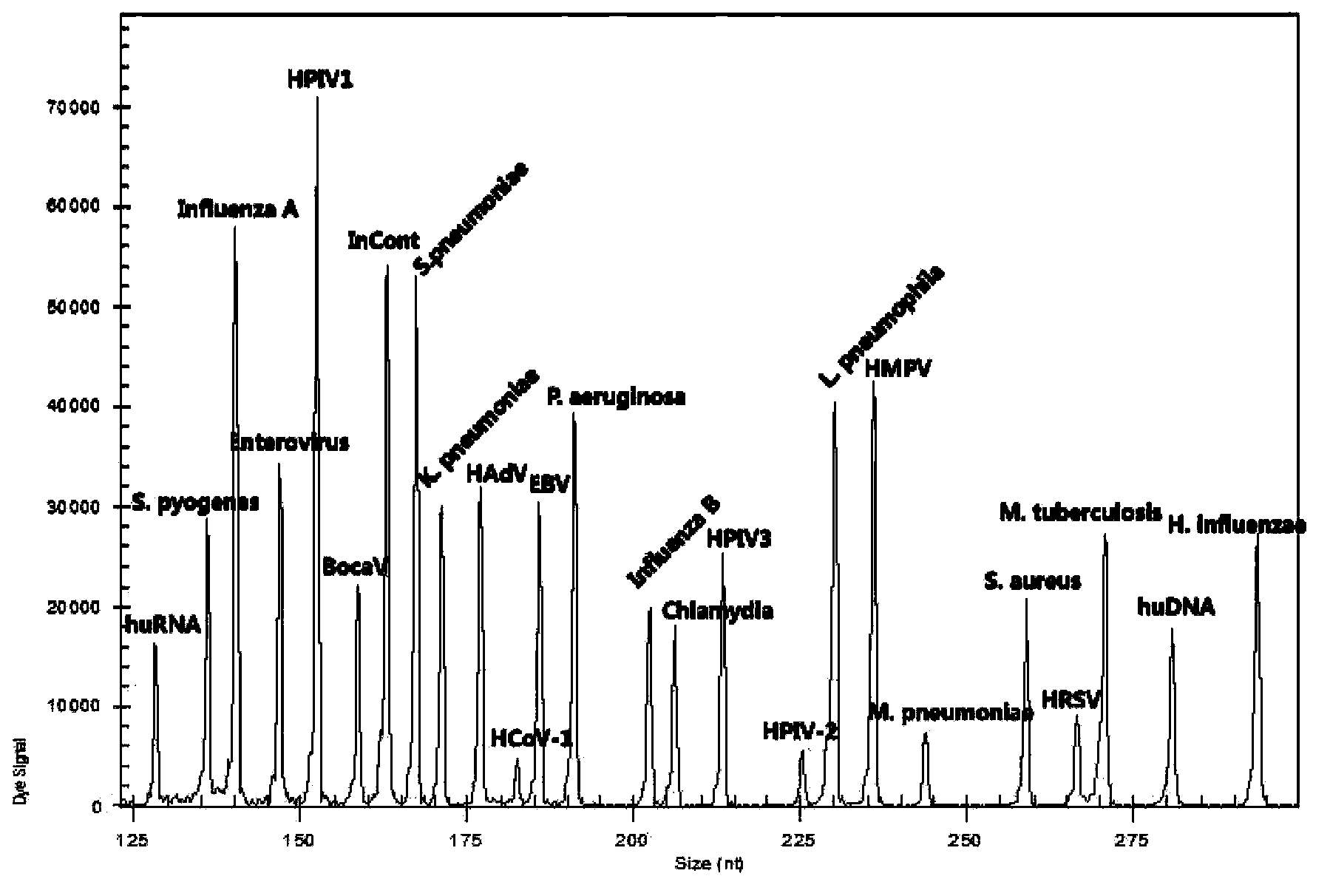
Kit for synchronously detecting twenty-two respiratory tract pathogens and detection method of kit
ActiveCN103074451AEnsuring Quality JudgmentsImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting twenty-two respiratory tract pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of the nine respiratory tract pathogens, and a reverse primer of a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-6 (sequence identifier number 1-6), and the PCR primer comprises forward and reverse PCR amplification primers of the rest thirteen respiratory tract pathogens, a forward and reverse amplification primer of a human DNA internal reference, a forward and reverse PCR amplification primer of a reaction internal reference, PCR amplification primers of the nine respiratory tract pathogens, and a PCR amplification primer of the human RNA internal reference, and has a gene sequence shown as SEQ ID NO. 7-44. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
Qualitative and quantitative analysis method for element sulfur in liquefied petroleum gas
ActiveCN102788856ALow detection limitQuantitative ion signal is obviousComponent separationStructure analysisGas liquid chromatographic
The present invention discloses a qualitative and quantitative analysis method for element sulfur in liquefied petroleum gas (LPG) by using gas chromatography-mass spectrometry combination, and relates to the field of analytical chemistry. According to the qualitative method, a scanning way is adopted to analyze a large amount of LPG samples; according to analysis of the resulting mass spectrometry spectrogram, a mass spectrometry standard library search is combined, comparison with an element sulfur standard sample retention time is performed, and element sulfur structure analysis is performed. According to the quantitative method, an internal standard is adopted, n-hendecane is adopted as an internal standard substance, and a quadratic fit standard curve is established by selecting an ion monitoring way to carry out quantitative analysis. A LPG sample requiring detection is treated by a toluene solvent, and then is directly subjected to structure and content analysis of element sulfur. The method of the present invention has characteristics of low detection limit, high accuracy, no interference during analysis, and short analysis time, and provides strong technical supports for guidance of normal LPG production and prevention of processing apparatuses and transport equipment from sulfur corrosion.
Owner:CHINA PETROLEUM & CHEM CORP
Kit for synchronously detecting fifteen hemorrhagic fever pathogens and detection method of kit
ActiveCN103074452AMonitor reaction efficiencyEnsuring Quality JudgmentsMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting fifteen hemorrhagic fever pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of the nine hemorrhagic fever pathogens and a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-10 (sequence identifier number 1-10), and the PCR primer comprises forward and reverse PCR amplification primers of the rest six hemorrhagic fever pathogens, a human DNA internal reference and a reaction internal reference, and PCR amplification primers of the nine hemorrhagic fever pathogens and the human RNA internal reference, and has a gene sequence show as SEQ ID NO. 10-36. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
Analysis method of free sterol components in edible oil sample and swill-cooked dirty oil identification method
InactiveCN104267123AReduce dosageTo meet the needs of rapid pre-processingComponent separationBiotechnologyPlant sterol
The invention relates to an analysis method of free sterol components in an edible oil sample and a swill-cooked dirty oil identification method. The analysis method comprises the following steps: carrying out solid phase extraction on the edible oil sample to be tested to separate out the free sterol in the sample, carrying out silylation derivatization on the free sterol, injecting a comprehensive two-dimensional gas chromatography-time-of-flight mass spectrum into the free sterol for detection, and realizing qualitative and quantitative analysis on the free sterol components in the edible oil sample. Compared with a traditional sterol compound analysis method, the analysis method has the characteristics of high sensitiveness, rapidness and high efficiency, an adulterated edible oil identification and swill-cooked dirty oil identification method is established through analyzing a proportional relation between phytosterol in cholesterol in different edible oil as well as a proportional relation between the cholesterol and other specific phytosterol, and the authenticity identification of the edible oil and the identification of the swill-cooked dirty oil can be realized.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
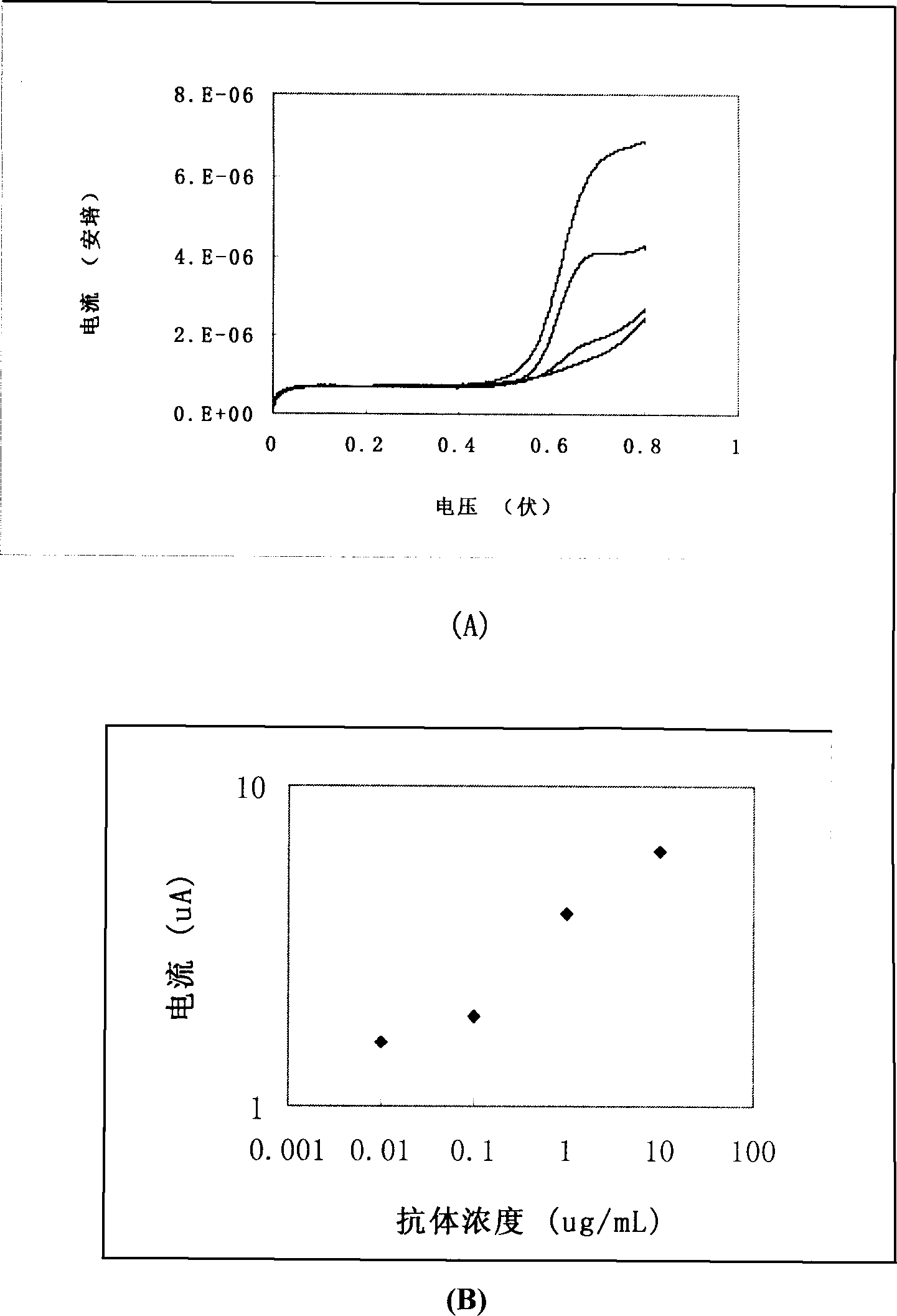
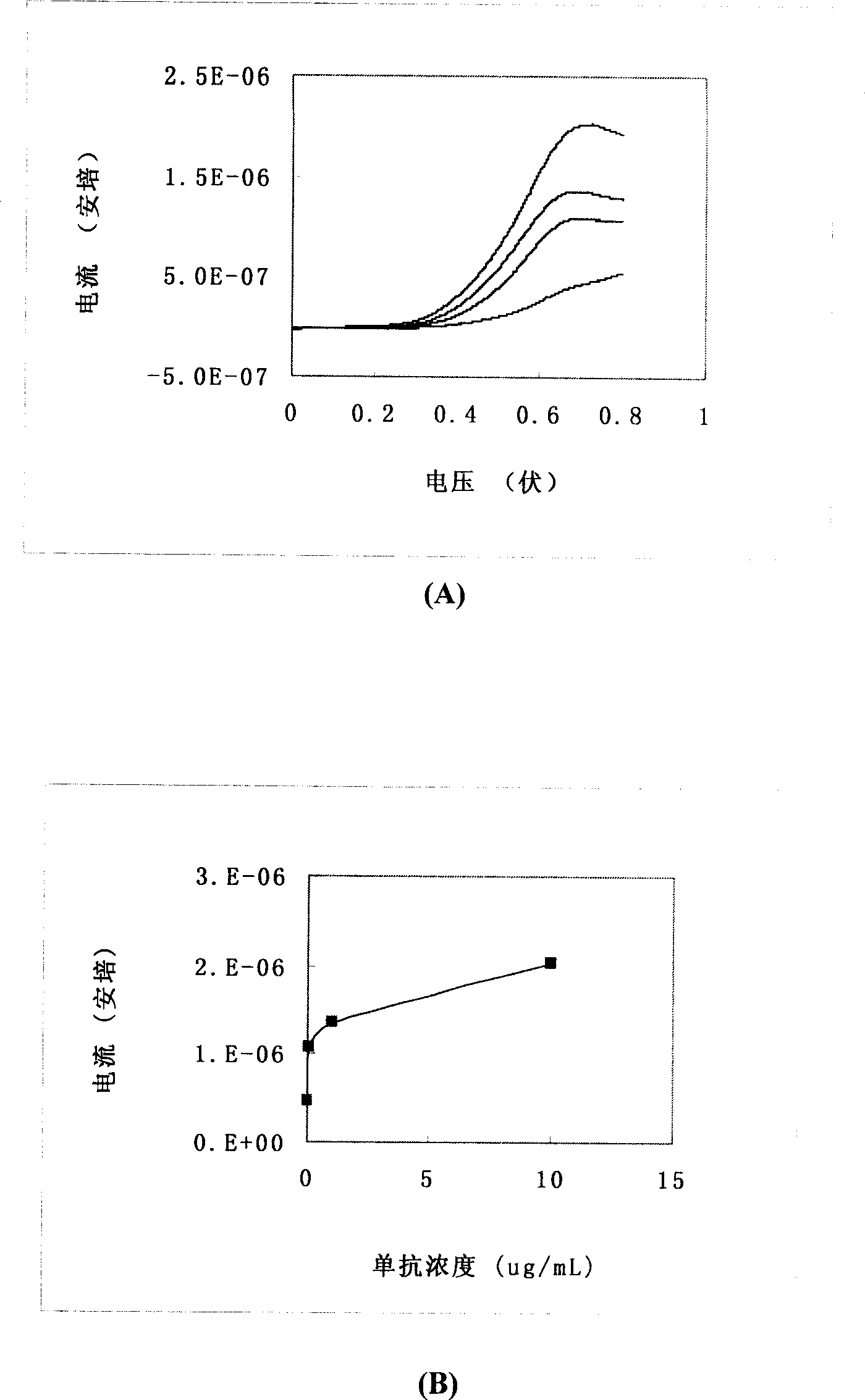
Paper strip for quantitative electrochemical biological test
