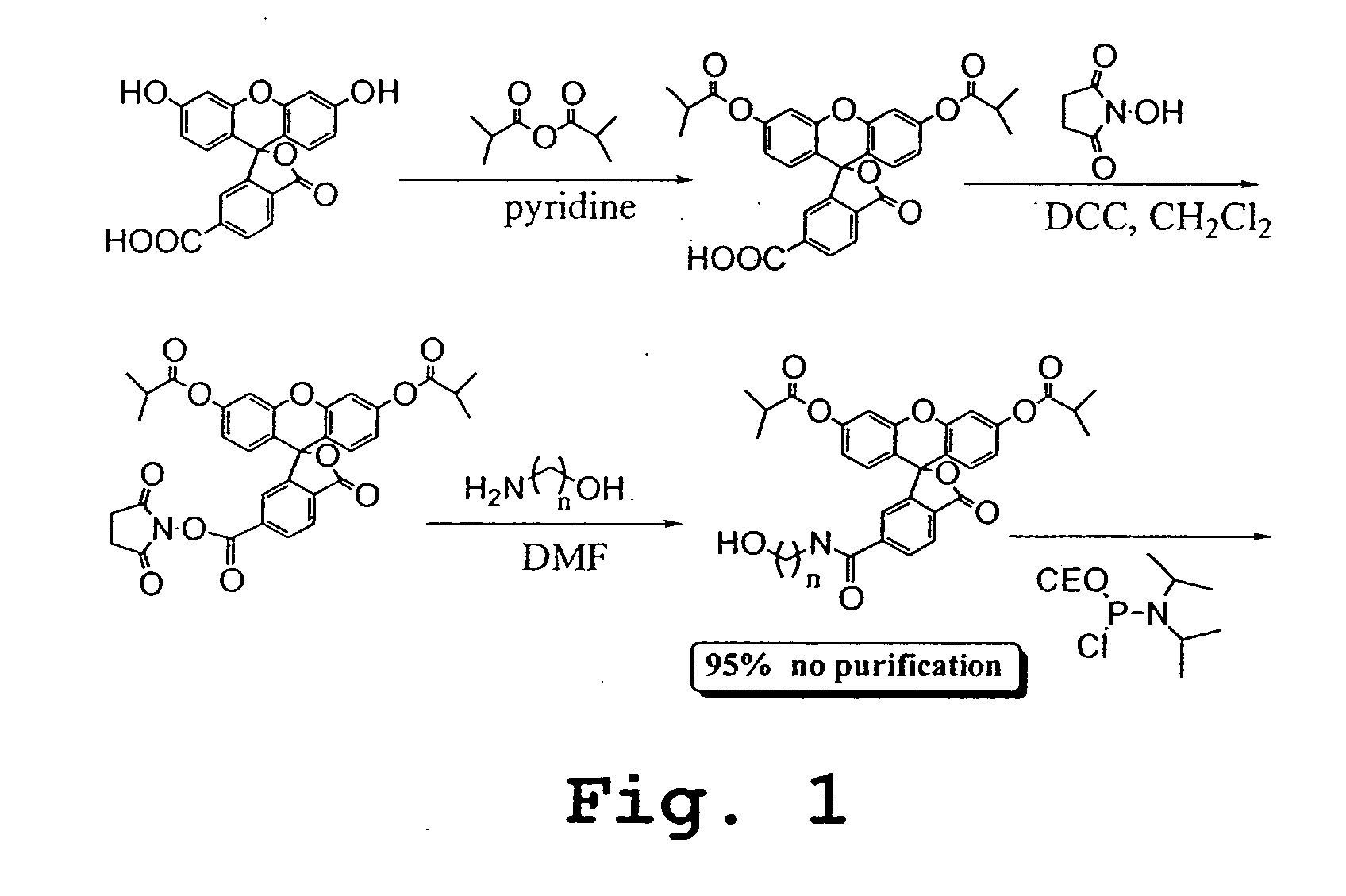
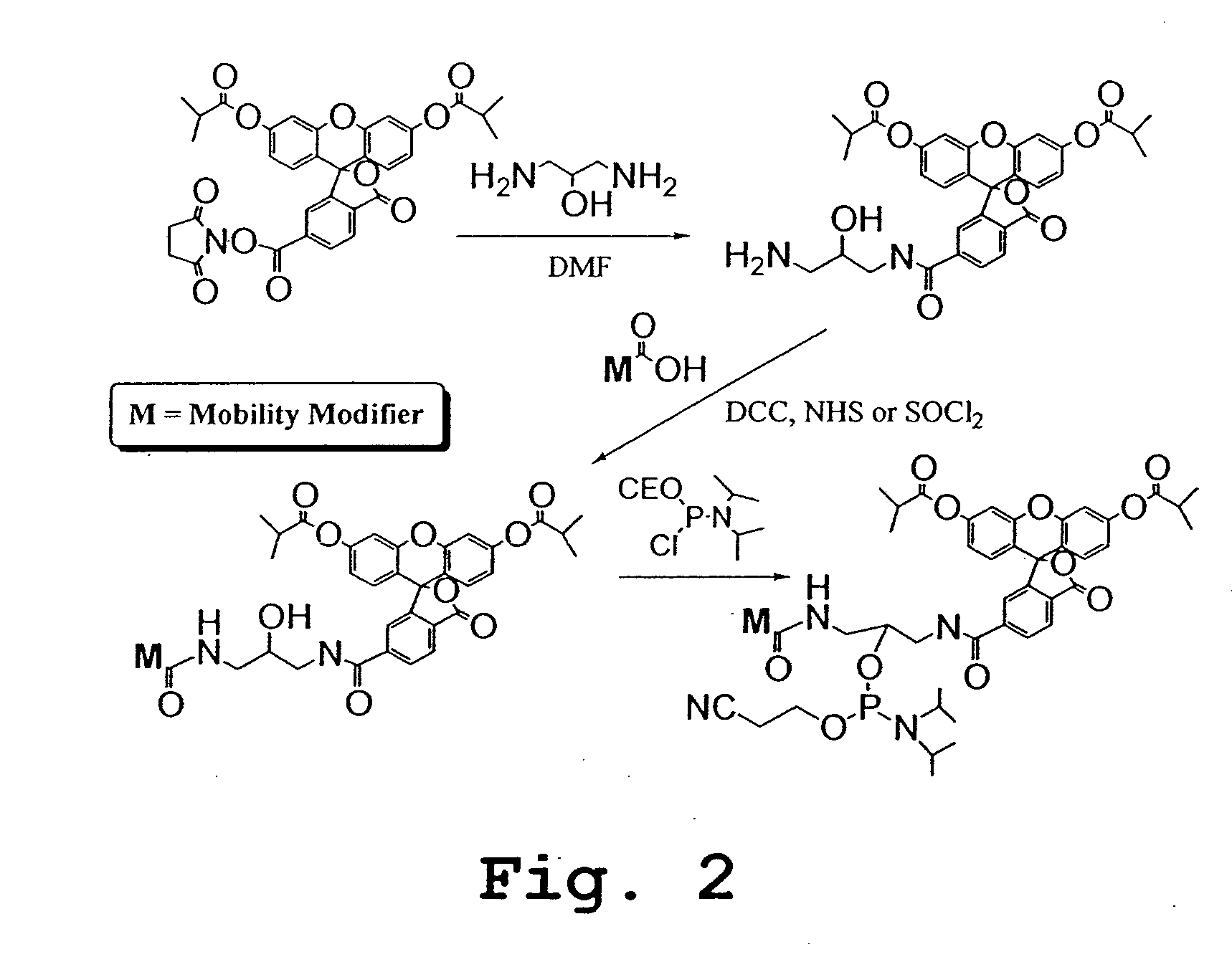
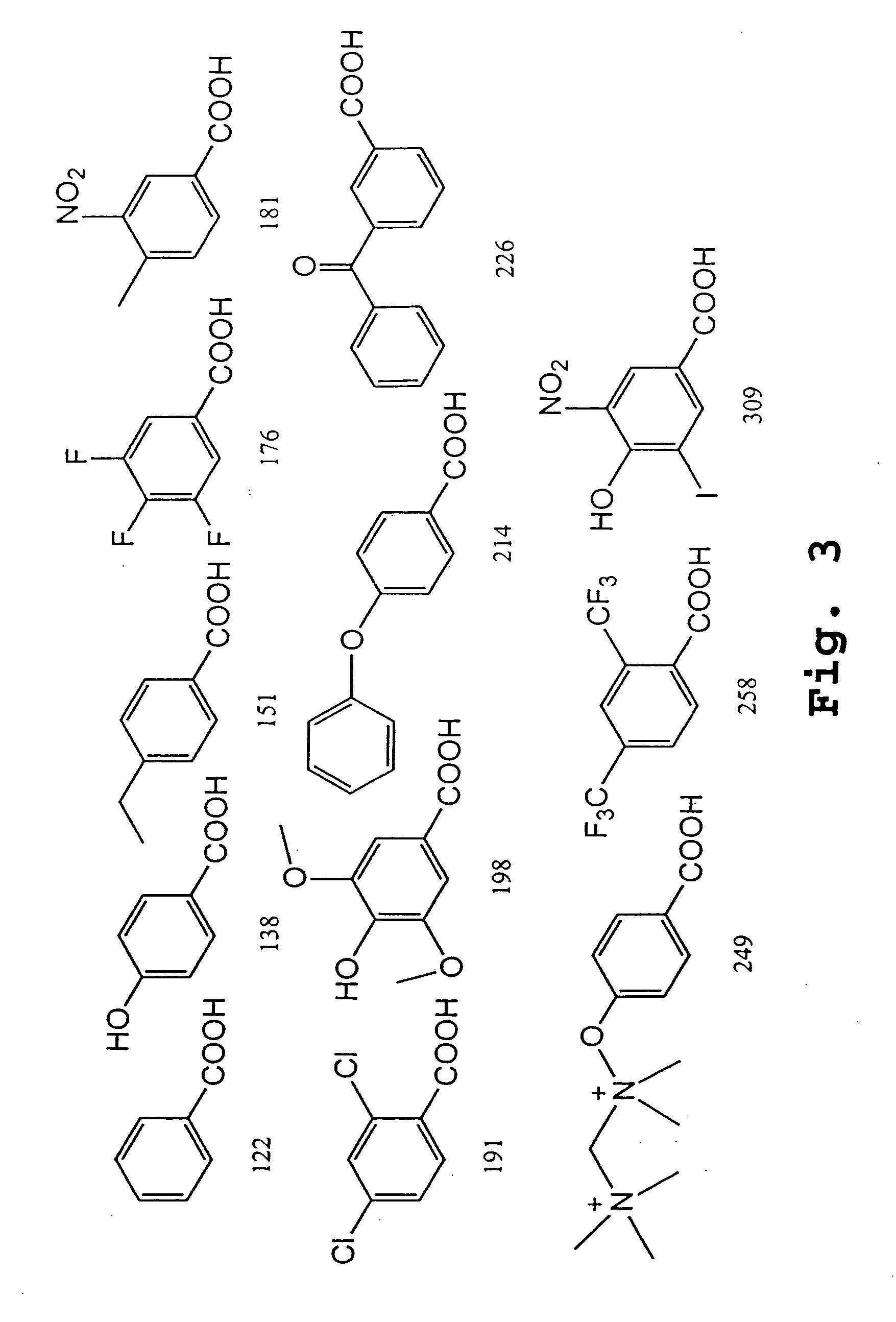
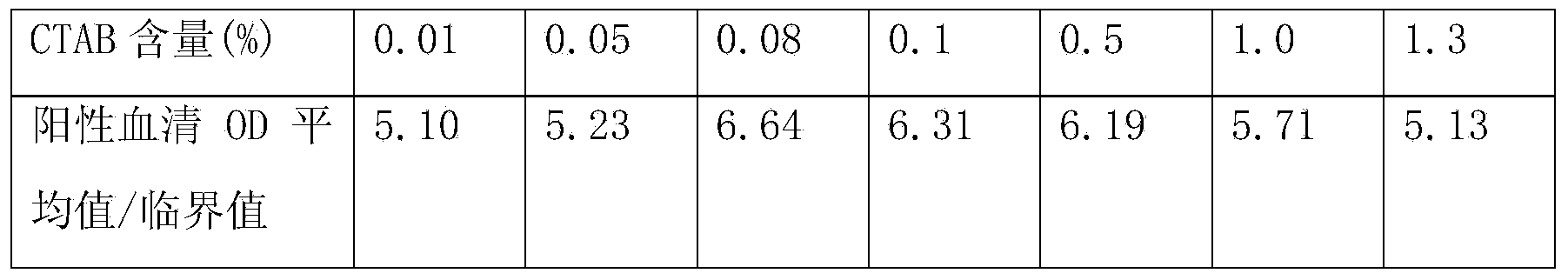
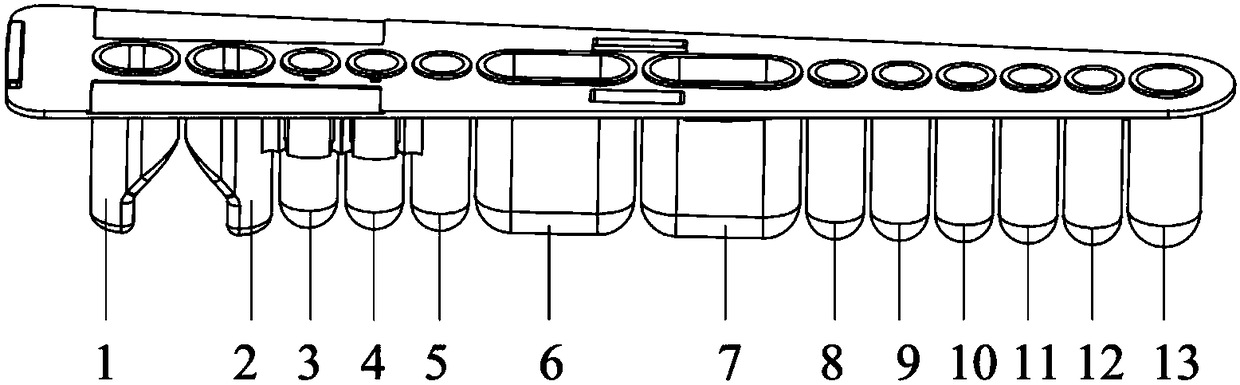
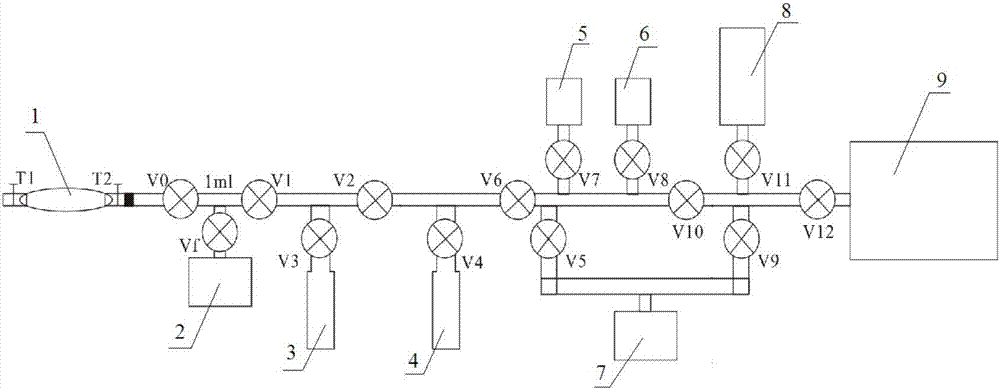
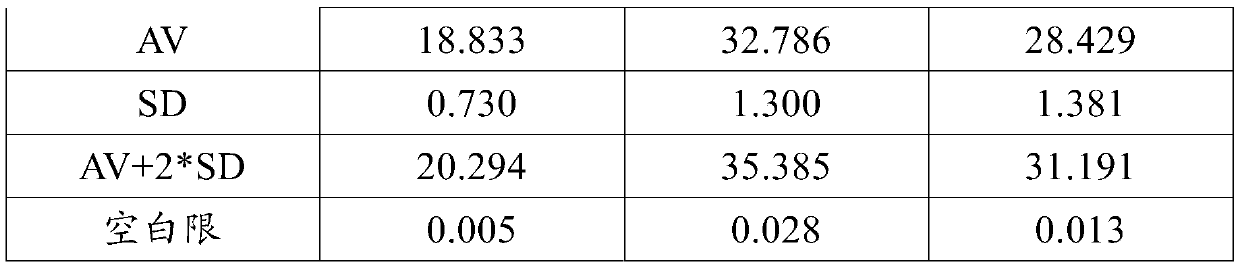
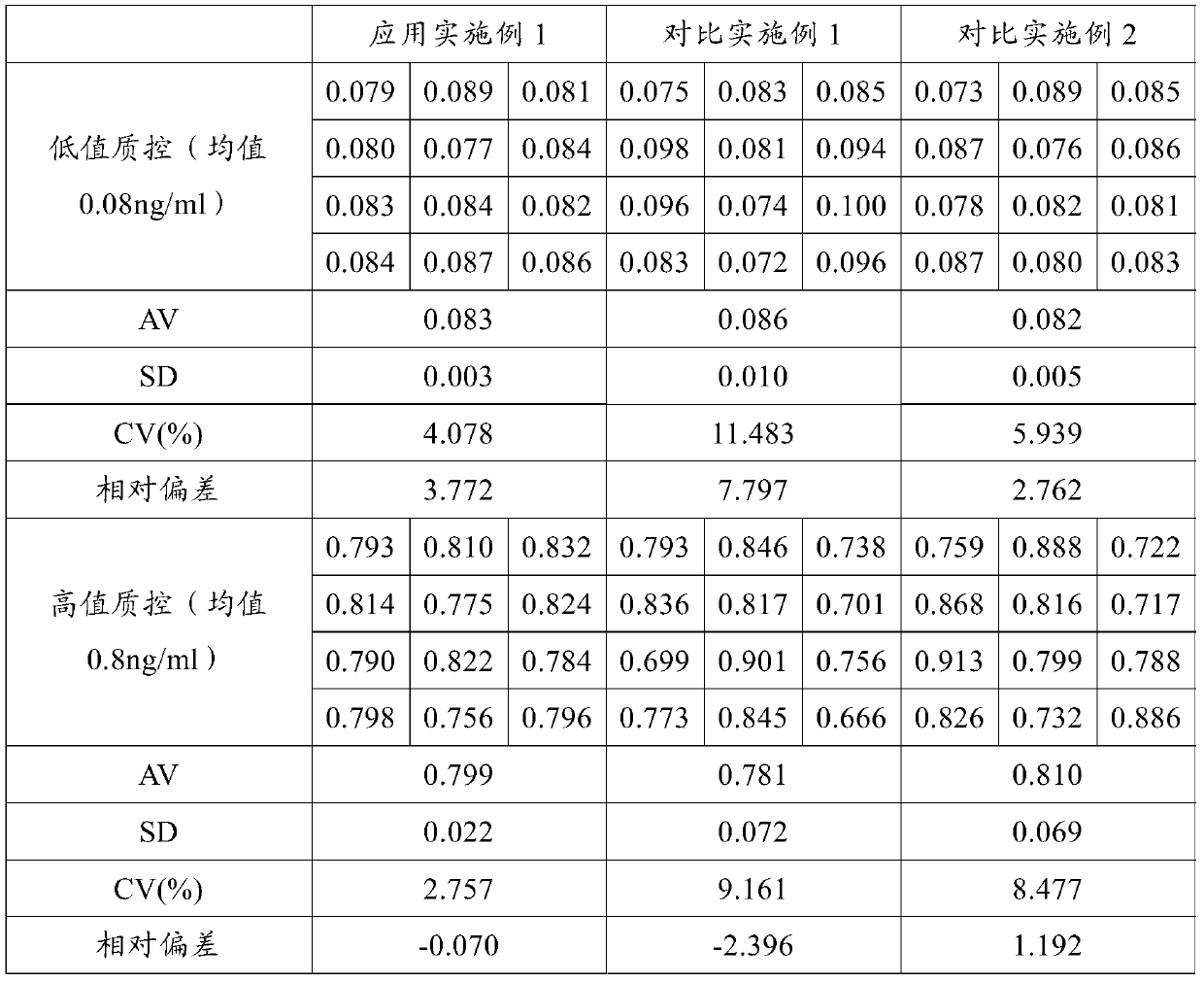
Patents
Literature
81results about How to "Reduce background" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Selected amplification of polynucleotides
InactiveUS20070128635A1ConservingReduce backgroundMicrobiological testing/measurementFermentationNucleotideDouble strand
The invention provides methods and compositions for selectively amplifying one or more target polynucleotides in a sample. In one aspect, a plurality of selection oligonucleotides are provided that are capable of simultaneously annealing to separate regions of a target polynucleotide to form a complex that is enzymatically converted into a closed double stranded DNA circle that incorporates the sequence region between the two separate regions. Sequences that fail to form such complexes may be removed by nuclease digestion and the sequences of the remaining DNA circles may be amplified by a variety of techniques, such as rolling circle replication after nicking, PCR amplification after linearization, or the like.
Owner:MACEVICZ STEPHEN C
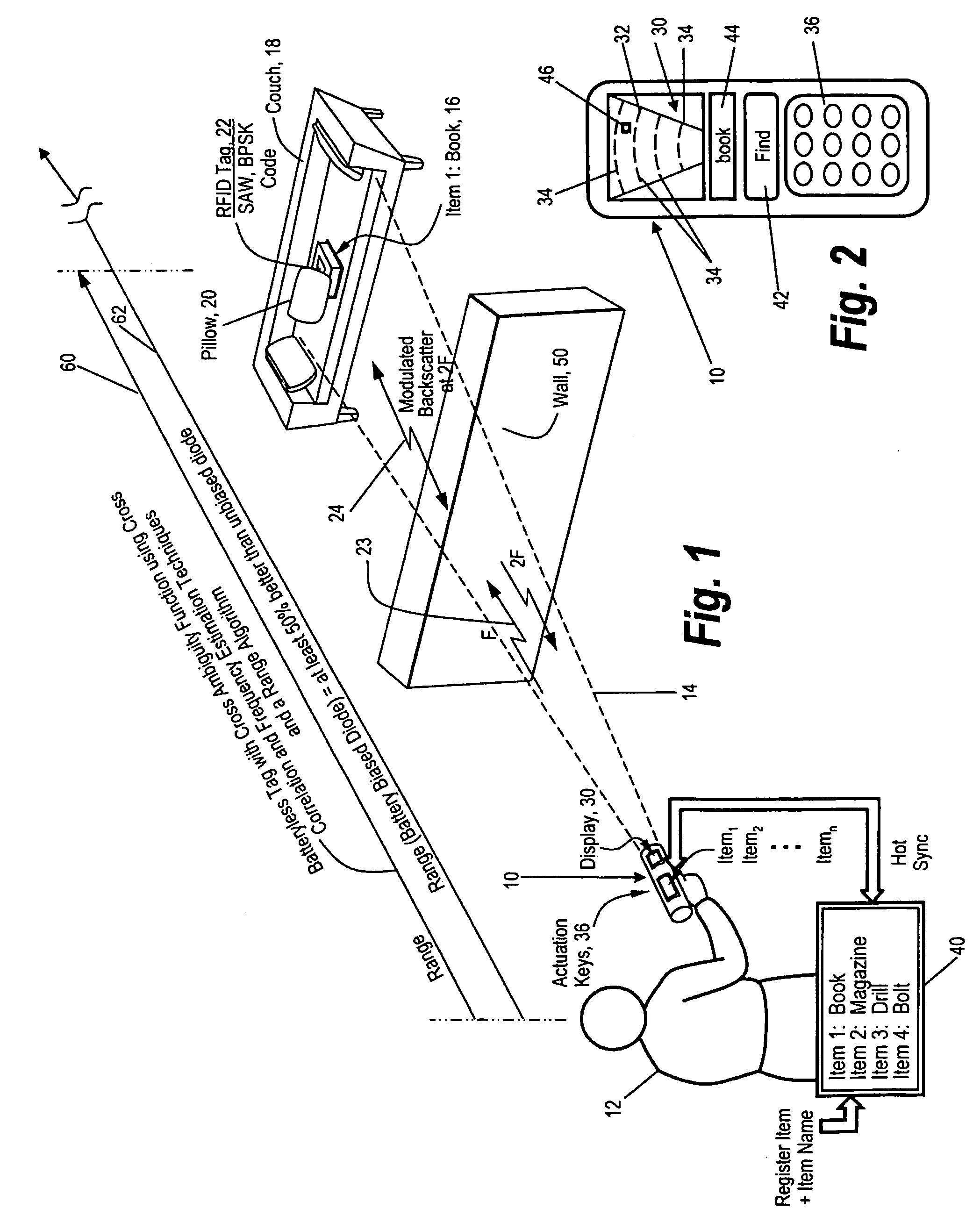
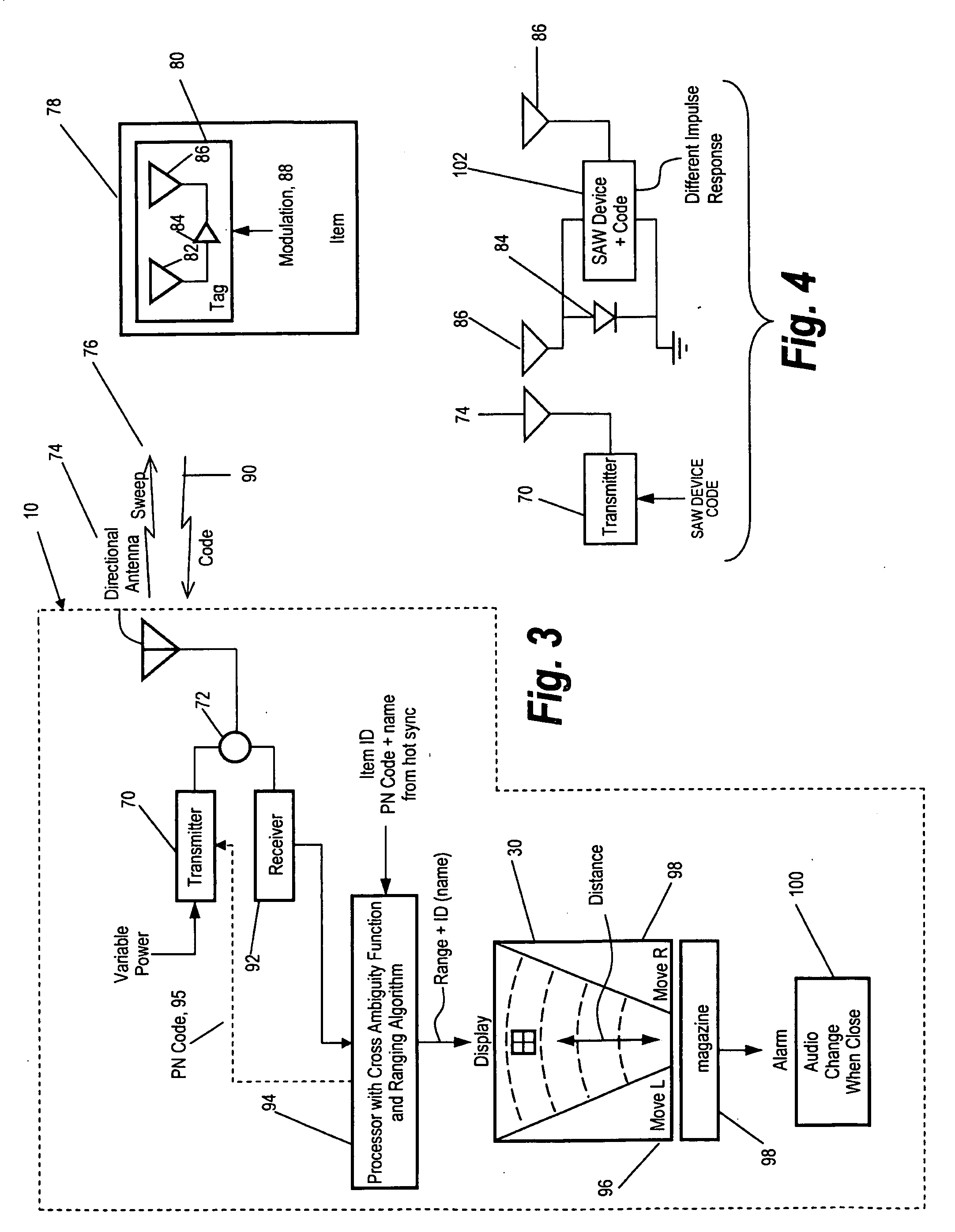
Method and apparatus for locating items
ActiveUS20100188211A1Reduce backgroundEase requirementFrequency-division multiplex detailsTime-division multiplexUrban environmentWorld Wide Web
A convenient handheld locator is provided for locating an item in an urban environment in which the locator is programmed to search for and locate specific items, with the detected item being displayed on the locator as to its identity or name, also displaying where the item is relative to the locator, as to position and range.
Owner:RADIOFIDO
Nucleic acid respiratory syncytial virus vaccines
InactiveUS6019980AReduce backgroundGood immune protectionSsRNA viruses negative-senseGenetic material ingredientsNucleotideViral Vaccine
Vectors containing a nucleotide sequence coding for an F protein of respiratory syncytial virus (RSV) and a promoter for such sequence, preferably a cytomegalovirus promoter, are described. Such vectors also may contain a further nucleotide sequence located adjacent to the RSV F protein encoding sequence to enhance the immunoprotective ability of the RSV F protein when expressed in vivo. Such vectors may be used to immunize a host, including a human host, by administration thereto. Such vectors also may be used to produce antibodies for detection of RSV infection in a sample.
