Method for detecting activation peroid markers of T lymphocyte in human peripheral blood
A lymphocyte and human peripheral blood technology, applied in the field of medical testing, can solve the problems that the test results cannot be directly compared, the absolute number of antigens or molecules cannot be expressed, and the qualitative analysis of fluorescence intensity cannot accurately reflect the characteristics of dynamic changes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 The expression levels of CD69, CD25 and CD71 in healthy people
[0028] ①Sample collection: 5 healthy volunteers were selected, 3ml of peripheral venous blood was drawn, and placed in K 2 EDTA anticoagulant tube;
[0029] ②Take 100 μl anticoagulant blood, add 2ml RPMI1640 cell culture medium and 20μl ConA stimulator, 37°C, 5% CO 2 Cultivate in the incubator for 24h;
[0030] ③ Add 2 μl each of antihuman-CD3-PerCP-5.5, antihuman-CD69PE, antihuman-CD25PE and antihuman-CD71PE, incubate in the dark for 30 minutes, then add 3ml of erythrocyte lysate, and let stand in the dark for 10 minutes
[0031] ④ Centrifuge at 477g for 10min, discard the supernatant;
[0032] ⑤Add 2ml of PBS to wash, centrifuge at 477g for 10min, discard the supernatant, and repeat this process twice;
[0033] ⑥Add 100μl PBS to resuspend the cells, to be tested;