InactiveCN1908645AQuantitative fastEasy to quantifyMicrobiological testing/measurementBiological testingSolid substrateElectrochemistry
The disclosed quantitative detective test paper for bio-combination reaction is prepared as following: based on solid substrate, preparing electrode firstly; adding a layer of film with fixed catching molecules, wherein the target molecule competes with the catching molecule and another enzyme-mark molecule to combine together, and it uses the electrochemical signal of reaction product for detection. This invention is simple and fast, and has wide application.
Owner:郭良宏
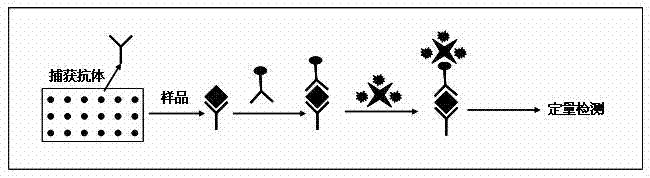
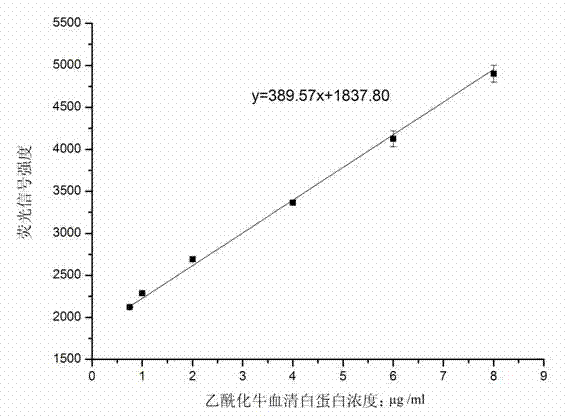
Method for quantitatively detecting protein acetylation level
InactiveCN103048471AOvercome weak affinityOvercoming the disadvantages of being difficult to efficiently enrich acetylated proteinsBiological testingFluorescence/phosphorescenceProtein AcetylationAntibody affinity
The invention belongs to the technical field of biology, and particularly discloses a method for quantitatively detecting a protein acetylation level. The method particularly comprises the steps of preparing protein chips, drawing standard curves and detecting actual samples. The method overcomes the defects that antibodies have weak affinities and acetylated protein is difficult to enrich efficiently when acetylation antibodies are subjected to immune precipitation enriching directly; the protein acetylation level can be quantitatively detected quickly and simply; the used sample amount is less; the difference comparison among the different samples is facilitated; the study of an acetylation difference spectrogram provides a technology platform for establishing high-flux protein acetylation difference spectral analysis; and a predictive value of an acetylation state for liver cancer metastasis can be investigated.
Owner:FUDAN UNIV
Quantitative detection reagent and quantitative detection method for urinary protein liquid
InactiveCN102680701AQuantitatively accurateQuantitative fastColor/spectral properties measurementsBiological testingBiologyUrine sample
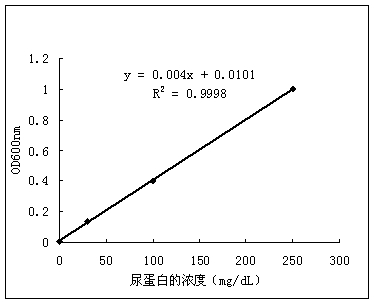
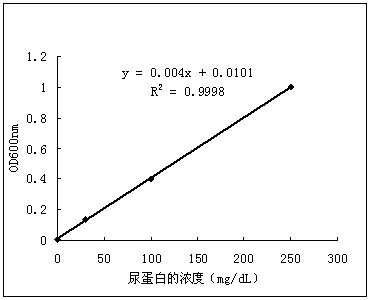
The invention discloses a quantitative detection reagent and a quantitative detection method for urinary protein liquid. The reagent is obtained by mixing pyrogallol solution with an intermediate solution. The intermediate solution is obtained by dissolving a certain amount of succinic acid, sodium benzoate and sodium molybdate in distilled water. By combining an automatic analyzer with the quantitative detection reagent for the urinary protein liquid, quantitative and accurate content of protein in a urine sample can be provided, so that accurate information can be provided to clinical doctors, and quantitative, automatic and fast detection of the urine sample can be achieved.
Owner:JIAXING JIUQIJIU BIOLOGICAL TECH
Kit for synchronously detecting twenty-two respiratory tract pathogens and detection method of kit
ActiveCN103074451BEnsuring Quality JudgmentsImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting twenty-two respiratory tract pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of the nine respiratory tract pathogens, and a reverse primer of a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-6 (sequence identifier number 1-6), and the PCR primer comprises forward and reverse PCR amplification primers of the rest thirteen respiratory tract pathogens, a forward and reverse amplification primer of a human DNA internal reference, a forward and reverse PCR amplification primer of a reaction internal reference, PCR amplification primers of the nine respiratory tract pathogens, and a PCR amplification primer of the human RNA internal reference, and has a gene sequence shown as SEQ ID NO. 7-44. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
Preparation method of gold and silver nanowire SERS sensor for detecting lung cancer marker miR-196a and sensor
ActiveCN109946285AEasy to operateGood repeatabilityMaterial nanotechnologyRaman scatteringSignalling moleculesLung cancer
The invention relates to a preparation method of a gold and silver nanowire SERS sensor for detecting lung cancer marker miR-196a and the SERS sensor. The method comprises the following steps: 1) mixing gold nanoparticles with positive charge and silver nanowires with negative charge; and modifying the gold nanoparticles with positive charge to the silver nanowire with negative charge by electrostatic adsorption so as to prepare gold and silver double-metal nanowires; 2) coupling the gold and silver double-metal nanowires prepared in step 1) to an amination type polishing surface of silicon wafer to prepare an SERS base on which the gold and silver nanowires are uniformly and densely arranged; 3) modifying a raman signaling molecule 5-FAM marked hairpin structural DNA probe on the surfaceof the SERS base so as to form the SERS sensor. The method has the advantages of being high in sensitivity, high in specificity, simple in assembling process, and quick in detection.