Owner:CONNAUGHT LAB
Superresolution Optical Fluctuation Imaging (SOFI)
ActiveUS20100303386A1Improve resolutionReduce backgroundTelevision system detailsImage enhancementImage resolutionStatistical analysis
Statistical analysis techniques based on auto- and cross-correlations / cumulants, of image stacks of fluctuating objects are used to improve resolution beyond the classical diffraction limit and to reduce the background. The time trajectory of every pixel in the image frame is correlated with itself and / or with the time trajectory of an adjacent pixel. The amplitude of these auto- or cross-correlations / cumulants of each pixel, at a given time lag or averaged or integrated over an interval of time lags, is used as the intensity value of that pixel in the generated superresolved optical fluctuation image.
Owner:SOFAST
Multiplexed analysis by chromatographic separation of molecular tags
InactiveUS20050048553A1Reduce backgroundConvenient multiplexing capabilityComponent separationMicrobiological testing/measurementChemistryChromatographic separation
Methods and kits are disclosed for determining, either in a homogeneous or heterogeneous assay format, one or more target analytes in a sample using binding compositions coupled to molecular tags by cleavable linkages. Generally, an assay mixture is formed comprising a sample and a reagent comprising multiple such binding compositions under conditions that permit stable complexes to form between the binding compositions and analytes. In one aspect of the invention, the interaction between the binding compositions and their respective binding sites brings a cleavage-inducing moiety into close proximity to cleavable linkages or provides a recognizable substrate for a cleavage-inducing moiety. In this way, one or more molecular tags for each of the analytes are released from the complexes. Released molecular tags are chromatographically separated and the presence and / or amount of the target analytes are determined based on the analysis of the released and separated molecular tags.
Owner:CHENNA AHMED +4
Hepatitis C virus antigen-antibody combined detection kit and detection method
ActiveCN103630690AImprove blood consumptionImprove securityBiological material analysisAntigenTherapy Evaluation
