Immunofluorescence kit for quantitatively detecting blood vessel endothelial marker CD105
A technology for quantitative detection of vascular endothelium, applied in the direction of biological testing, measuring devices, material inspection products, etc., can solve the problems of long operation time, only qualitative, poor accuracy, etc., and achieve broad market prospects, reasonable design, and convenient use Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The structure of the immunofluorescence assay kit of the present invention;
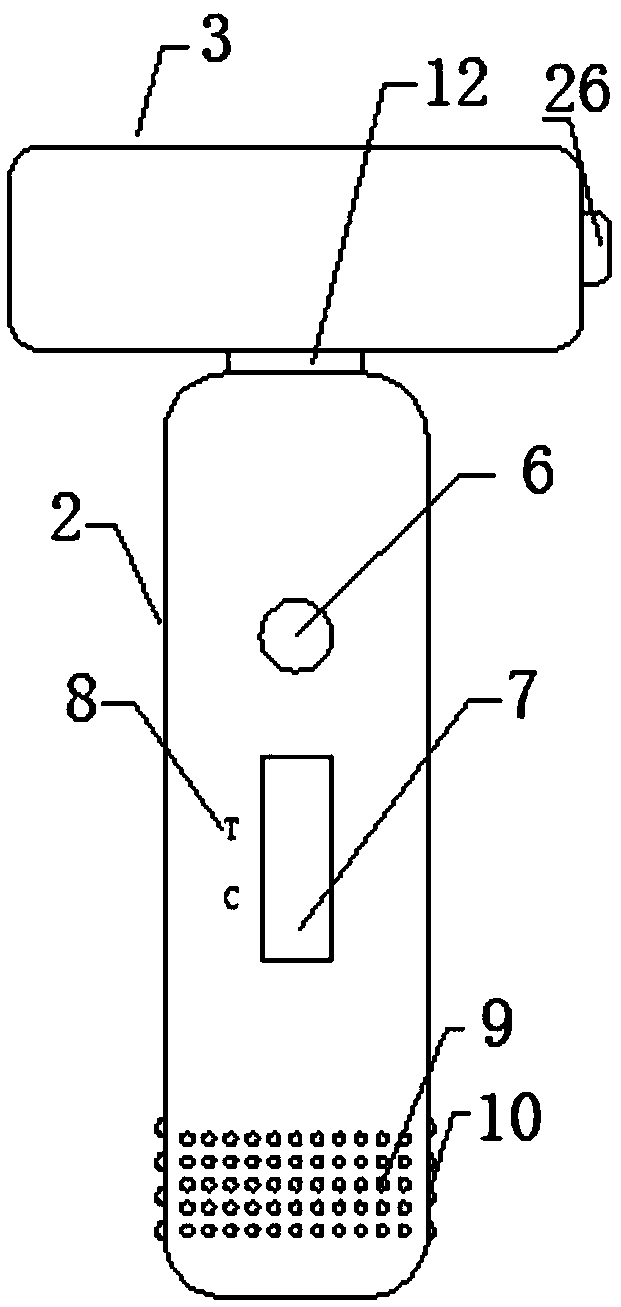
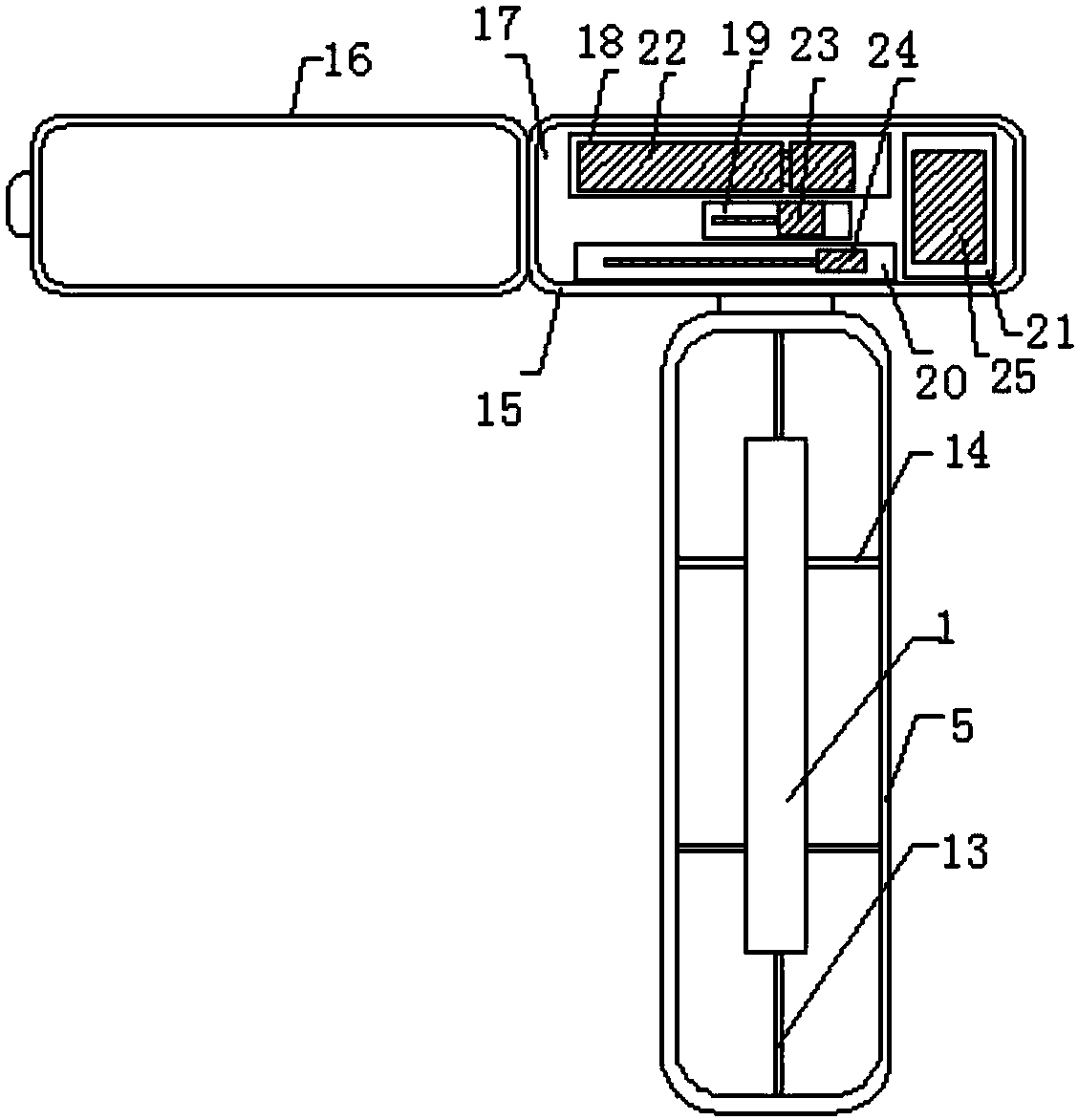
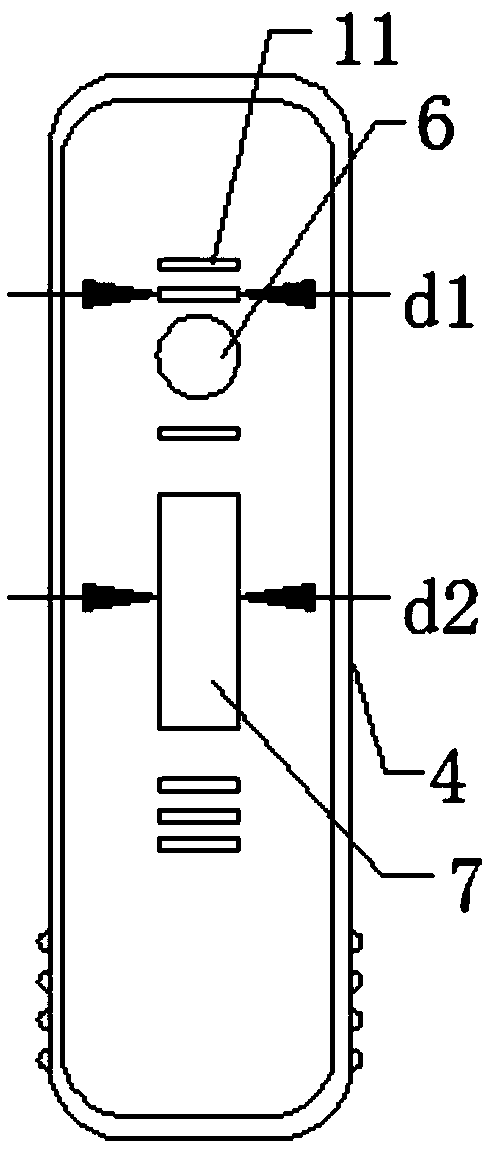
[0028] Such as Figure 1-4 As shown, an immunofluorescence kit for quantitatively detecting the vascular endothelial marker CD105 includes a kit body and a test strip disposed in the kit body, the kit body includes a first box body, a second box body Body and the connecting strip connecting the first box body and the second box body. The first box body includes an upper shell and a lower shell. The upper shell and the lower shell are connected by clamping. Sample hole, observation window, one side of the observation window is provided with