Owner:YANGZHOU UNIV
Fluorescent quantitation calculation method based on recombinase-aid isothermal nucleic acid amplification (RAA) method
ActiveCN107016258ARapid Amplification TestQuantitative fastProteomicsGenomicsFood safetyMathematical model
The invention discloses a fluorescent quantitation calculation method based on a recombinase-aid isothermal nucleic acid amplification (RAA) method. A set of mathematical models and calculation methods are established for the RAA nucleic acid amplification technical features, quantitative detection can be conducted accurately to expand the initial template quantity, the RAA nucleic acid amplification technology is better applied and popularized, and the RAA technology is more rapidly, accurately and widely applied to detection and scientific research in the fields of modern agriculture, medicine, food safety, inspection and quarantine, disease control and prevention, environmental microbiological identification and the like.
Owner:宁波奇天基因科技有限公司
Method for detecting activation peroid markers of T lymphocyte in human peripheral blood
InactiveCN103604919AQuantitative fastQuantitatively accurateFluorescence/phosphorescenceCell Surface AntigensMicrosphere
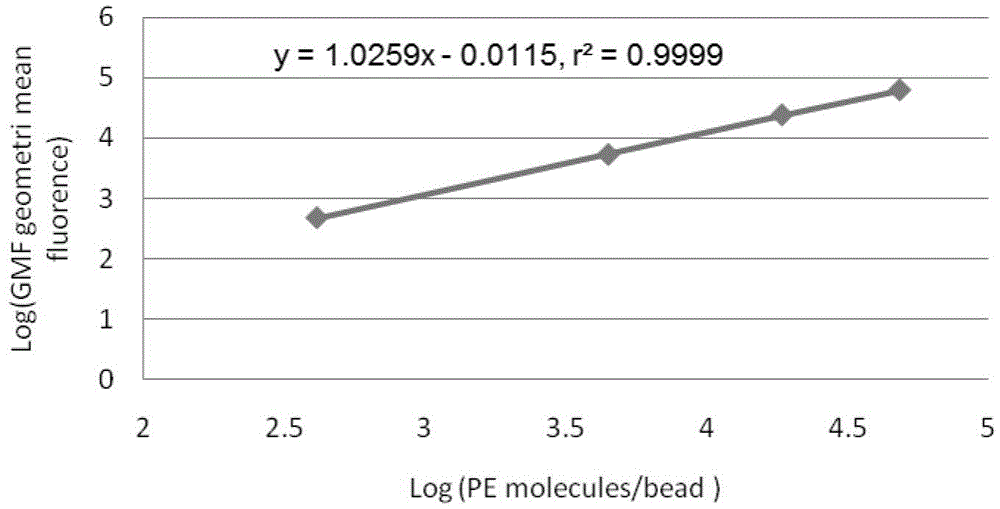
The invention belongs to the technical field of medical tests, and relates to a method for simultaneously determining activation markers CD69, CD25 and CD71 of T lymphocyte in human peripheral blood by employing quantitative flow cytometry. The method comprises the following steps: firstly, cultivating and stimulating peripheral blood; then carrying out dyeing treatment on the cultivated and stimulated peripheral blood, and carrying out red blood cell cracking and washing on the dyed sample; and finally carrying out quantitative flow detection on the washed sample. Meanwhile, a standard curve is built according to QuantiBRITE PE microspheres, and specific expression quantity of antigens on each cell surface is calculated through the standard curve. By adopting the method disclosed by the invention, the antigen expression quantity of the CD69, CD25 and CD71 at the surface of the human T lymphocyte can be accurately and sensitively determined, and immunological characteristics of the detected T cells can be reflected. Thus, certain immunological basis and detection index are supplied for clinical research.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Quantitative analysis method of nitrocellulose component in propellant
The invention discloses a quantitative analysis method of a nitrocellulose component in a propellant, which comprises the following steps: after treating a propellant sample, extracting with organic ether at 55-70 DEG C; soaking organic ether insoluble substance in water while keeping the water temperature at 40-60 DEG C; drying the organic ether insoluble substance subjected to organic ether extraction and water soaking, cooling and weighing; and determining the nitrogen element content in the organic ether insoluble substance by using a secondary-combustion nitrogen element analysis meter of which the sample size is 80-100mg, and comparing with the nitrogen content in the raw material nitrocellulose to obtain the content of the nitrocellulose component in the propellant. The method is direct, quick and accurate, and is applicable to quantification of the nitrocellulose component in mono-base and double-base propellants as well as modified double-base propellants and composite propellants.
Owner:XIAN MODERN CHEM RES INST
A method for rapid detection of phenyllactic acid isomers by reversed-phase high performance liquid chromatography
InactiveCN102288703ASimple and fast operationGood reproducibilityComponent separationHplc methodEnantiomer
The invention relates to a method for analyzing DL-3-phenyllactic acid enantiomer by a reversed phase high performance liquid chromatogram and belongs to the technical field of high performance liquid chromatogram analysis. The method comprises the following steps of: dissolving cyclodextrin into buffer solution to obtain cyclodextrin buffer solution containing a chiral selective agent; dissolving a sample into distilled water or the cyclodextrin buffer solution; and separating two enantiomers of D-(+)-3-phenyllactic acid and L-(-)-3-phenyllactic acid by the reversed phase high performance liquid chromatogram. The cyclodextrin serves as a chiral agent, is low in background absorption, low in price and readily available, and adopts a reversed phase C18 chromatographic column commonly used in the liquid chromatogram instead of an expensive chiral chromatographic column. Compared with the prior art, the method has the advantages of low detection cost, high separation efficiency, high detection sensitivity, no toxicity and low consumption of the reagent, environment friendliness, simpleness and convenience for operation, and capacity of realizing chiral separation of the enantiomers under the inversed phase condition. By the method, the medicine synthetical intermediate DL-3-phenyllactic acid enantiomer can be detected rapidly and the produced phenyllactic acid can be detected qualitatively and quantitatively.
Owner:JIANGNAN UNIV
Kit capable of synchronously detecting eighteen kinds of fever with eruption pathogens and detection method thereof
ActiveCN103146841AEnsuring Quality JudgmentsImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesReverse transcriptasePolymerase L
The invention discloses a kit capable of synchronously detecting eighteen kinds of fever with eruption pathogens and a detection method thereof. The kit comprises diethylpyrocarbonate (DEPC) water, 5*reverse transcriptase (RT) buffer solution, reverse transcription primers, reverse transcriptase, X solution, 10* polymerase chain reaction (PCR) buffer solution, PCR primers, 25m M magnesium chloride solution, deoxyribonucleic acid (DNA) polymerase and positive reference substances. The kit is characterized in that the reverse transcription primers comprise five kinds of fever with eruption pathogens and ribonucleic acid (RNA) internal reference RT primers, and the sequences are shown as SEQ ID NO.1-NO.6. The PCR primers comprises the remaining thirteen kinds of fever with eruption pathogens, herpes simplex virus 1, 2 universal type, human DNA internal reference, forward and reverse PCR amplification primers of a reaction internal reference, and the five kinds of fever with eruption pathogens and PCR amplification primers of a human RNA internal reference. The gene sequence is shown as SEQ ID NO.7-NO.44. The kit has the advantages of being strong in specificity, high in sensitivity, high in flux, strong in reliability, low in cost, and free from false-negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
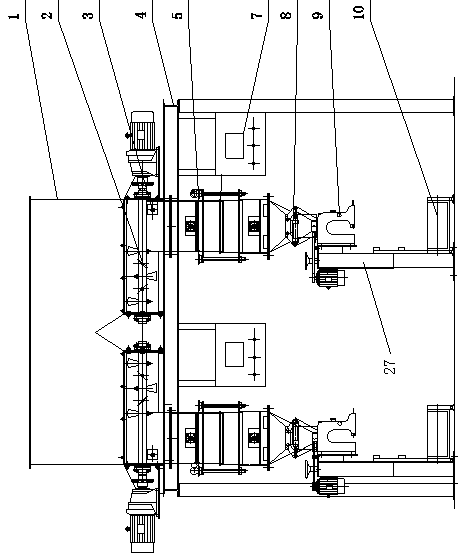
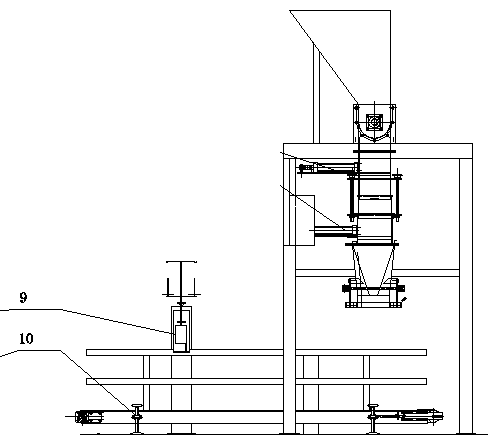
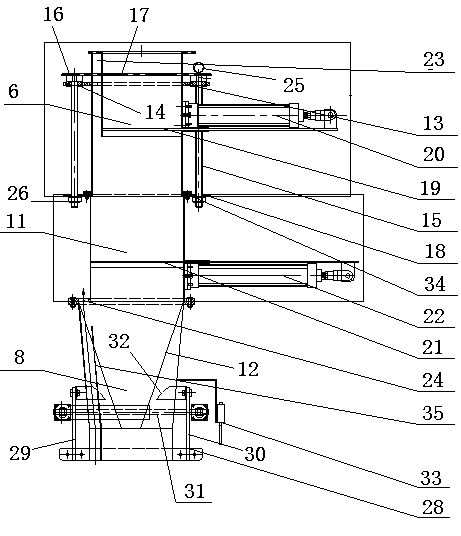
Volumetric type automatic quantitative packing machine and capacity regulating device thereof
PendingCN107719725AReduce labor intensityConducive to online loadingSolid materialFertilizer plantEngineering
The invention discloses a capacity regulating device for a packing machine. The capacity regulating device comprises an upper capacity body and a lower capacity body which sleeve with each other, wherein a charge hole is formed in the upper part of the upper capacity body; a discharge nozzle is arranged on the lower part of the lower capacity body; an upper flange is fixedly arranged outside the upper capacity body; a plurality of nuts are fixedly arranged on the upper flange; a vertical lead screw is screwed into each nut; a chain wheel is fixedly arranged at the upper end of each lead screw;chain wheels of each lead screw are synchronously connected through a chain, and a regulating device is arranged at the upper end of one lead screw; a lower flange is fixedly arranged outside the lower capacity body, the lower end of the lead screw is inserted into each light hole of the lower flange, and the lower end of the lead screw is equipped with an anti-dropping choke plug. The capacity regulating device realizes quantitative packing on solid materials according to the volume, and overcomes a phenomenon that packing capacity is affected by moisture changes of the material. Especiallyfor quantitative packing for a nursery substrate fertilizer plant, quantifying uniformity is high, filling speed is high and production efficiency is high, so that online loading of fertilizer enterprises is facilitated, and the handing cost and reservoir capacity occupation are reduced.