The invention discloses a hepatitis C virus (HCV) antigen-antibody combined detection kit. The kit comprises a microwell plate coated with an HCV chimeric antigen and an HCV monoclonal antibody, a sample diluent, an HCV antigen-antibody combined detection enzyme working fluid, an HCV abzyme working fluid, an HCV antigen enzyme working fluid, a substance A fluid, a substance B fluid, a 20-times concentrated washing fluid and a stopping fluid. The invention also discloses preparation and usage of the key components of the kit, such as the microwell plate of the HCV chimeric antigen and the HCV monoclonal antibody, the sample diluent, and the diluent of enzyme working fluid. The kit and detection method, disclosed by the invention, are able to be used for detecting the HCV antigen and antibody at the same time, or individually detecting the situation of the HCV antigen during the early hepatitis c or the acute infection period, or before the antibody is produced, or when an antigen-antibody compound is produced, or individually detecting the situation of the HCV antibody after the antibody is produced; the kit and detection method can be applied to early HCV detection and therapy evaluation, so as to provide important detection evaluation index for clinical guideline.
Owner:山东莱博生物科技有限公司
Kit for quantitative detection on O type foot-and-mouth disease virus antibody through fluorescence immunoassay magnetic particles
InactiveCN108107220AAdequate responseIncrease binding areaBiological testingBiotin-streptavidin complexSorbent
The invention discloses a kit for quantitative detection on an O type foot-and-mouth disease virus antibody through fluorescence immunoassay magnetic particles. The kit consists of O type foot-and-mouth disease virus antibody negative serum, O type foot-and-mouth disease virus antibody positive serum, VP1 coating magnetic beads, a biotinylation goat-anti-pig antibody, a streptavidin marking fluorescent substance, a cleaning solution and an enhancing solution. The magnetic beads used in the kit have relatively large binding areas, so that the detection range is greatly increased, the reaction time is shortened, and the sensitivity is improved. The kit has a relatively wide stimulation spectrum and a relatively narrow emitting spectrum, the cost can be reduced, and the sensitivity can be improved; compared with a conventional fluorescent substance, the kit is relatively wide in detection range and relatively good in specificity. Due to adoption of a streptavidin-biotin signal amplification system, the detection sensitivity is further improved, and the kit is relatively high in sensitivity when being compared with ELISA (Enzyme-Linked Immuno Sorbent Assay) and chemiluminiscence. Together with a full-automatic detector, on-site automatic operation can be achieved, one or more samples can be simultaneously detected, and the kit is simple, convenient and rapid to operate and low in price.