a detection mark, and the upper surface of the upper shell is also provided with a number of anti-skid protrusions, which are arranged in a rectangular array, and a number of anti-skid strips are provided on both sides of the lower end of the upper shell , a number of anti-slip strips are arranged equidistantly. There are clamping strips on the inner surfac...
Embodiment 2
[0037] The preparation method of the immunofluorescence assay kit of the present invention;
[0038] An immunofluorescence kit for quantitative detection of vascular endothelial marker CD105 comprises the following preparation steps:
[0039] (1) Preparation of FITC-labeled monoclonal antibody I: Dilute CD105 monoclonal antibody I with PBS buffer (0.05M, pH=7.2) containing 0.05mol / l NaCl at 4°C to make the final CD105 monoclonal antibody I The concentration is 10 mg / ml, add FITC fluorescein to the CD105 monoclonal antibody Ⅰ solution, the ratio of FITC fluorescein to CD105 monoclonal antibody Ⅰ is 1:50, stir at 4°C for 12 hours, add half-saturated ammonium sulfate, centrifuge, and Remove the precipitate, then dialyze with PBS buffer (0.01M, pH=7.2) to remove ammonium sulfate, add 0.01w / v% thimerosal to the prepared FITC-labeled antibody, divide into 1ml ampoules, and store at 4°C;
[0040] (2) Preparation of monoclonal antibody binding pad: Dilute the FITC-labeled CD105 monoc...
Embodiment 3
[0045] The preparation method of the immunofluorescence assay kit of the present invention;
[0046] An immunofluorescence kit for quantitative detection of vascular endothelial marker CD105 comprises the following preparation steps:
[0047] (1) Preparation of FITC-labeled monoclonal antibody I: Dilute CD105 monoclonal antibody I with PBS buffer (0.05M, pH=7.2) containing 0.15mol / l NaCl at 4°C to make the final CD105 monoclonal antibody I The concentration is 20 mg / ml, add FITC fluorescein to the CD105 monoclonal antibody Ⅰ solution, the ratio of FITC fluorescein to CD105 monoclonal antibody Ⅰ is 1:40, stir at 4°C for 16 hours, add half-saturated ammonium sulfate, centrifuge, and Remove the precipitate, then dialyze with PBS buffer (0.01M, pH=7.2) to remove ammonium sulfate, add 0.01w / v% thimerosal to the prepared FITC-labeled antibody, divide into 1ml ampoules, and store at 4°C;
[0048] (2) Preparation of monoclonal antibody binding pad: Dilute the FITC-labeled CD105 monoc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com