Owner:平原博越机械科技有限公司
Kit for rapid identification of nocardia and using method of kit
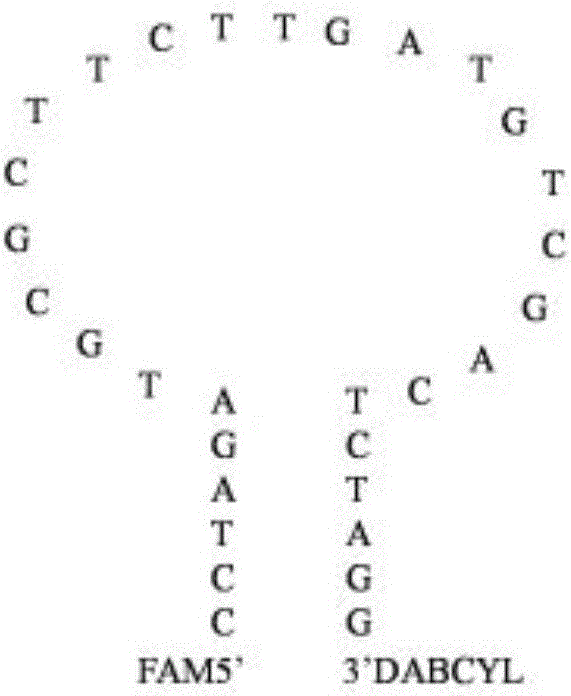
InactiveCN106755346AResolve quick identificationReduce identification costsMicrobiological testing/measurementMicroorganism based processesRapid identificationNocardia
The invention discloses a kit for rapid identification of nocardia. The kit is characterized in that a probe design sequence is shown as FAM-CCTAGA TGCGCTTCTTGATGTCGAC TCTAGG-DABCYL; and composition of the kit is shown as the Description. In accordance with the design, identification and diagnosis can be conducted on the nocardia without sequencing, so that the rapid identification of pathogenic actinomycetes is achieved, difficulties and bottlenecks in the identification and the diagnosis are solved, identification cost is reduced and operations are simplified; and in addition, influence to a PCR amplification reaction is avoided.
Owner:GUIZHOU MEDICAL UNIV
Method for simultaneously quantifying two viable pathogenic bacteria in penaeus vanmamei
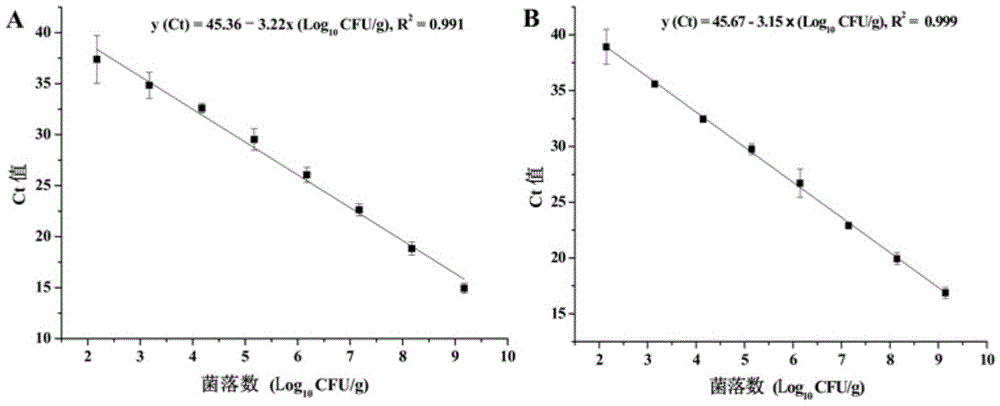
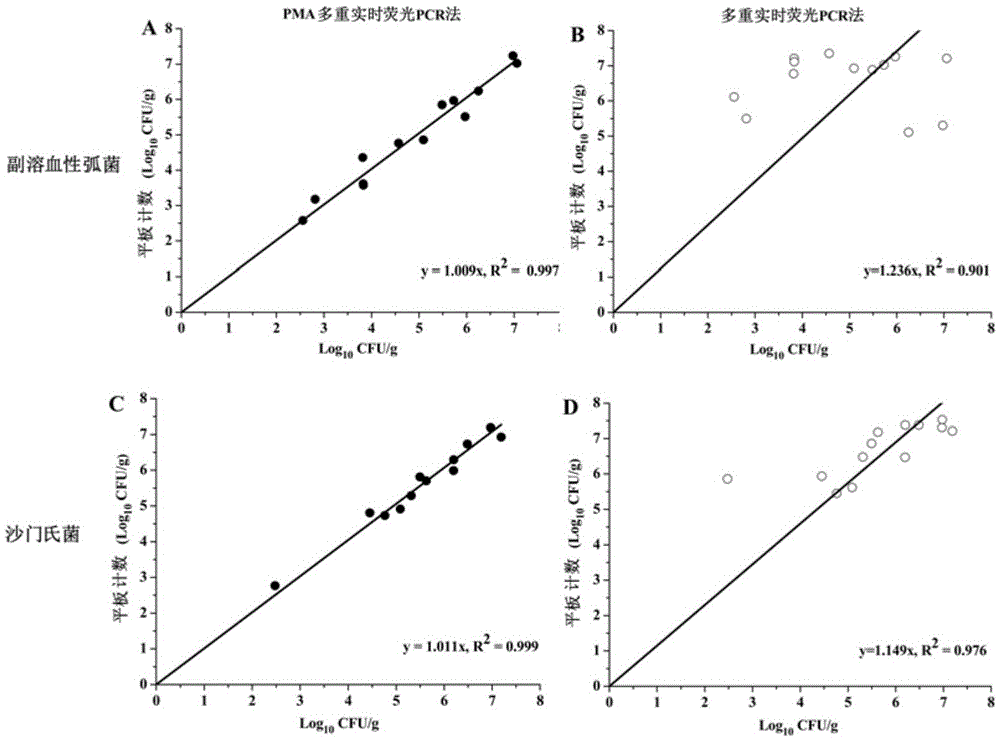
InactiveCN105543367AQuantitative fastOptimal treatment conditionsMicrobiological testing/measurementMicroorganism based processesFluorescenceStandard curve
The invention discloses a method for simultaneously quantifying two viable pathogenic bacteria in penaeus vanmamei. The method includes the following steps that propidium monoazide mother liquid is added into to-be-detected penaeus-vanmamei-liquid homogenate, and propidium monoazide sample mixed liquid is obtained; the sample mixed liquid is subjected to dark treatment to be exposed and centrifuged at a high speed, and the bacteria are collected; DNA of the bacteria is extracted; a multi-fluorescence quantitative PCR amplified reaction is carried out with the extracted DNA as a template and t1h genes of vibrio parahaemolyticus and orgC genes of salmonella bacteria as target genes,, and corresponding circulation threshold values are output; the circulation threshold values are compared with a standard curve of the vibrio parahaemolyticus and a standard curve of the salmonella bacteria respectively, and the viable-bacterium concentration of the vibrio parahaemolyticus and the viable-bacterium concentration of the salmonella bacteria are obtained. According to the method, the multi-fluorescence quantitative PCR technology and PMA dye are creatively combined, and the aim that the two important viable pathogenic bacteria in the penaeus vanmamei are rapidly quantified is achieved.
Owner:SHANGHAI OCEAN UNIV
Anti-Mullerian hormone quantitative measurement reagent and method based on nanometer up-converting phosphor method
ActiveCN107340398AQuick checkAccurate detectionDisease diagnosisBiological testingPhosphorRare earth
The invention relates to an anti-Mullerian hormone quantitative measurement reagent and method based on a rare earth nanometer up-converting phosphor method. UCP particles in which the up-converting phosphor method is adopted are used as a biomarker, and an anti-AMH antibody is specifically marked. Through the adoption of the anti-Mullerian hormone quantitative measurement reagent and method based on the up-converting phosphor method, the content of AMH in a measured sample can be quickly, simply, quantitatively and accurately measured, and a detection basis is provided for evaluation of the female ovarian reserve function.