Owner:GUANGZHOU BIOKEY HEALTH TECH CO LTD
Positron annihilation lifetime spectrometer
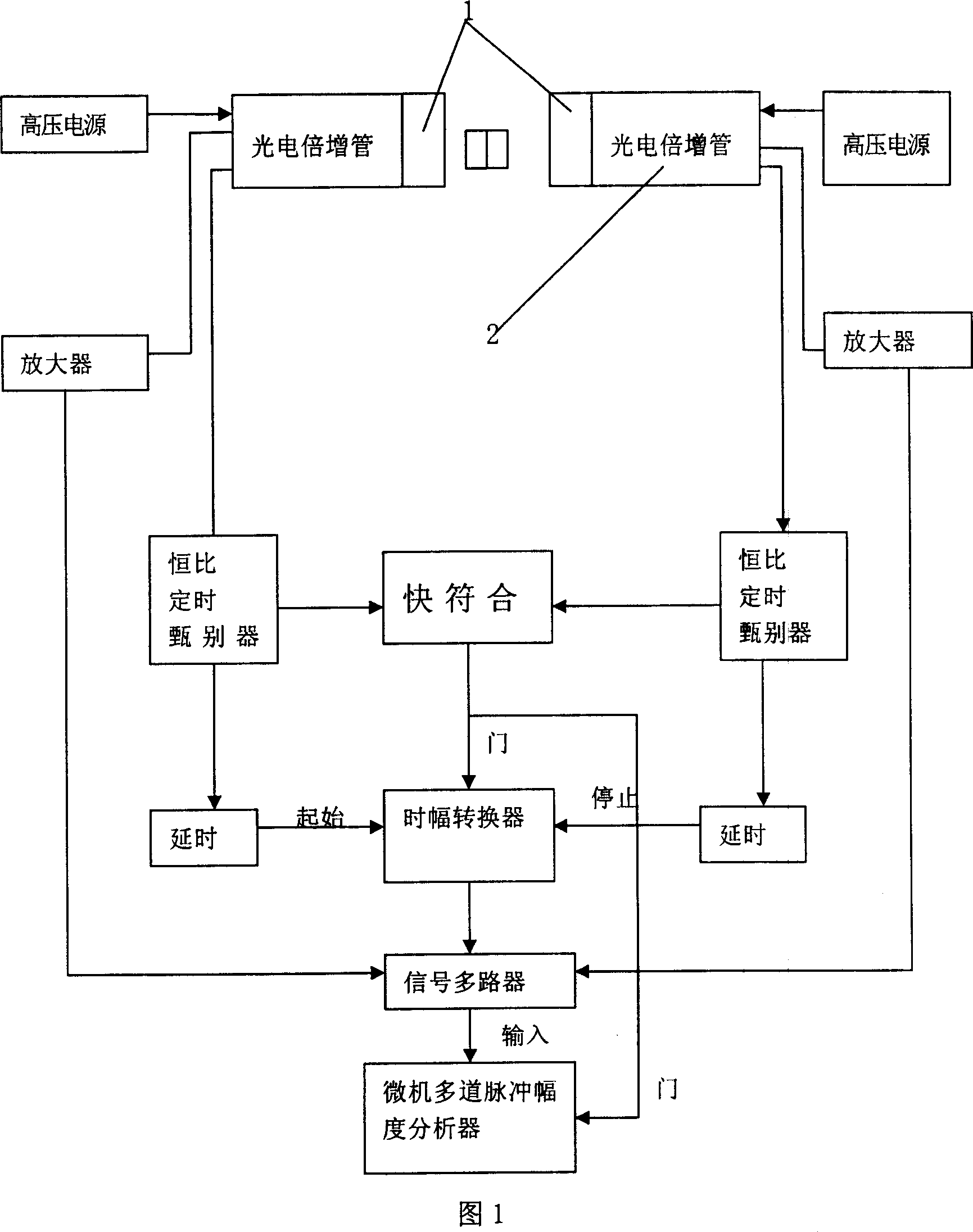
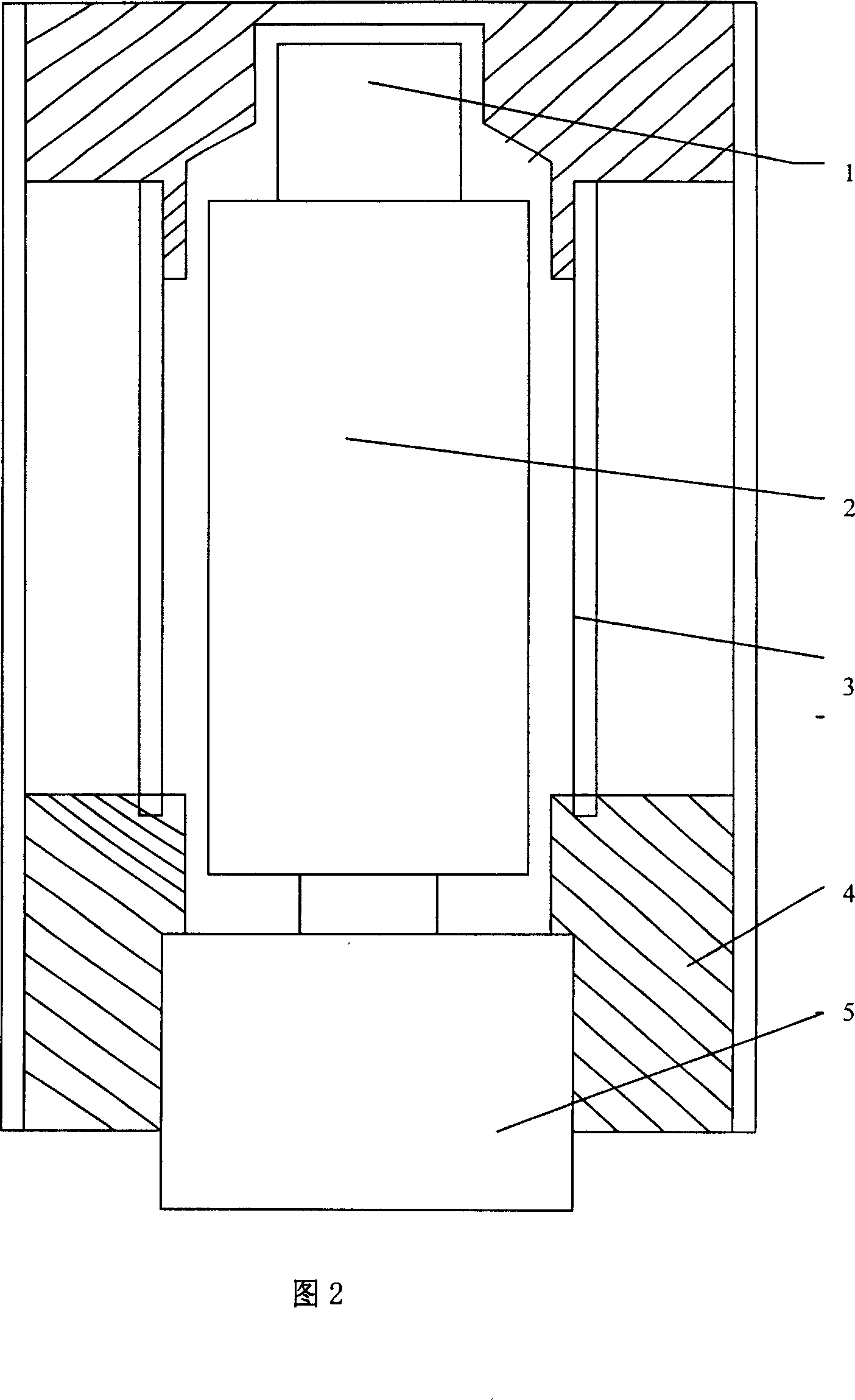
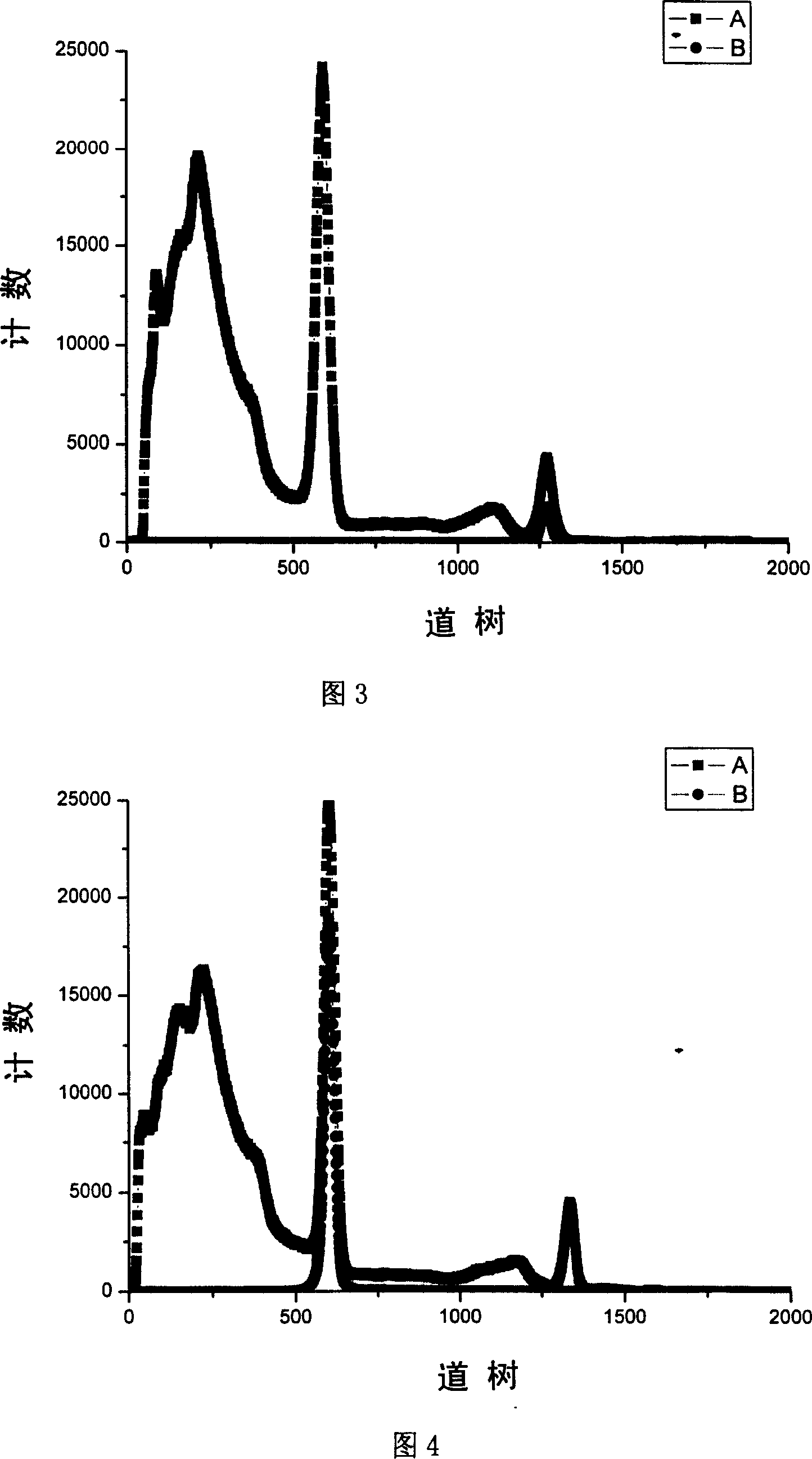
InactiveCN101013095AFast time responseReduce backgroundMaterial analysis by transmitting radiationEnergy windowImage resolution
It is a positron annihilation lifetime spectroscopy measurement spectrometer, which is characteristic of the positron annihilation lifetime spectroscopy using lanthanum chloride crystals as the scintillator detector. The lanthanum chloride crystals not only have fast response time, but also have more superior energy resolution than the BaF2 crystals. Therefore, it can use the energy window to select circuit, and restrict the signals from the start and stop road detectors in a very narrow energy range, and it can effectively exclude the effect of gamma radiation on the measurement process of other sources (sample materials, environment, etc.), greatly reducing the lifetime spectrum ending, and it enables the spectrometer detector to maintain fast response time, and meanwhile has better energy resolution, which can process the positron annihilation lifetime spectroscopy measurement under the strong gamma-ray interfere, in order to extract positron annihilation lifetime spectroscopy effective information.
Owner:TSINGHUA UNIV
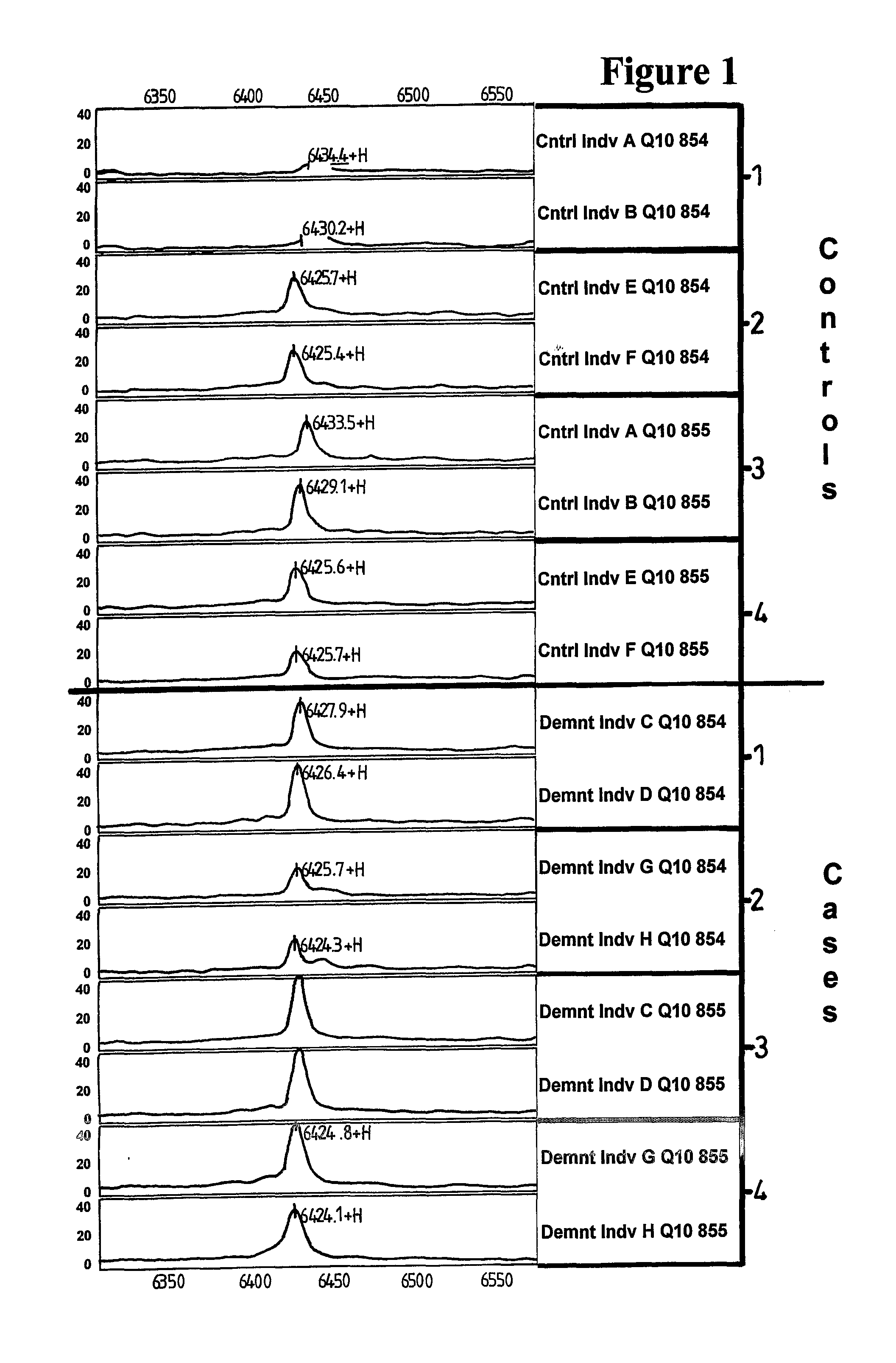
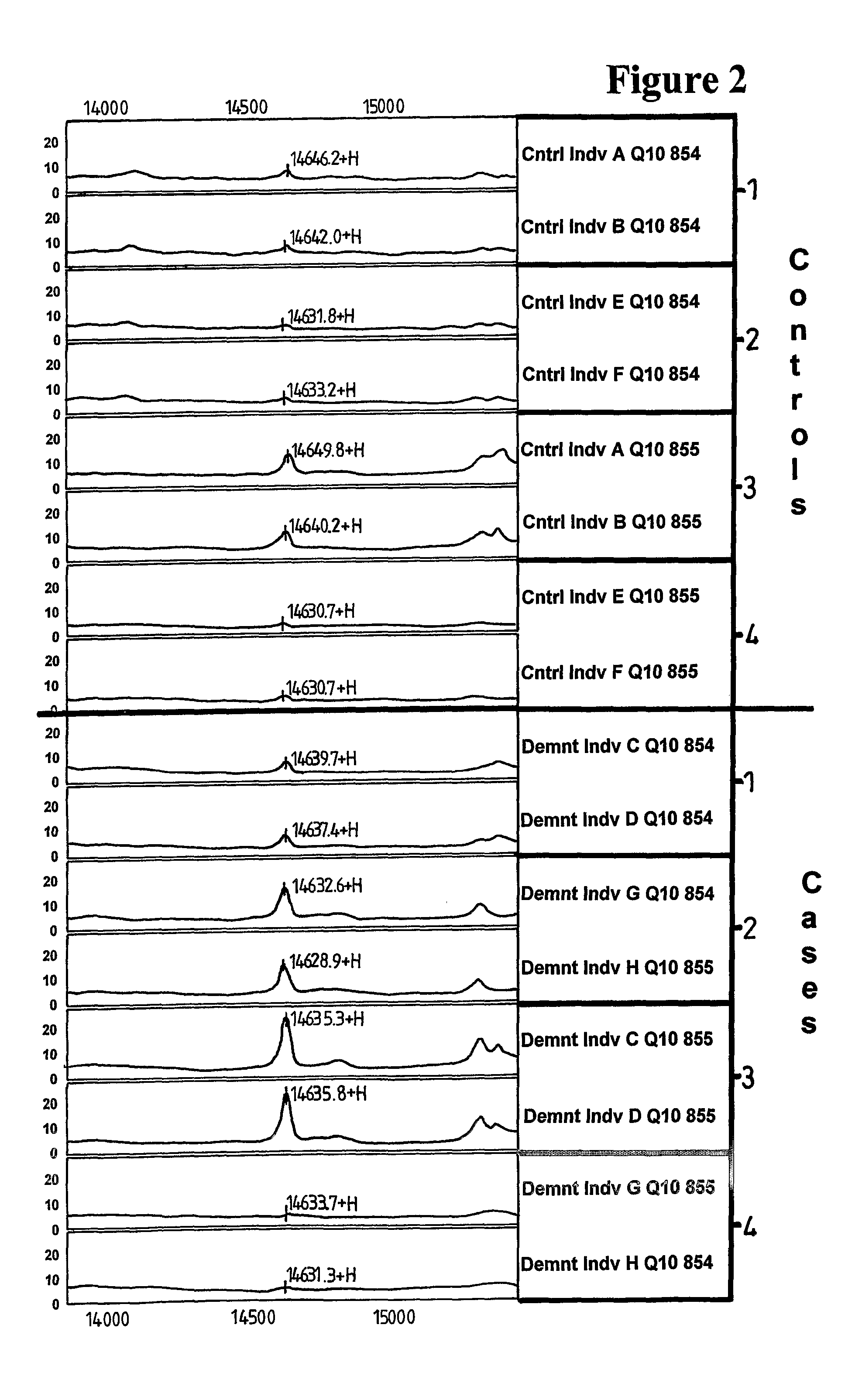
Methods and Compositions Relating to Alzheimer's Disease
ActiveUS20080070995A1Reduce backgroundEnhancing likely responseBiocideNervous disorderProtein identificationMedicine
Methods and compositions relating to Alzheimer's disease are provided. Specifically, proteins that are differentially expressed in the Alzheimer's disease state relative to their expression in the normal state are provided. Proteins associated with Alzheimer's disease are identified and described. Methods of diagnosis of Alzheimer's disease using the differentially expressed proteins are also provided, as are methods for the identification and therapeutic use of compounds for the prevention and treatment of Alzheimer's disease.
Owner:PROTEOME SCI +1
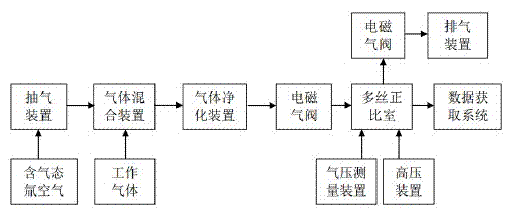
Method and system for measuring gas tritium based on multi-wire proportional chamber
InactiveCN102928864AImprove stabilityReduce backgroundRadiation intensity measurementEngineeringHigh pressure
The invention provides a method and a system for measuring a gas tritium based on a multi-wire proportional chamber. The method comprises the following steps that: A, an exhaust device discharges air in the multi-wire proportional chamber, and a gas pressure measuring device monitors the air pressure in the multi-wire proportional chamber; B, the exhaust device extracts an air sample containing gas tritium, the air sample is mixed with a working gas in a certain ratio through a gas mixing device, and then the mixed is output to a gas purifying device; C, the mixed gas is purified by the gas purifying device and is output to the multi-wire proportional chamber through an electromagnetic valve; and D, the anode wire of the multi-wire proportional chamber is connected with a positive high pressure, a beta-ray generated from the decay of the gas tritium entering the