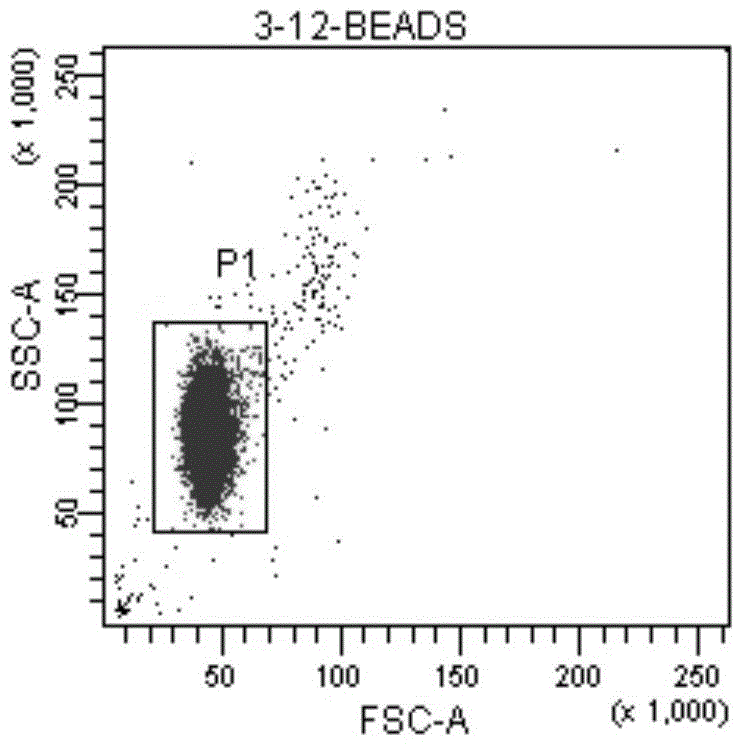
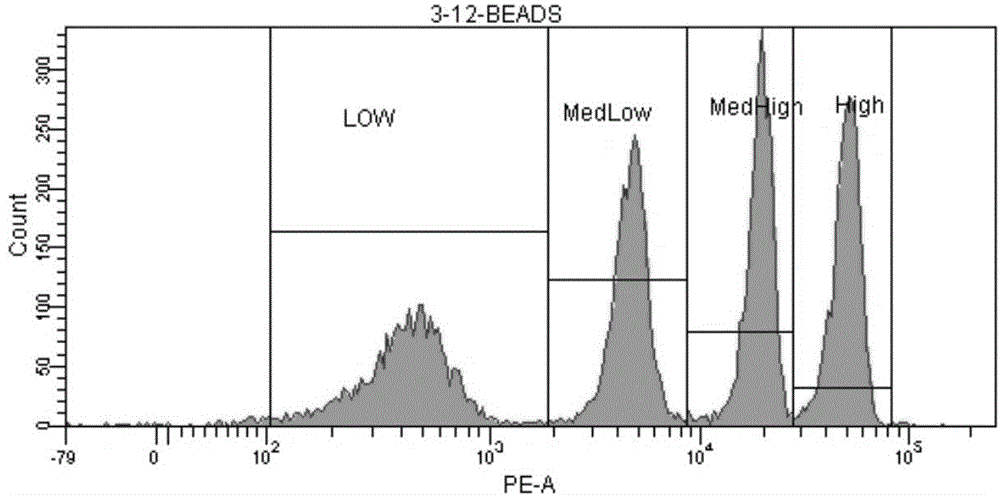
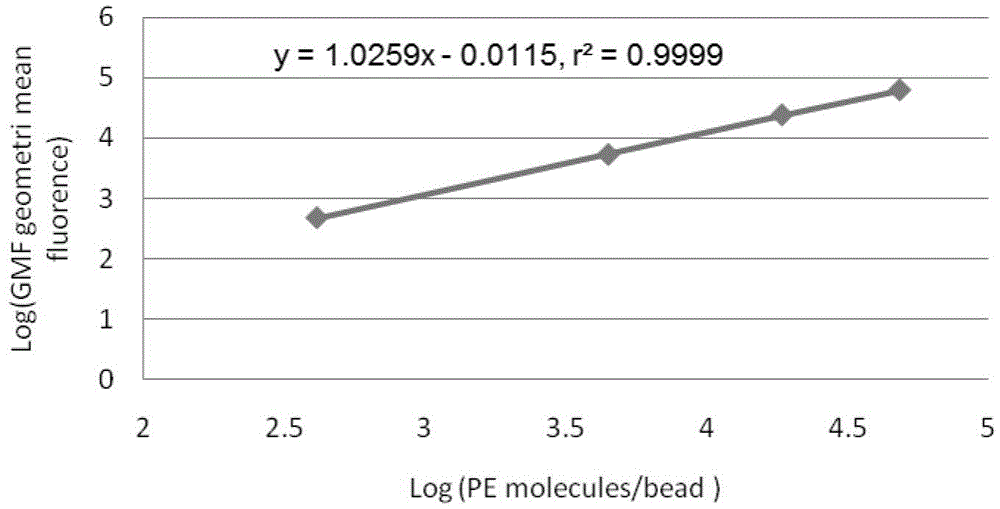
[0034] ⑦Preparation of standard microsphere solution: add 1ml of PBS to the measurement tube containing QuantiBRITE PE microsp...
Embodiment 2
[0040] Example 2 Expression of CD69, CD25 and CD71 in peripheral blood of kidney transplant patients
[0041] ①Sample collection: 5 kidney transplant patients were selected, 3ml of peripheral venous blood was drawn, and placed in K 2 EDTA anticoagulant tube;
[0042] ②Take 100μl anticoagulant blood, add 2ml RPMI1640 medium and 20μl ConA stimulator, 37℃, 5%CO 2 Cultivate in the incubator for 24h;
[0043]③ Add 2 μl each of antihuman-CD3-PerCP-5.5, antihuman-CD69PE, antihuman-CD25PE and antihuman-CD71PE antibodies, incubate in the dark for 30 minutes, then add 3ml of erythrocyte lysate, and let stand in the dark for 10 minutes
[0044] ④ Centrifuge at 477g for 10min, discard the supernatant;
[0045] ⑤Add 2ml of PBS to wash, centrifuge at 477g for 10min, discard the supernatant, and repeat this process twice;
[0046] ⑥Add 100μl PBS to resuspend the cells, to be tested;
[0047] ⑦Preparation of standard microsphere solution: add 1ml of PBS to the measurement tube containing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com