Owner:BEIJING HOTGEN BIOTECH CO LTD +1
Fluorescent quantitative PCR method for detecting vibrio parahaemolyticus causing acute hepatic pancreatic necrosis
PendingCN110106264AImprove stabilityReduce backgroundMicrobiological testing/measurementMicroorganism based processesVibrio parahaemolyticusBiology
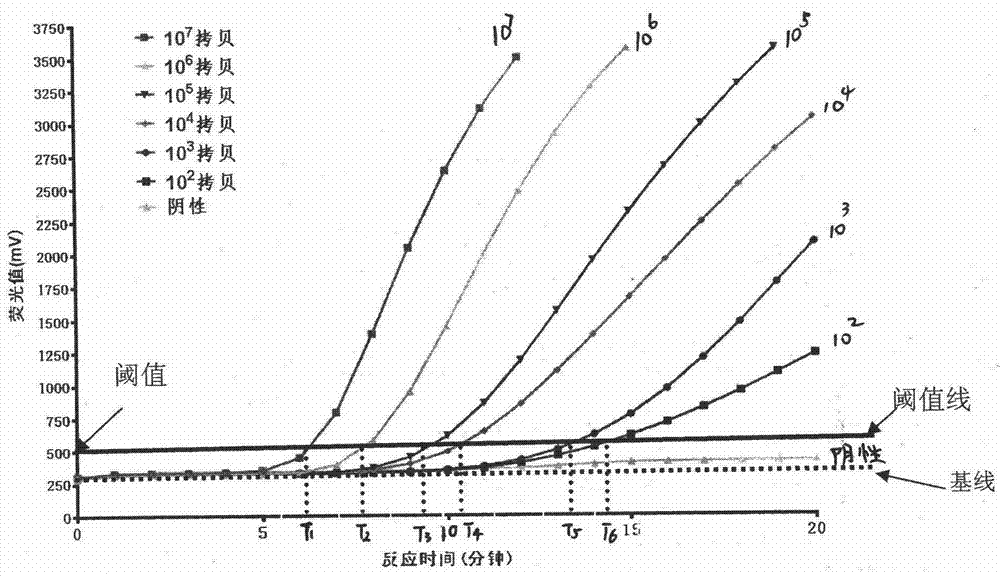
The invention discloses a fluorescent quantitative PCR method for detecting vibrio parahaemolyticus causing acute hepatic pancreatic necrosis. The method specifically comprises the following steps of(1) taking the PirAVp gene of VpAHPND as a detection target gene and designing a specific primer and a TaqMan-MGB probe; (2) extracting the DNA of the VpAHPND, constructing a recombinant plasmid, preparing a standard substance, and storing at -20 DEG C for use; (3) performing a quantitative PCR, and establishing a standard curve and a standard equation between the logarithm of the initial templatecopy number and the threshold cycle number; (4) detecting the to-be-detected sample with the primer and the probe in the step (1),and based on the measured threshold cycle number, according to the standard curve, calculating the copy number of the VpAHPND in the to-be-detected sample. The Fluorescent quantitative PCR method for detecting the VpAHPND for non-diagnostic purposes established by theinvention has the advantages of high sensitivity, strong specificity, good reproducibility, rapid quantification and the like, and can be used for clinically detecting the VpAHPND of shrimp samples and water samples.
Owner:GUANGXI ACADEMY OF FISHERY SCI
Method for rapidly quantifying synthetic cathinone drugs in urine
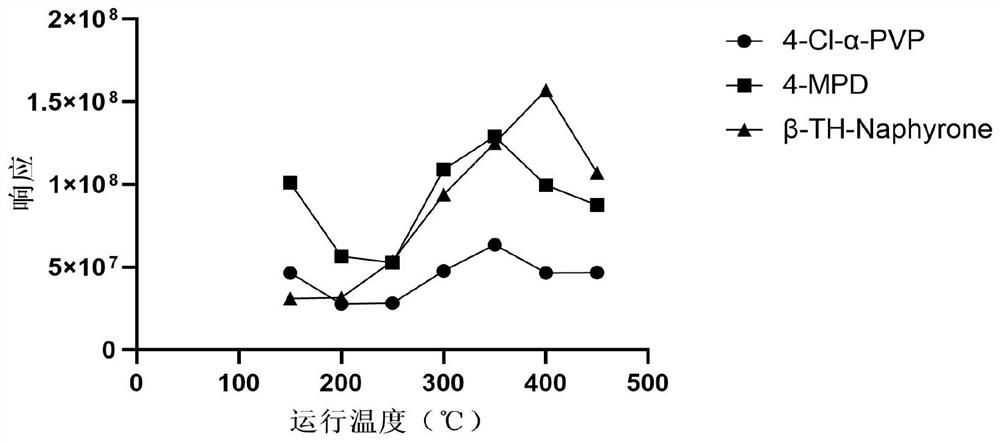
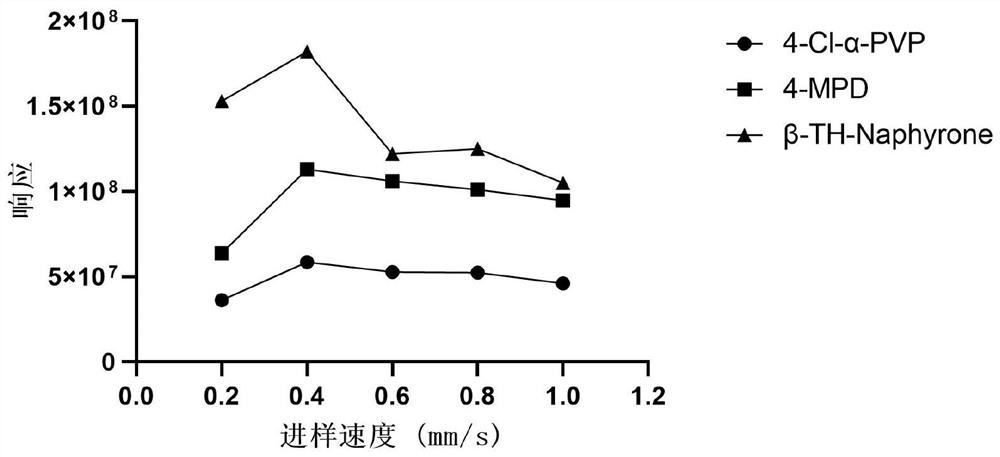
ActiveCN113804746ARapid quantitationHigh sensitivityPreparing sample for investigationMaterial analysis by electric/magnetic meansForensic PharmacyResolution (mass spectrometry)
The invention relates to a method for rapidly quantifying synthetic cathinone drugs in urine. The method comprises the following steps: by taking methcathinone-D3 and SKF525A as internal standard substances, extracting synthetic cathinone drugs in urine by using hydrophobic magnetic beads through a magnetic dispersive solid-phase extraction (MDSPE), rapidly quantifying by combining a real-time direct analysis high-resolution mass spectrometry (DART-HRMS), and obtaining a quantifying result within one minute after sample introduction, wherein a to-be-detected substance is good in stability under various conditions, the correlation coefficient is larger than 0.99, the deviation of precision and accuracy is smaller than 15%, rapid quantification of the synthesized cathinone drugs in urine is achieved, detection is rapid and convenient, and a new and effective technical support can be provided for rapidly and quantitatively synthesizing cathinone drugs in forensic poison analysis.
Owner:SHANGHAI CRIMINAL SCI TECH RES INST
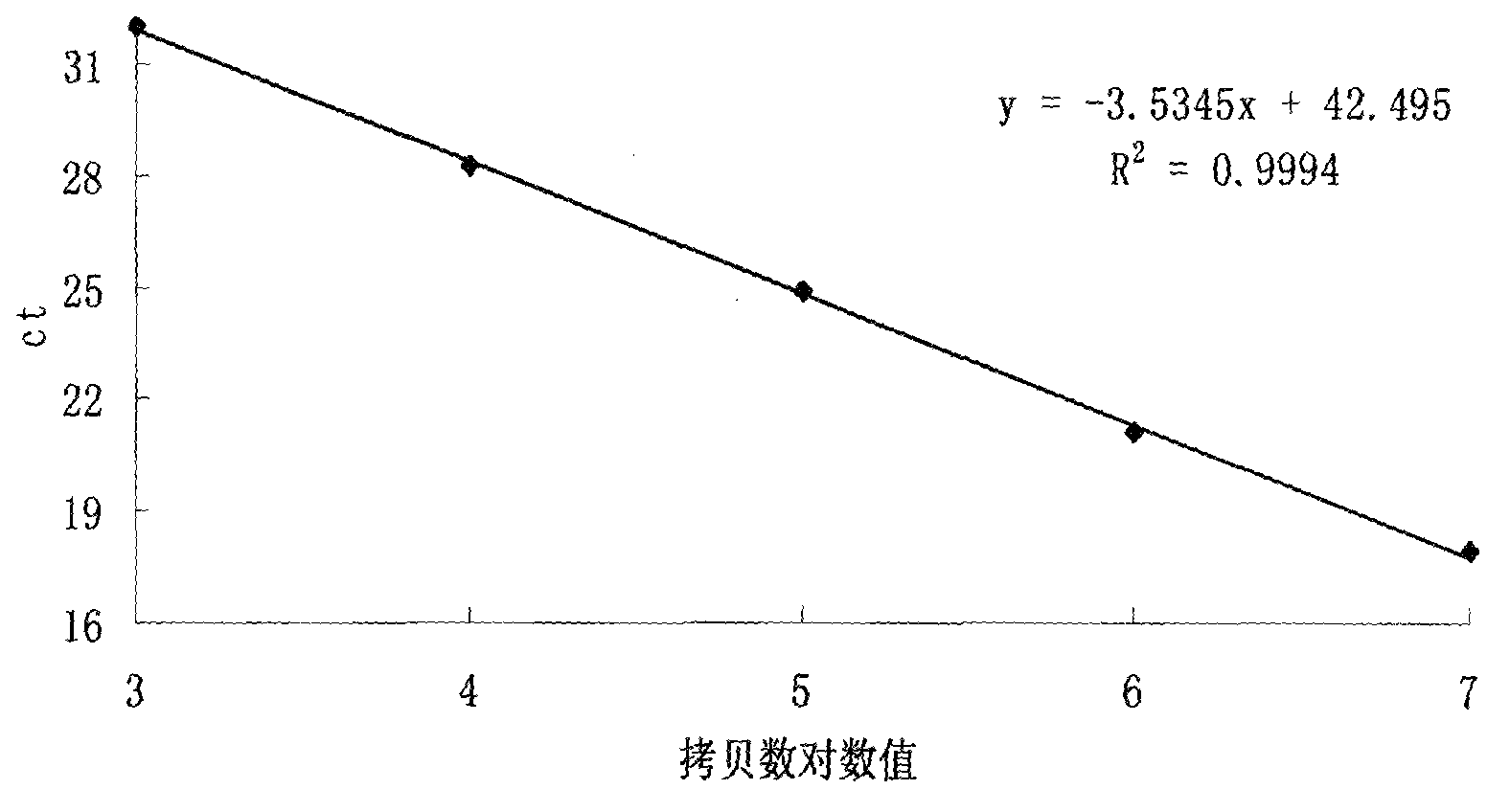
Detection method for dose of ionizing radiation on human peripheral blood lymphocytes
InactiveCN103805683AGood stability and repeatabilityShorten the timeMicrobiological testing/measurementPeripheral blood lymphocyteStandard curve
The present invention relates to a detection method for a dose of ionizing radiation on human peripheral blood lymphocytes. The detection method mainly comprises: designing primers and a Taqman-MGB probe according to a lymphocyte gdf15 gene expression sequence, and constructing a recombinant vector containing the lymphocyte gdf15 gene expression sequence as a standard substance; adopting the 10-fold serial diluted standard substance to carry out real-time fluorescence PCR, and drawing an absolute quantification standard curve; carrying out quantitative determination on the expression levels of the gdf15 gene of lymphocytes with different culture times after ionizing radiations with different doses to obtain a dose-effect fitting curve of the radiation dose and the gdf15 gene expression level; and carrying out quantitative determination on the expression level of the gdf15 gene of lymphocytes with the radiation dose requiring detection, and calculating the dose of the ionizing radiation on the lymphocytes requiring detection. According to the present invention, the dose of ionizing radiation on human peripheral blood lymphocytes can be rapidly and quantitatively detected so as to meet requirements of simpleness, rapid quantitation and high throughput, and the advantages can be provided when the large-scale radiation accident occurs.