multi-wire proportional chamber is electrically separated from the working gas, the avalanche amplification is carried out under the action of a high voltage electric field, a pulse signal is output, and the pulse signal is obtained and identified by a data obtaining system so as to realize the measurement of the gas tritium. According to the method, the defects of an existing gas tritium measuring method, such as working efficiency, precision and sensitivity in the measurement, are overcome, the measurement of the environment gas tritium is accurately realized, and active demands on the gas tritium measurement of an existing tritium environment are met.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Electrode for dielectrophoretic apparatus, dielectrophoretic apparatus, method for manufacturing the same, and method for separating substances using the electrode or dielectrophoretic apparatus
InactiveUS20050139473A1Improve signal-to-noise ratioLow backgroundSludge treatmentElectrostatic separatorsSubstance useElectrophoresis
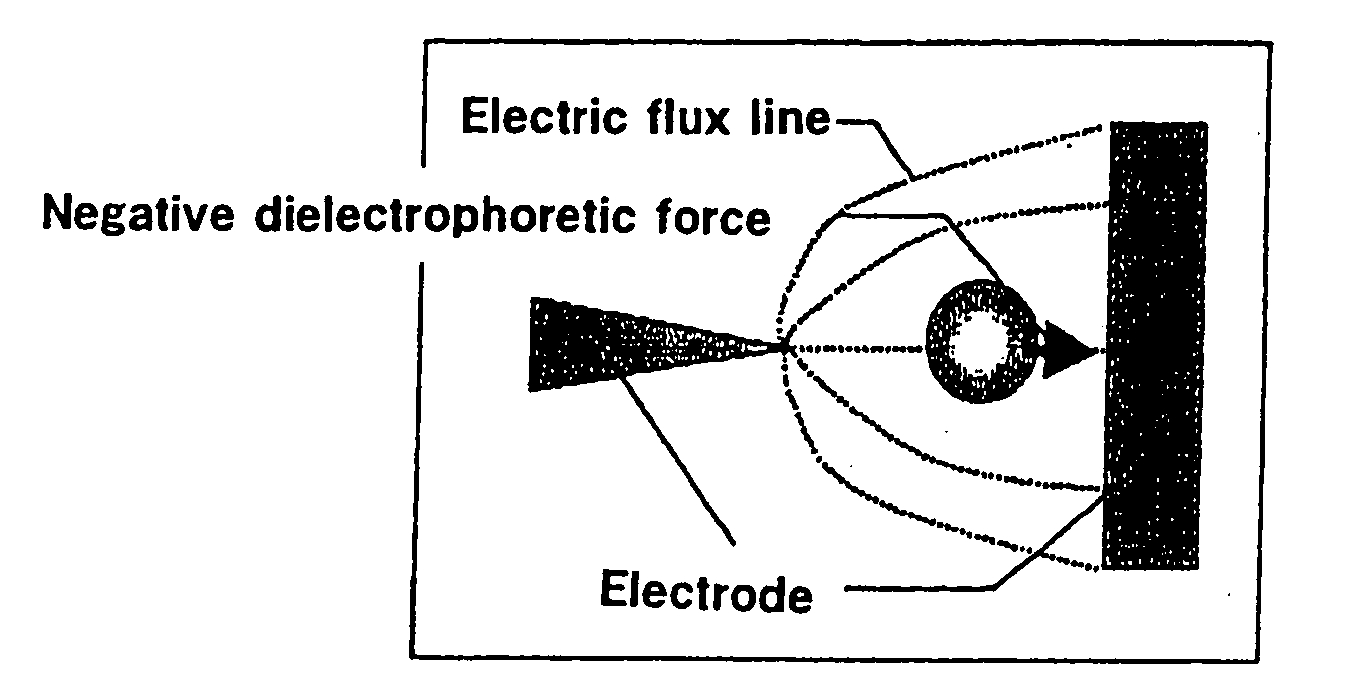
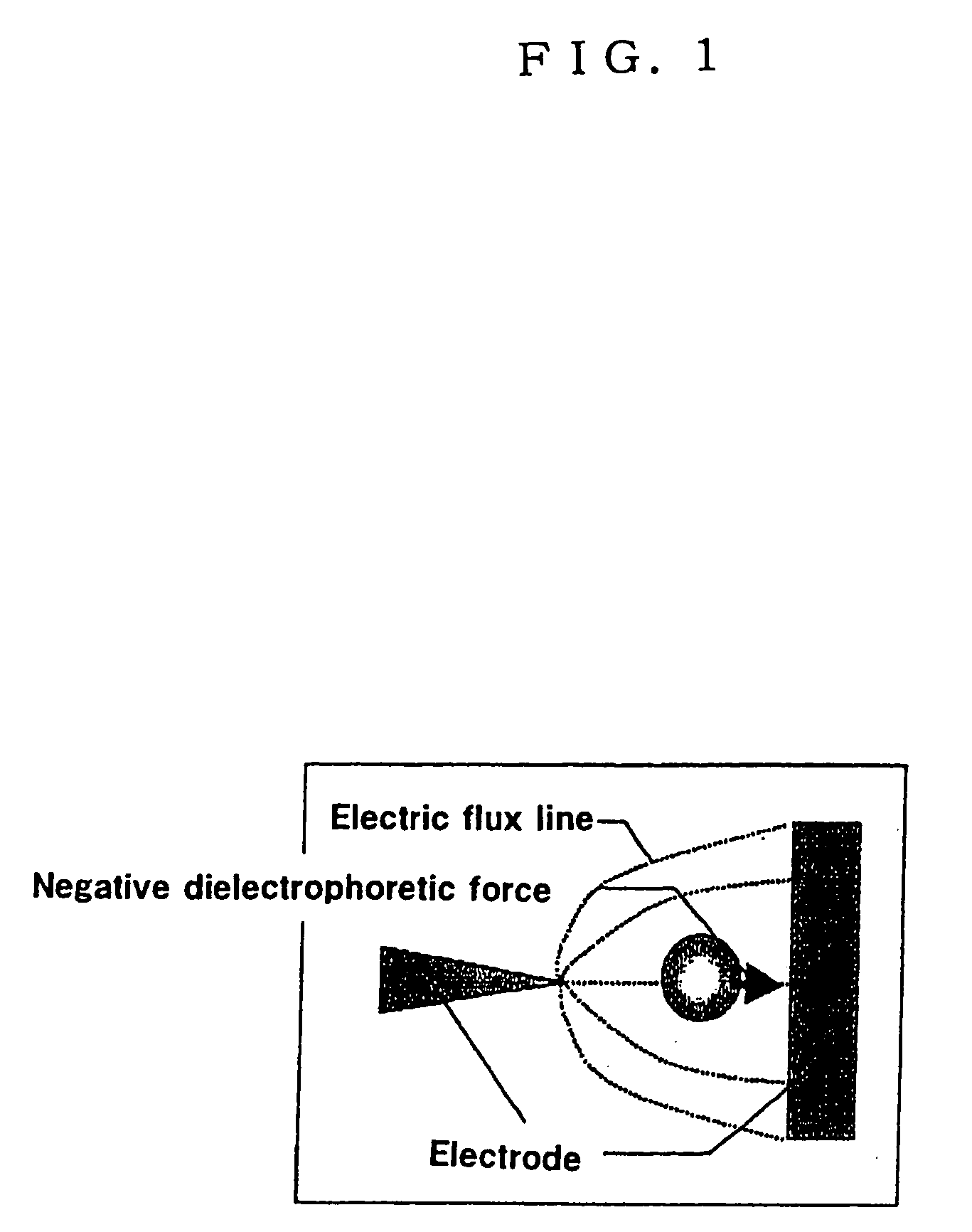
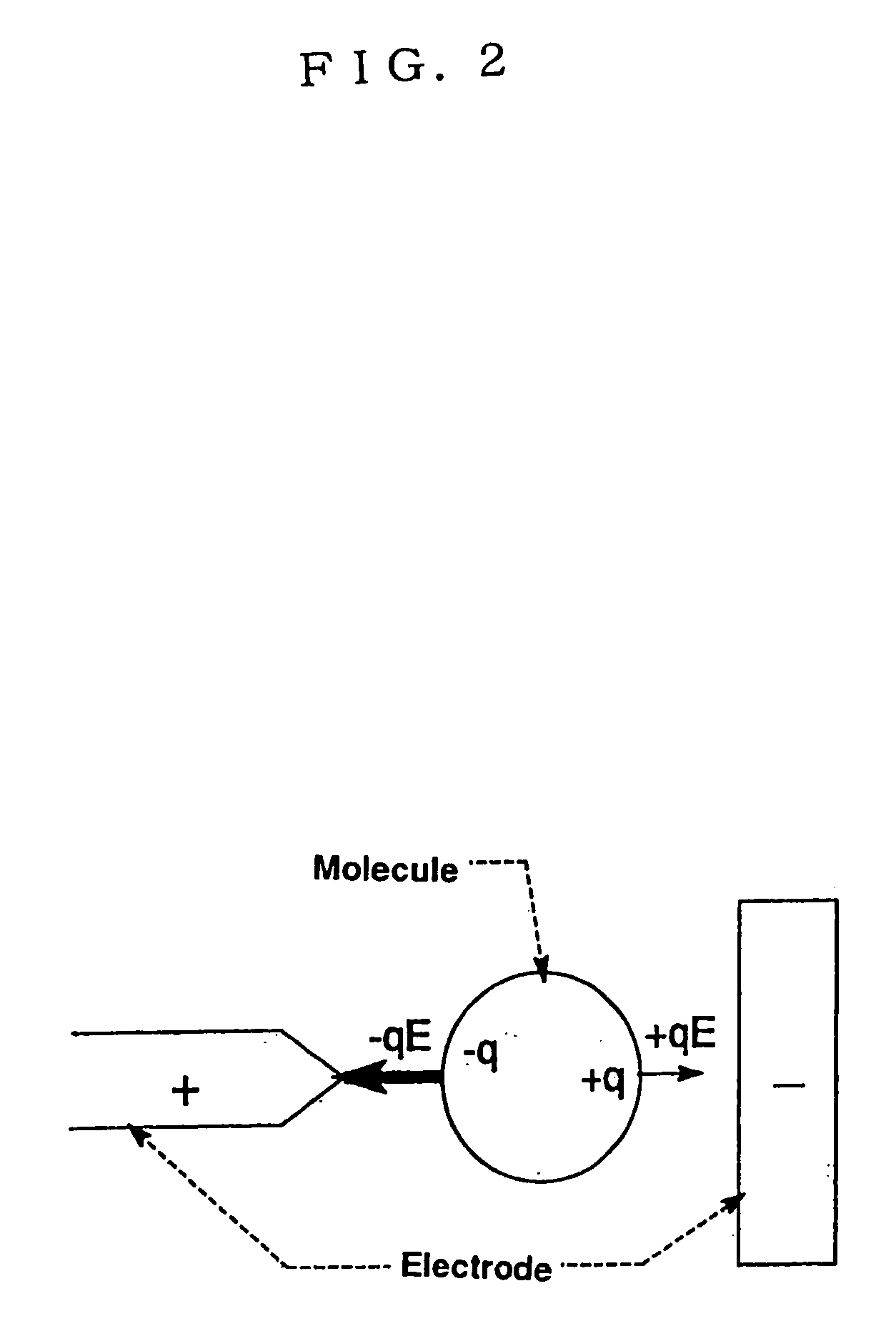
To provide an electrode for a dielectrophoretic apparatus in which a background detected by reflecting an excited light on an electrode present under the substance (molecule) is reduced and an S / N ratio is enhanced. Also, there is provided an dielectrophoretic apparatus, in an apparatus in which a liquid containing substances to be separated is present in a non-uniform electric field formed by a dielectrophoretic electrode, and separation is carried out by a dielectrophoretic force exerting on the substances, wherein the collecting ability of substances is enhanced. The present invention is characterized in that a vacant space is provided in an electrode whereby substances subjected to influence by a negative dielectrophoretic force can be concentrated in said vacant space of an electrode, or above or below portion of the space.
Owner:WAKO PURE CHEMICAL INDUSTRIES
Trace radioactive gas nuclide activity measuring method and device
ActiveCN104536031AReduce backgroundHigh detection sensitivityX/gamma/cosmic radiation measurmentBeta gamma coincidenceRadioactive gas
The invention relates to a trace radioactive gas nuclide activity measuring method and device. A hollow inflatable beta detector is filled with a radioactive gas nuclide sample. The inflatable beta detector is used for detecting radioactive gas nuclide beta-rays or internal conversion electrons efficiently. A gamma detector is used for measuring gamma-rays or x-rays in high resolution. A beta-gamma coincidence technology is used for lowering a system background. The trace radioactive gas nuclide activity measuring method and device achieves the technical aim that trace xenon isotope activity is detected in high resolution and high sensitivity.
Owner:北京放射性核素实验室
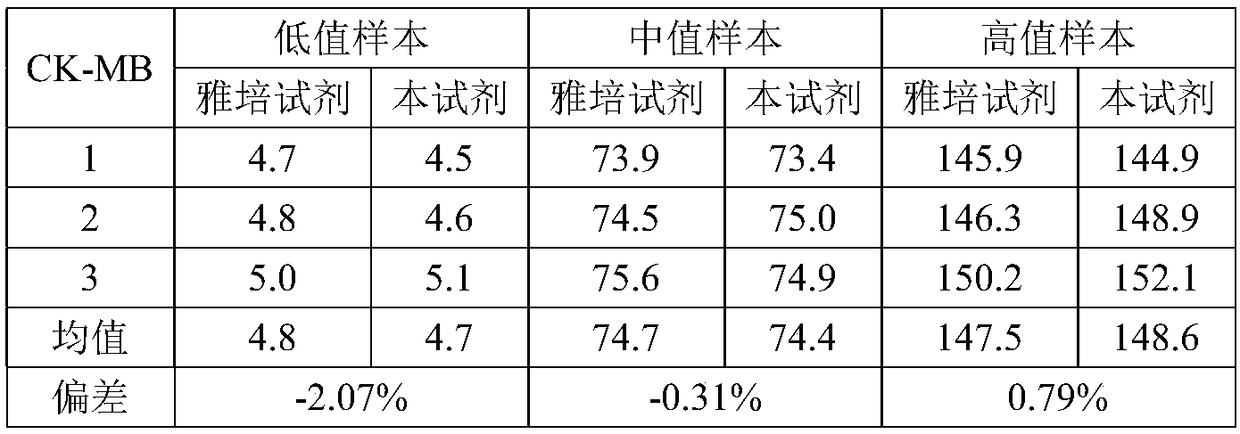
Magnetic bead time resolution fluorescence immunoassay quantitative determination CK-MB (creatine kinase-MB) kit