Owner:NAT INST FOR RADIOLOGICAL PROTECTION & NUCLEAR SAFETY CHINESE CENT FOR DISEASE CONTROL & PREVENTION
Reagent kit for real time fluorescence quantitative PCR detection of bacillus pyocyaneus
InactiveCN101429539BReal-time quantitativeReal-time quantitative detectionMicrobiological testing/measurementUpper urinary tract infectionCorneal ulcer
The invention relates to a kit for detecting presence of pathogen pseudomonas aeruginosa in patient samples such as clinical bacterial pneumonia, corneal ulcer, urinary tract infection, septicemia and the like and samples such as cosmetics, environmental monitoring objects and the like, in particular to a kit for early and quickly diagnosing pseudomonas aeruginosa infection by a real-time fluorescence quantitative polymerase chain reaction technique.
Owner:DAAN GENE CO LTD
Primer group for detecting mycoplasma hyopneumoniae, application of primer group and real-time fluorescent quantitative PCR detection method
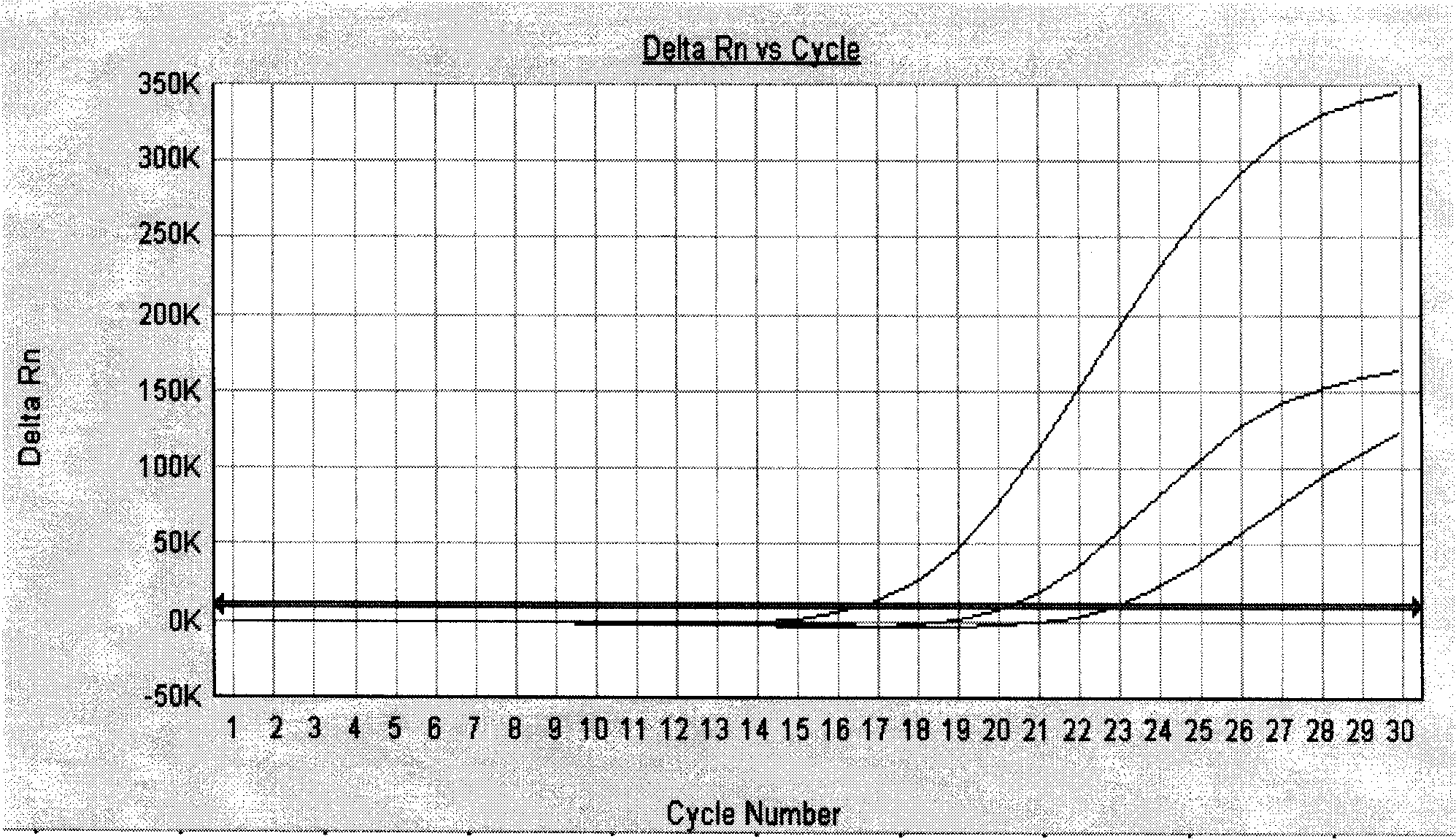
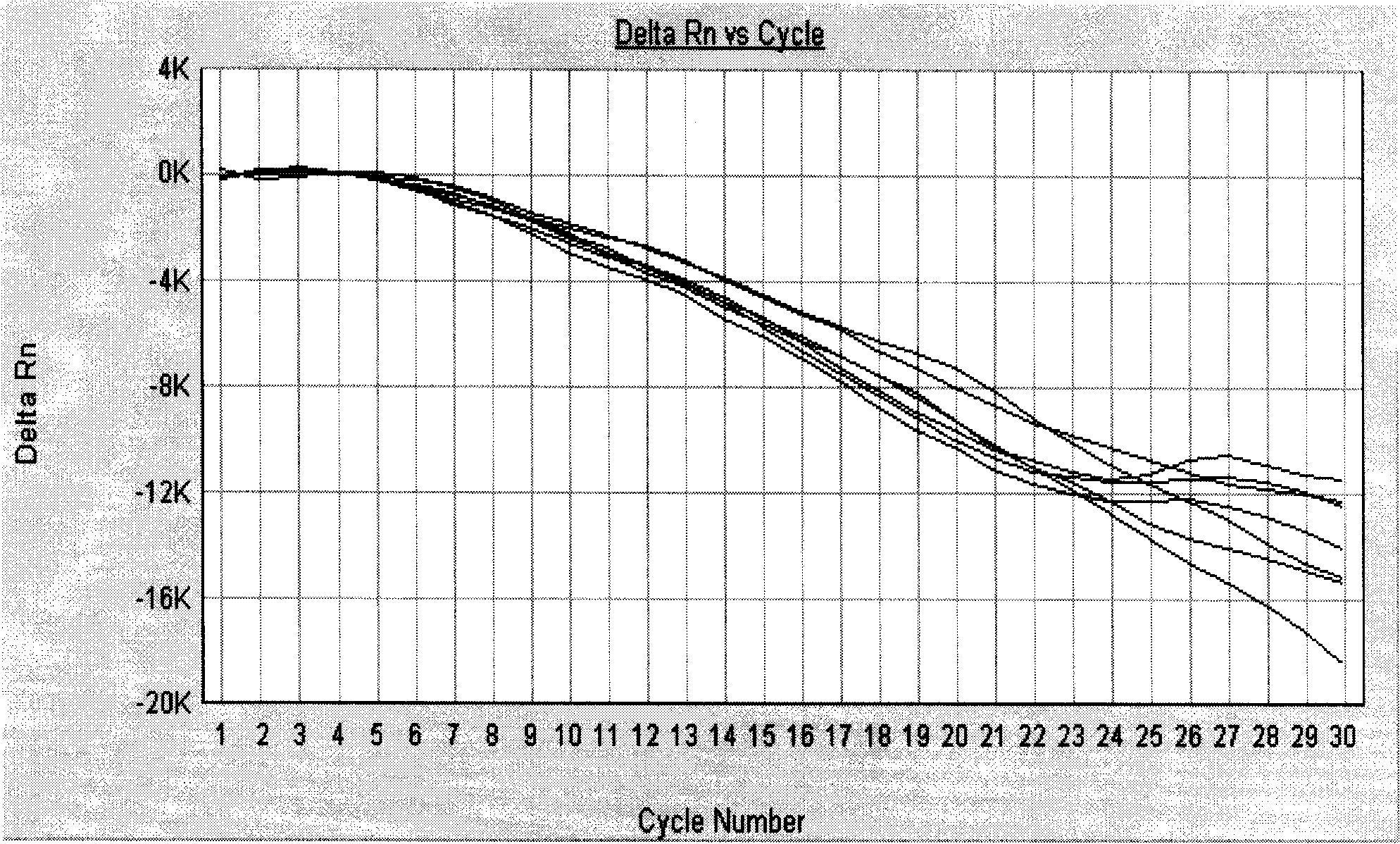
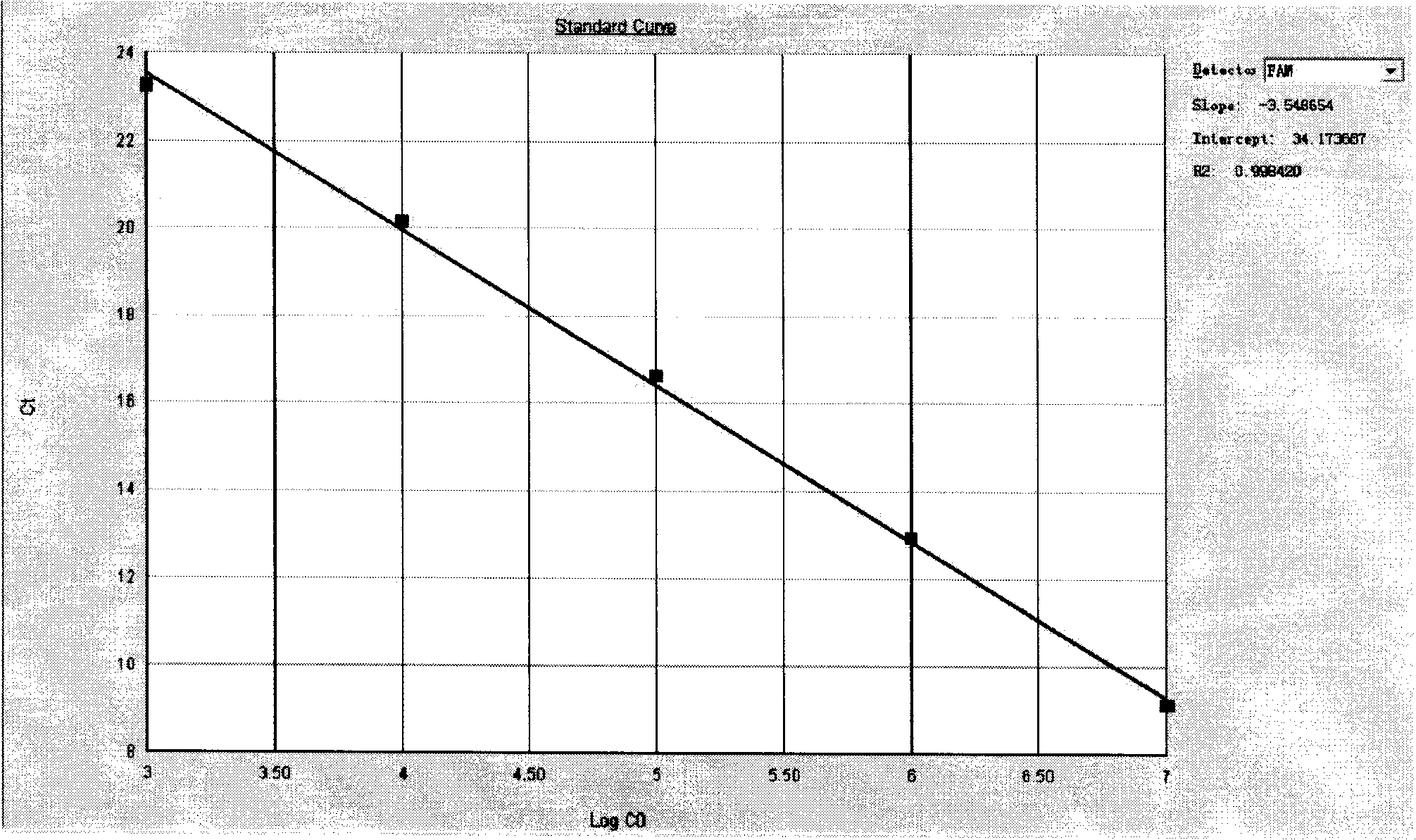
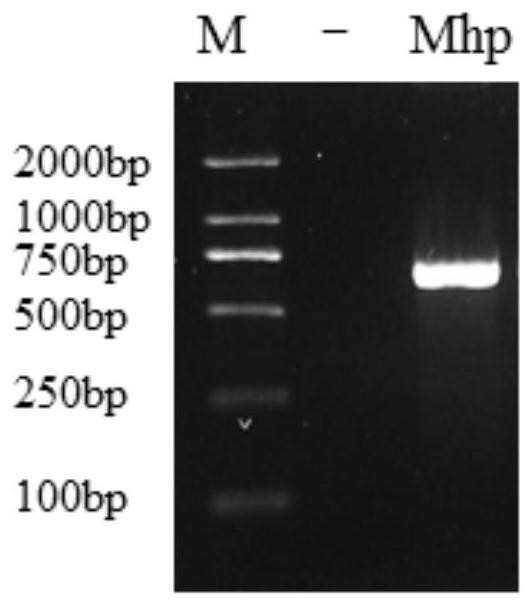
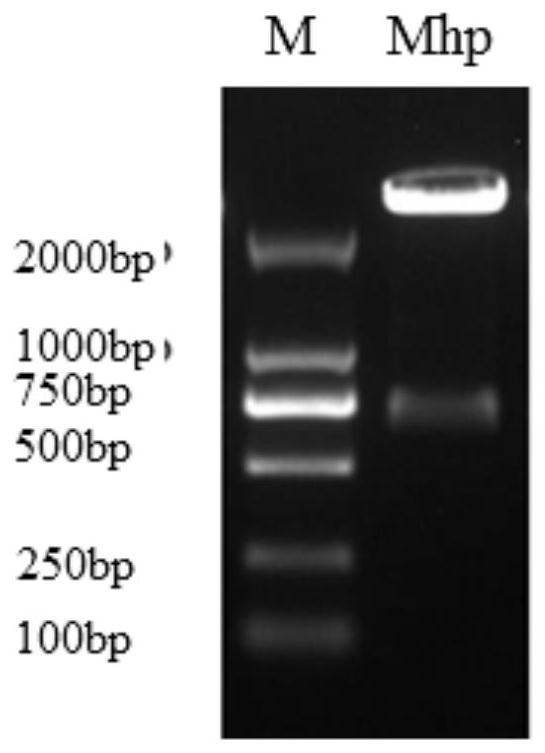
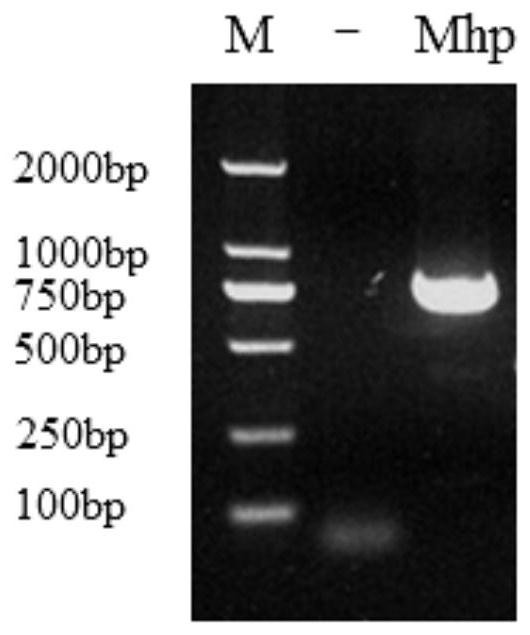
ActiveCN113308561AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processesMycoplasma hyopneumoniaeBioinformatics
The invention provides a primer group for detecting mycoplasma hyopneumoniae, application of the primer group and a real-time fluorescent quantitative PCR detection method, and relates to the technical field of biology. The primer group for detecting the mycoplasma hyopneumoniae comprises a first primer pair and a probe, has the characteristics of strong specificity and high sensitivity, and can be used for detecting the mycoplasma hyopneumoniae. The real-time fluorescent quantitative PCR detection method for the mycoplasma hyopneumoniae adopts the primer group, and comprises the following steps: firstly, performing real-time fluorescent quantitative PCR on a mycoplasma hyopneumoniae standard substance with a known initial copy number