The invention discloses a magnetic bead time resolution fluorescence immunoassay quantitative determination CK-MB kit. The CK-MB kit comprises an immunomagnetic bead coating a CK-MB monoclonal antibody, a CK-MB standardized product solution, a europium-marked CK-MB monoclonal antibody solution, washing liquid and enhancement liquid. The immunomagnetic bead coating the CK-MB monoclonal antibody isa covalent conjugate of a superpara magnetic bead modified by a functional group and with the diameter being 1-3 microns and the CK-MB monoclonal antibody. The kit has the high sensibility, the sensibility of CK-MB is 1ng / mL, and a blood serum (plasma) does not need to be diluted; the determination time is short, and a report can be resulted within 30 minutes; the demanding amount of the sample isless, and only 50 microliters are needed for one-time sample loading; and the kit is equipped with a full-automatic time resolution immune analysis meter, operation is easy, no artificial error exists, and labor is saved. The kit reasonably utilizes the space of a reagent strip, the structure of the reagent strip is more compact, the reagent strip can be transported more easily, and used conveniently, the operation is simple, and the stability is good.
Owner:GUANGZHOU BIOKEY HEALTH TECH CO LTD
Method and device for analyzing Xe isotope in methane-enriched natural gas
InactiveCN107167510ARealize analysisEasy to operatePreparing sample for investigationMaterial analysis by electric/magnetic meansAnalysis methodMoisture
The invention discloses a method and device for analyzing Xe isotope in methane-enriched natural gas, and relates to the technology of noble gas isotope analysis. The method comprises the following steps of Xe-enriching treatment by negative pressure extraction, moisture removal and low-temperature collection on external natural gas to obtain a to-be-detected sample which has the Xe content enough for detecting the Xe isotope information and takes Kr and Xe as main components from the external natural gas; purification treatment for the to-be-detected sample taking Kr and Xe as main components to obtain an analyzing sample which only contains Kr and Xe components; and separation treatment for the analyzing sample which only contains Kr and Xe to obtain an analyzing sample only containing Xe, and isotope analysis on the analyzing sample only containing Xe to obtain the isotope information of Xe in the natural gas sample. The method can be used for directly obtaining the Xe isotope content, has few influence factors in the analyzing process, and has the advantages of accurate result, high efficiency, simple operation and wide universality.
Owner:NORTHWEST INST OF ECO ENVIRONMENT & RESOURCES CAS
Biological chip aldehyde glass carrier
ActiveCN101486532AReduce backgroundUniform surfaceMicrobiological testing/measurementMicroscopesAntigenMicroarray cgh
The invention relates to a biochip glass slide, in particular to a biochip glass slide prepared by employing an aldehyde group method. The surface of the glass slide is decorated by aldehyde group, thus being convenient for the chemical combination between a nucleic acid probe decorated by amino-group, the antigen-antibody of protein property and other matters. The invention is suitable for the probes of nucleic acids, proteins, and other types, as well as template segment point system microarray chips, has firm and specific chemical bond combining characteristics, and provides the reliable carrier for preparing biochips.