to obtain a relationship between the copy number and a Ct value; then extracting DNA of a to-be-detected sample for real-time fluorescent quantitative PCR, and obtaining the target gene copy number in the to-be-detected sample through calculation. The detection method is high in specificity, good in repeatability, high in sensitivity, rapid in detection and accurate in quantification and has great significance in rapid quantitative detection of mycoplasma hyopneumoniae cultures, vaccine semi-finished products and the like.
Owner:天康制药(苏州)有限公司
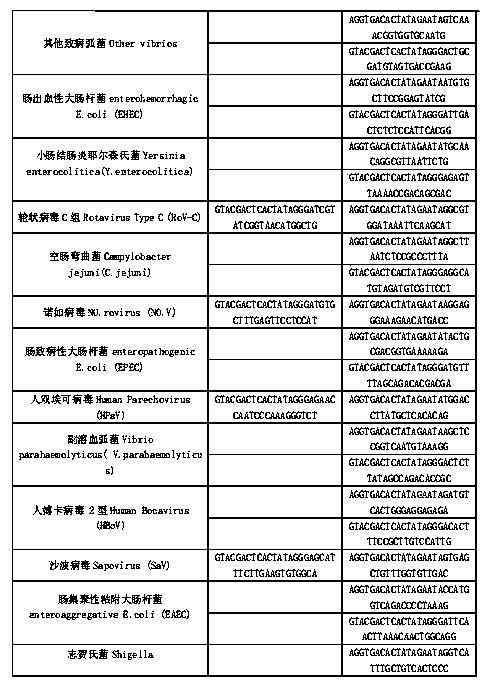
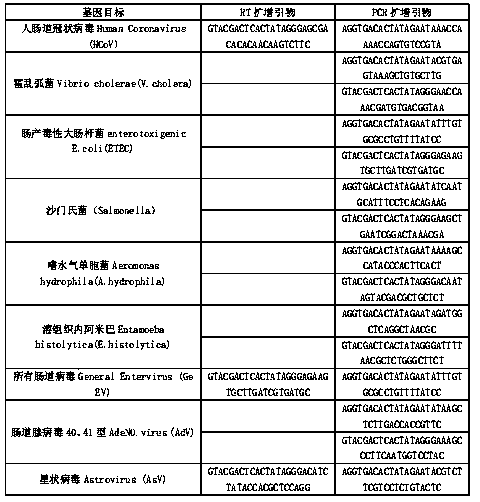
Kit for synchronously detecting thirty diarrhea pathogens and detection method of kit
ActiveCN103074450BStrong specificityImprove detection efficiencyMicrobiological testing/measurementMicroorganism based processesPositive controlReverse transcriptase
The invention discloses a kit for synchronously detecting thirty diarrhea pathogens and a detection method of the kit. The kit comprises DEPC (diethylpyrocarbonate) water, a 5*RT (reverse transcription) buffer, a reverse transcription primer, a reverse transcriptase, an X solution, a 10*PCR (polymerase chain reaction) buffer, a PCR primer, a 25mM magnesium chloride solution, a DNA (deoxyribonucleic acid) polymerase and a positive control, and is characterized in that the reverse transcription primer comprises RT amplification primers of eleven diarrhea RNA viruses and a human RNA (ribonucleic acid) internal reference, and has a gene sequence shown as SEQ ID NO. 1-12 (sequence identifier number 1-12), and the PCR primer comprises forward and reverse PCR amplification primers of the rest nineteen diarrhea pathogens, a human DNA internal reference and a reaction internal reference, and PCR amplification primers of eleven diarrhea RNA viruses and the human RNA internal reference, and has a gene sequence shown as SEQ ID NO. 13-66. The kit and the detection method have the advantages of high specificity, sensitivity, flux and reliability, low cost, and no false negative results.