Owner:GUANGZHOU DARUI BIOTECH
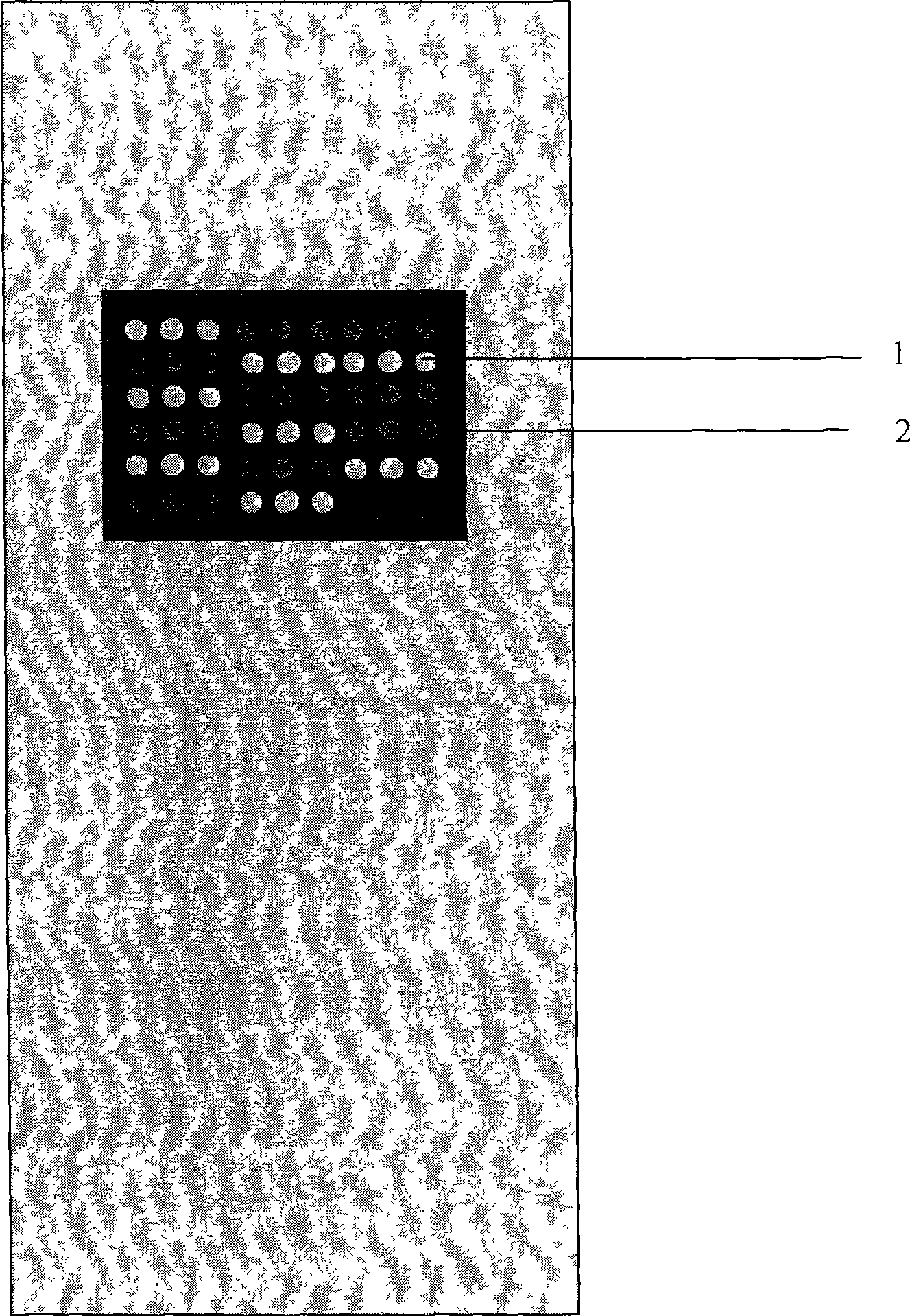
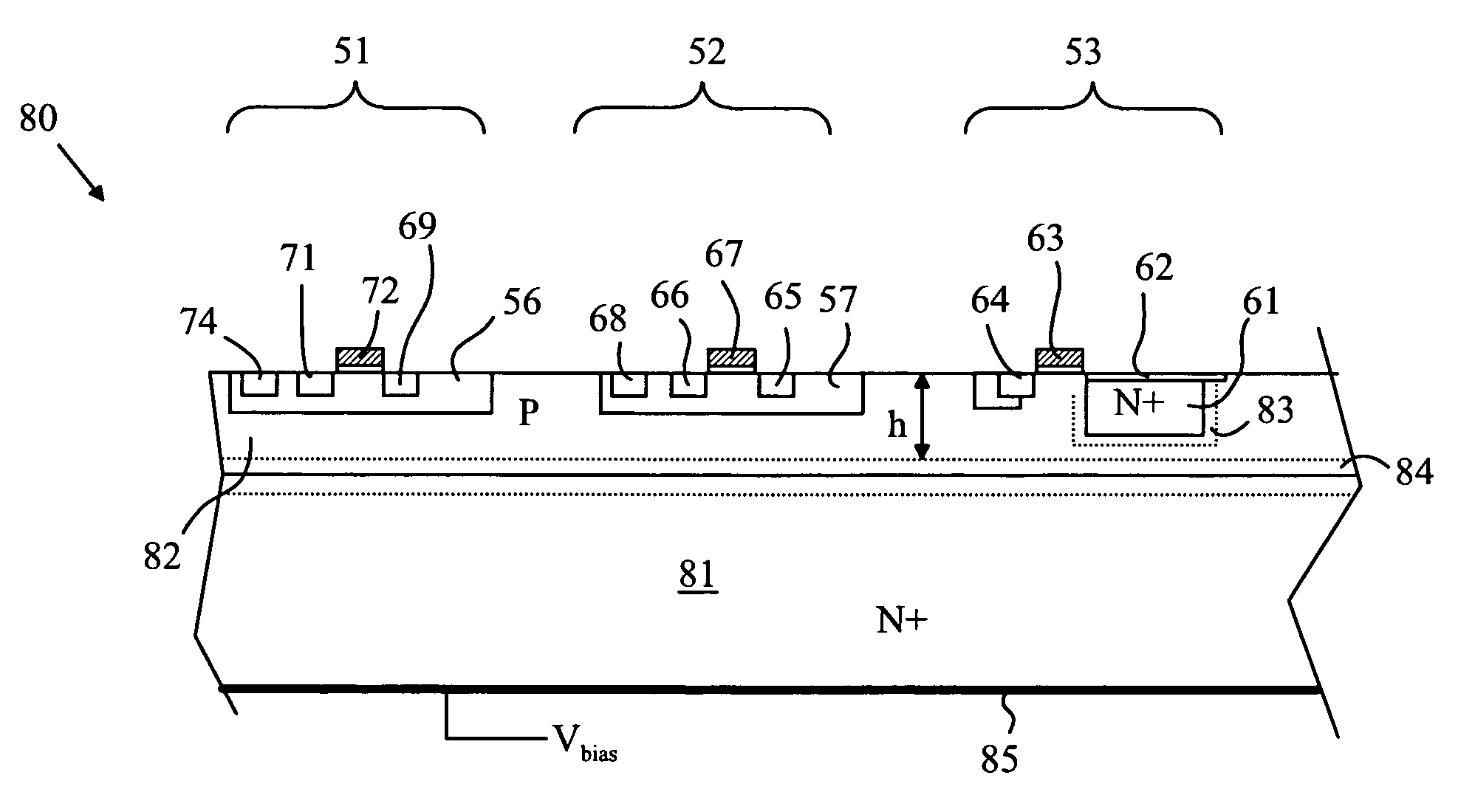
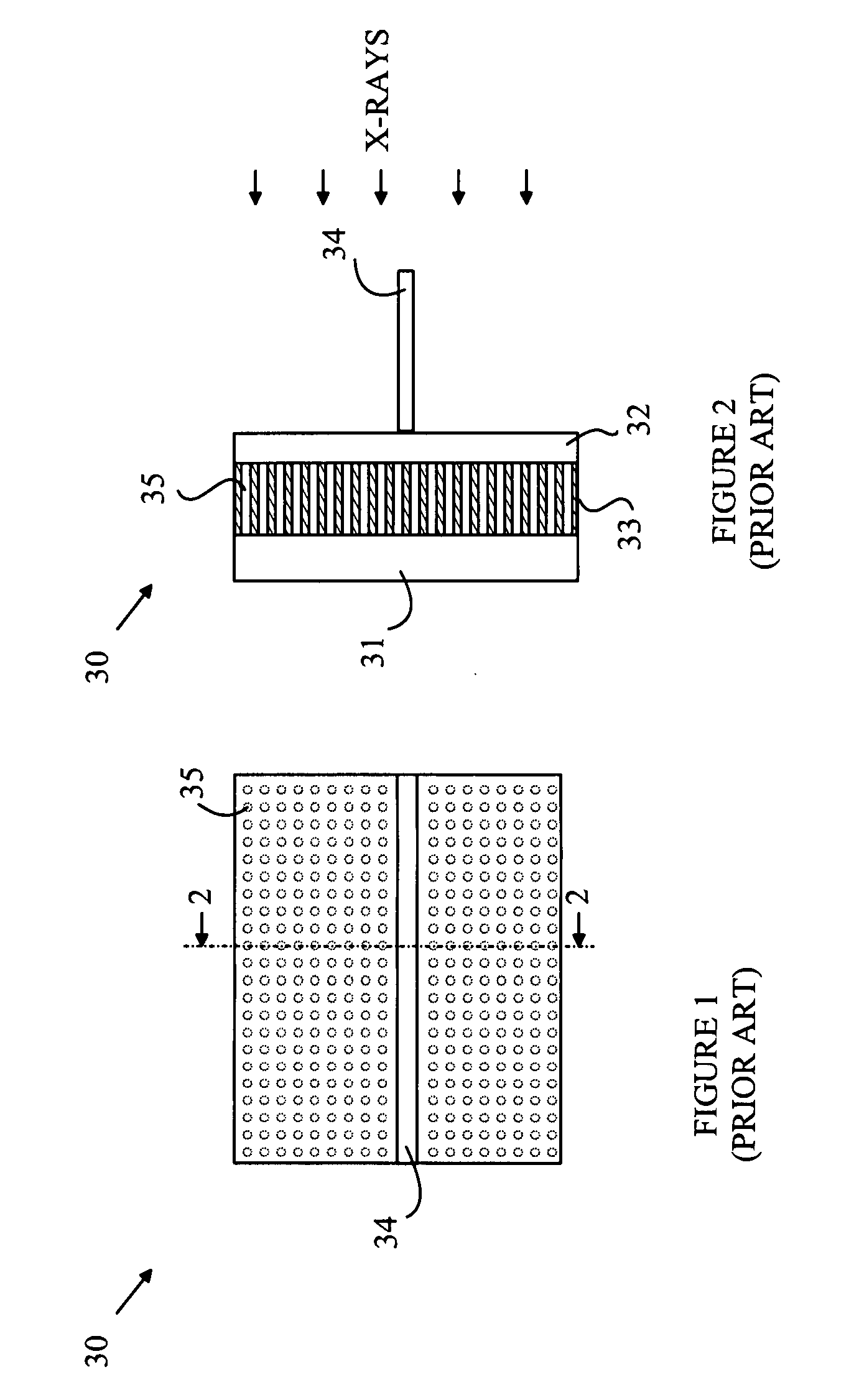
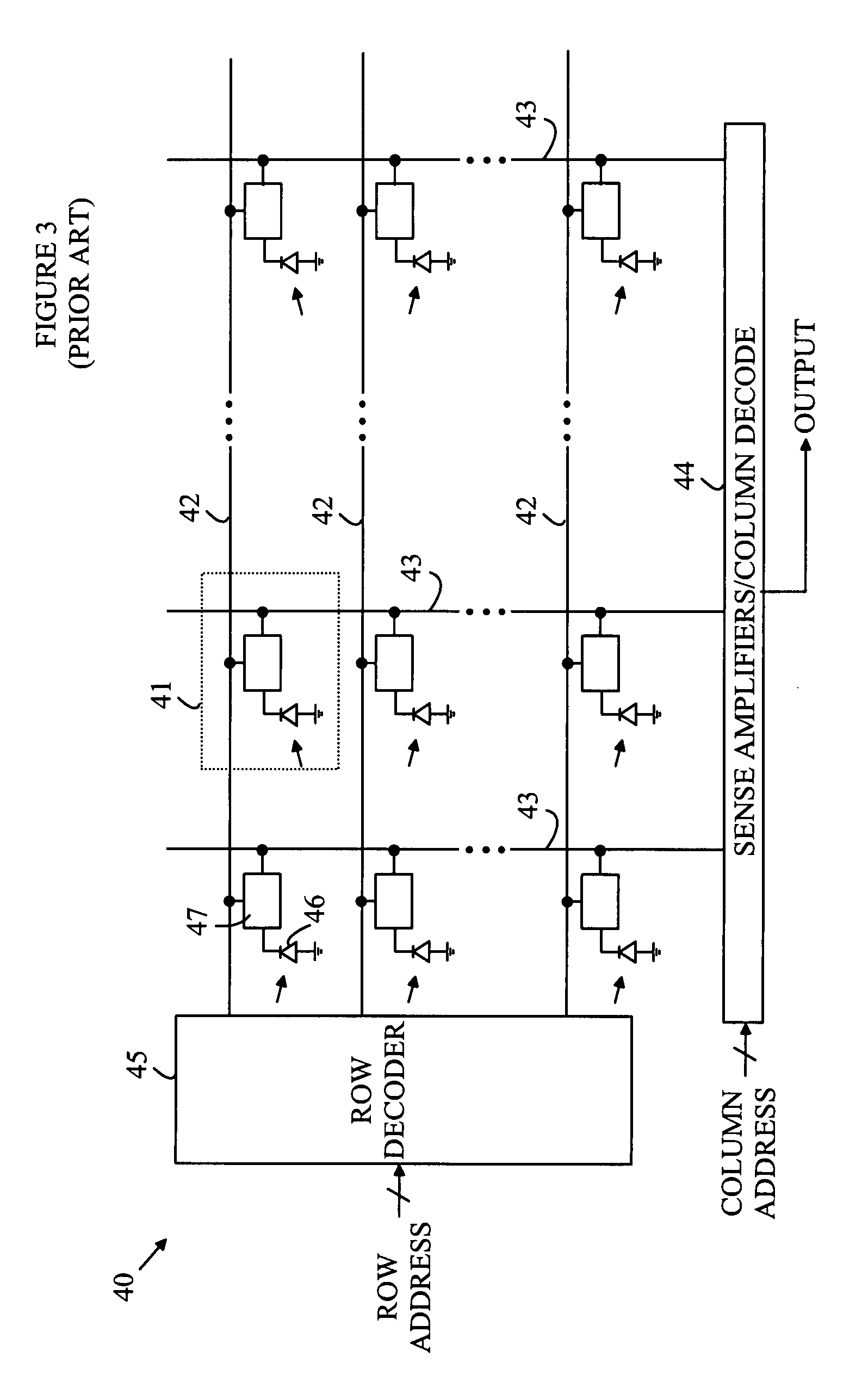
CMOS Detector with Reduced Sensitivity to X-Rays
InactiveUS20090108311A1Reduce backgroundSolid-state devicesMaterial analysis by optical meansPhysicsCMOS
An imaging array and method for operating the same is disclosed. The imaging array includes a semiconductor substrate having an epitaxial layer of semiconductor material deposited on a first surface thereof. A plurality of photodiodes is formed in a top surface of the epitaxial layer. The imaging array also includes a depletion layer underlying the photodiodes and disposed between the epitaxial layer and the semiconductor substrate. The depletion layer is connected to a power rail for removing electrons collected in the depletion layer. The depletion layer collects electrons generated by x-ray interactions in the substrate. The depletion layer can also be biased such that the depletion layer collects electrons collected by the photodiodes to provide a reset operation for the imaging array. The current flowing through the depletion layer can be used to generate a trigger signal indicating the start of an x-ray exposure.