Owner:NINGBO HEALTH GENE TECHNOLOGIES CO LTD
Immunofluorescence kit for quantitatively detecting blood vessel endothelial marker CD105
PendingCN109100505AReasonable designEasy to useBiological material analysisBiological testingFluorescenceBlood vessel
The invention discloses an immunofluorescence kit for quantitatively detecting blood vessel endothelial markers CD105. The immunofluorescence kit comprises a kit body and a test strip arranged in thekit body; the kit comprises a second body, a trace blood-collecting vessel, a peripheral blood taking needle, a sucker, and an alcohol prep pad are arranged in the second body, full process of the blood drawing and blood test can be accomplished once; a detection line and a quality control line are arranged on the test strip; when being loaded on a sample pad, a to-be-detected sample moves forwardthrough capillary action, firstly reacts with a fluorescent mark antibody on a conjugate pad to form a fluorescent mark antibody-antigen compound; the to-be-tested sample continuously move forward, and combines with an antibody II cladded by the detection line to form the fluorescent mark antibody-antigen-antigen sandwich compound to immobilize on the detection line; the redundant fluorescent mark antibody and the rabbit anti-mouse IgG are combined and immobilized, and then detected through a fluorescence detector. The immunofluorescence kit disclosed by the invention has the advantages of being quantitative, fast, simple and convenient, and sensitive, and extensive in market prospect.
Owner:NINGBO AUCHEER BIOTECHNOLOGY CO LTD
Method for quantitatively detecting free amino acid in clinical sample
InactiveCN106353424AEasy to removeEfficient separationComponent separationChromatographic separationAlcohol
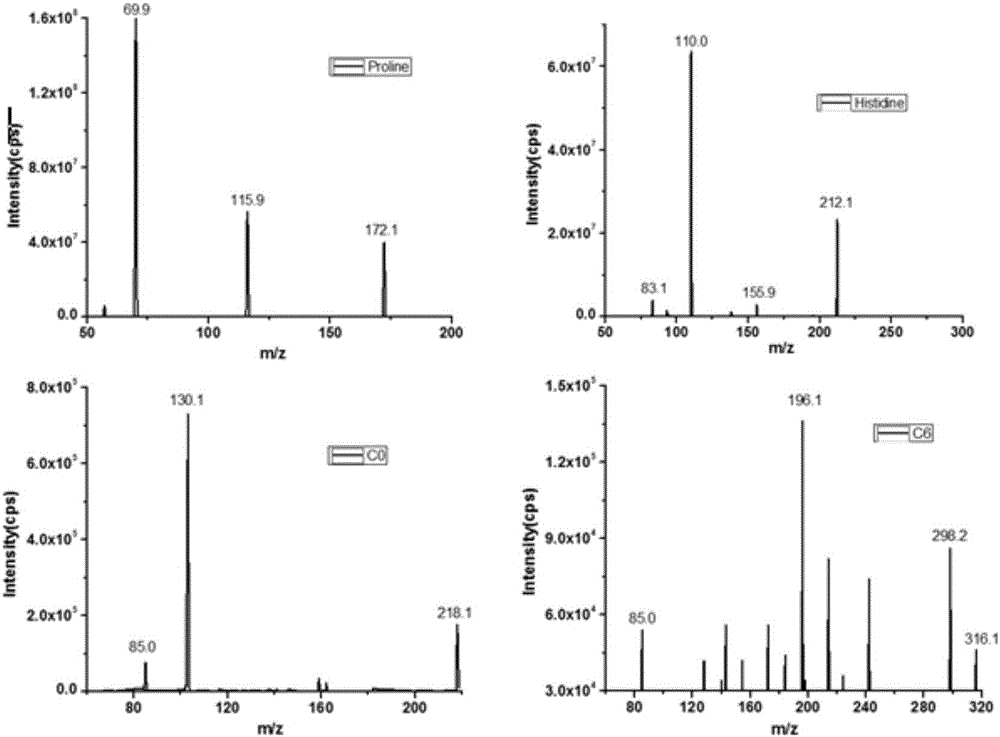
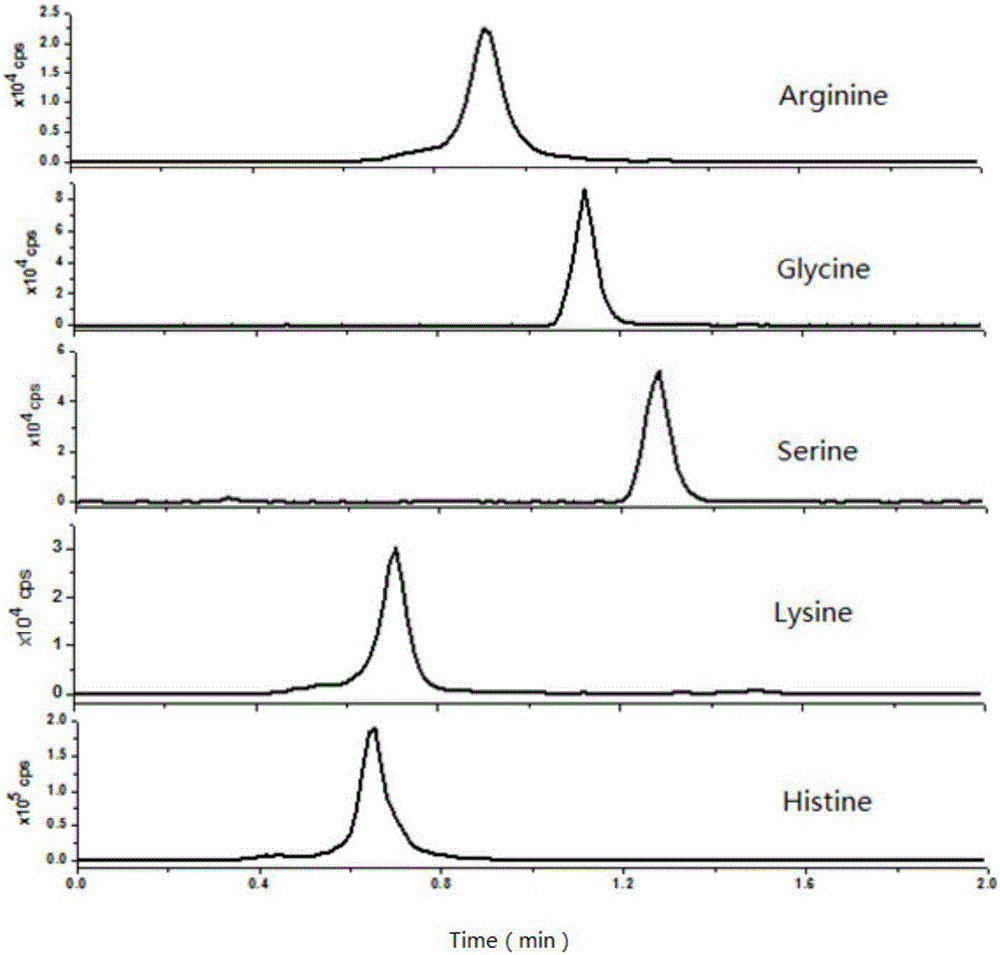
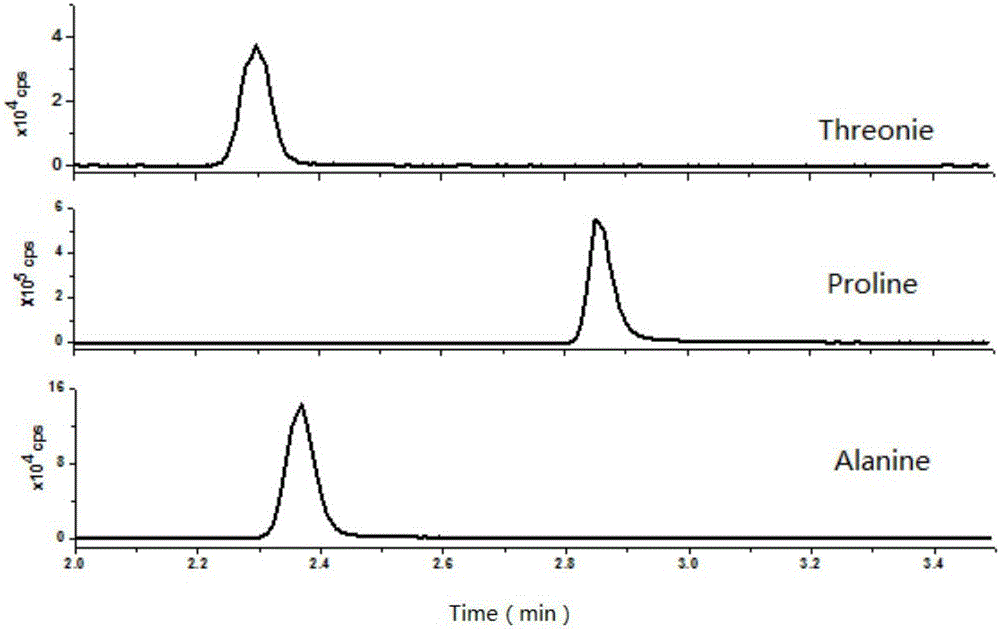
The invention discloses a method for quantitatively detecting a free amino acid in a clinical sample, and belongs to the field of analysis and detection. According to the method, an acidic methanol solution is used for extracting the free amino acid in the clinical sample, then the property of the amino acid is changed through alcohol derived carboxyl, and deuteration agent derived amino acid serves as an internal standard instead of deuteration amino acid to be favorable for chromatographic separation and quantitative mass spectrometric detection. The operation of the method is simple, the amino is basically separated within a short period of time, the interference of a matrix is greatly reduced, the cost is low, the specificity is good, and the method is suitable for quantitative detection of the free amino acid in the clinical sample.
Owner:武汉生物技术研究院有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com