Owner:BAE SYST IMAGING SOLUTIONS
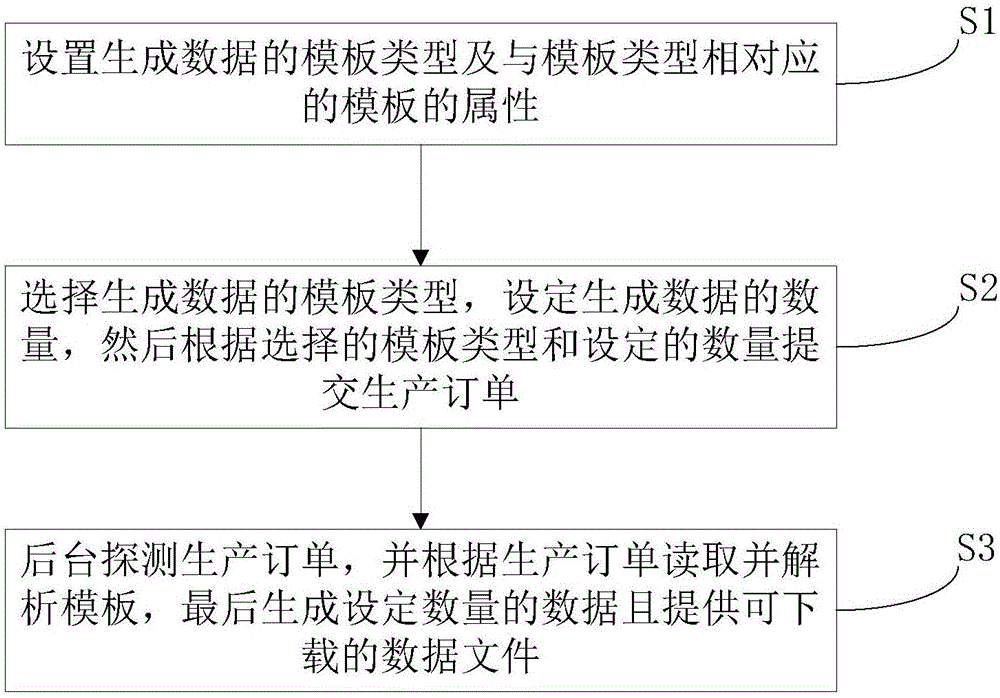
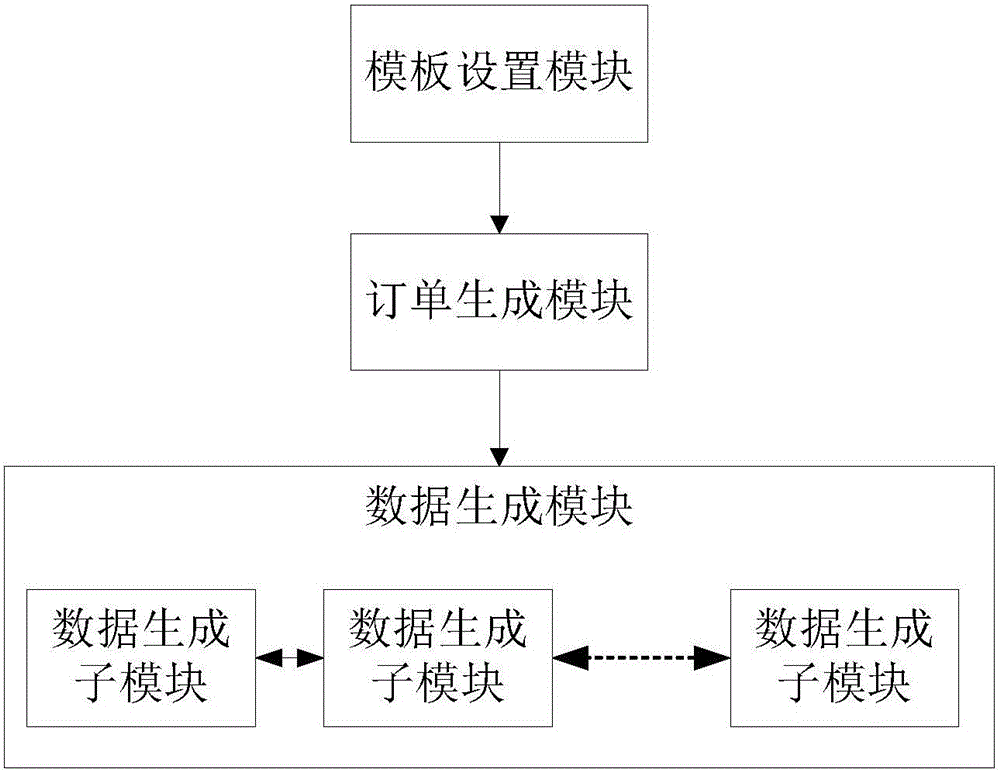
Method and system for generating data in batches
InactiveCN106815366AEasy maintenanceEasy to modifyRelational databasesFile/folder operationsOrder formData file
The invention relates to a method and system for generating data in batches and belongs to the field of data generation. The operation can be more convenient while the data generating efficiency can be improved. The method comprises the steps that a template type for data generation and attributes of a template corresponding to the template type are arranged firstly; the template type for data generation is selected, the volume of data to be generated is set, and then a production order is submitted according to the selected template type and the set volume of data to be generated; the production order is detected on a background, the template is read and parsed according to the production order, and finally the set volume of data is generated and downloadable data files are provided. The method and system is used for achieving reuse in the data generating process and completing decoupling of production order submitting and the data generating process, and the data generating efficiency is improved.
Owner:北京思特奇信息技术股份有限公司
Kit for detecting biomarker of Alzheimer's disease and detection method thereof
PendingCN112162101AImprove corrosion resistanceImprove the coating processDisease diagnosisBiological testingHorse-radishAntiendomysial antibodies
The invention discloses a kit for detecting a biomarker of an Alzheimer's disease, and relates to the technical field of in vitro diagnosis. The kit comprises a horse radish peroxidase labeled biomarker antibody solution, an anti-biomarker antibody coated elisa plate, a concentrated washing solution, a luminous substrate solution, a calibration product and a quality control product. The inventionfurther discloses a preparation method of the elisa plate coated with the anti-biomarker antibody. The kit has the beneficial effects that on the basis of chemiluminiscence immunoassay, the elisa plate coated with the anti-biomarker antibody is introduced, a coating process of the anti-biomarker antibody is improved, the reaction sensitivity is increased, a reagent production process is simplified, production time is shortened, and the detection kit which is high in quality, low in price, stable, reliable, good in repeatability, small in batch difference and high in accuracy is provided for the market.
Owner:NANJING LEADING BIOMEDICAL TECH CO LTD
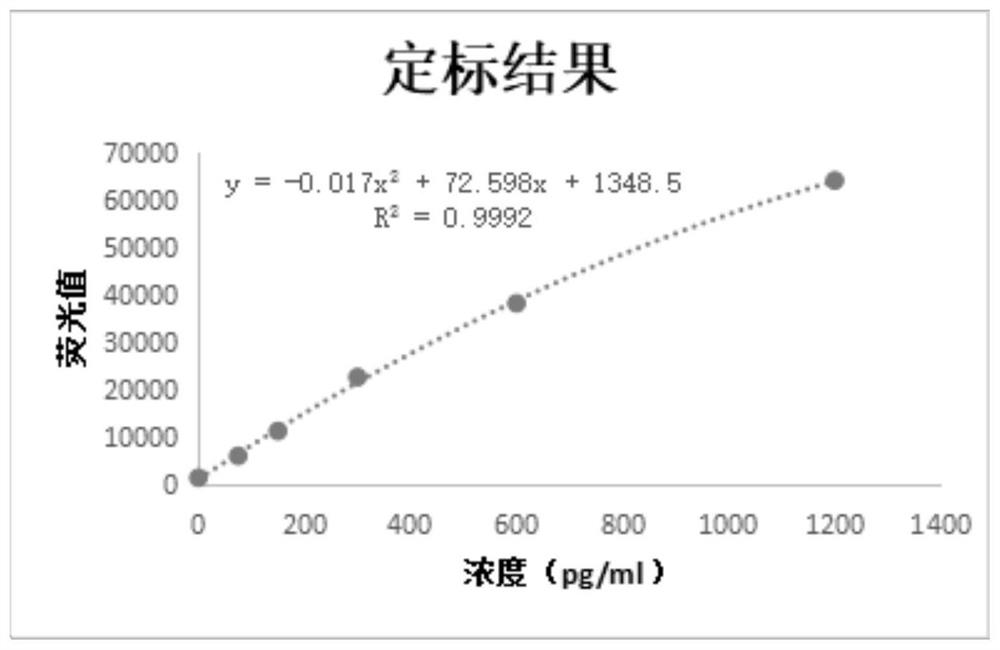
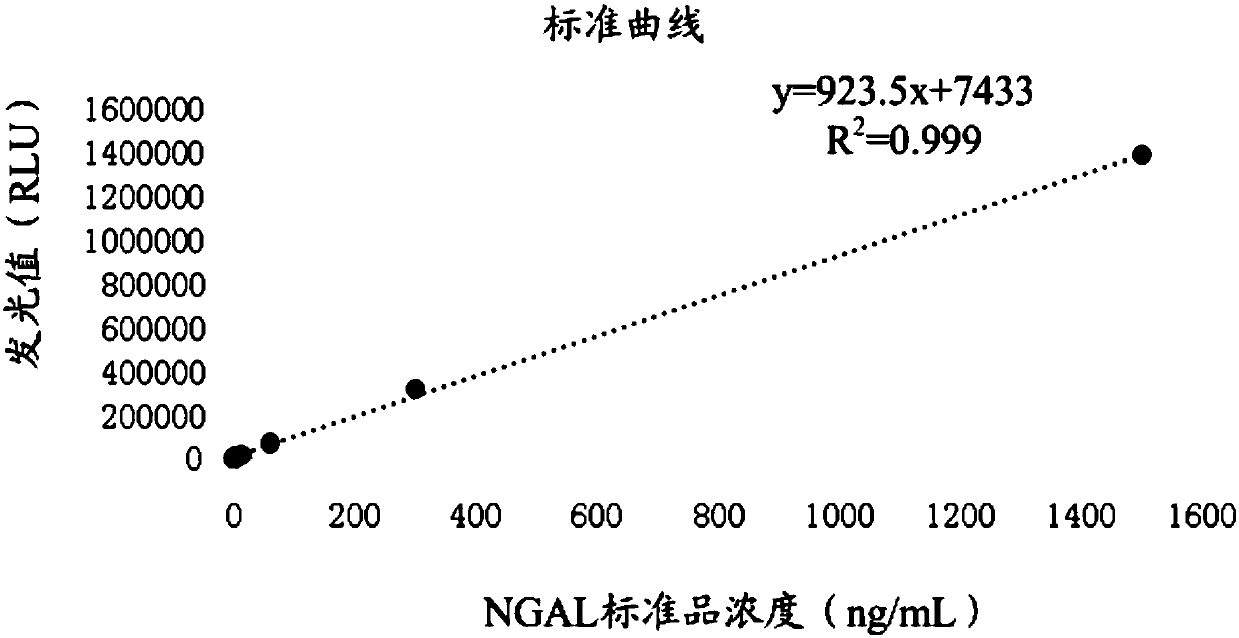
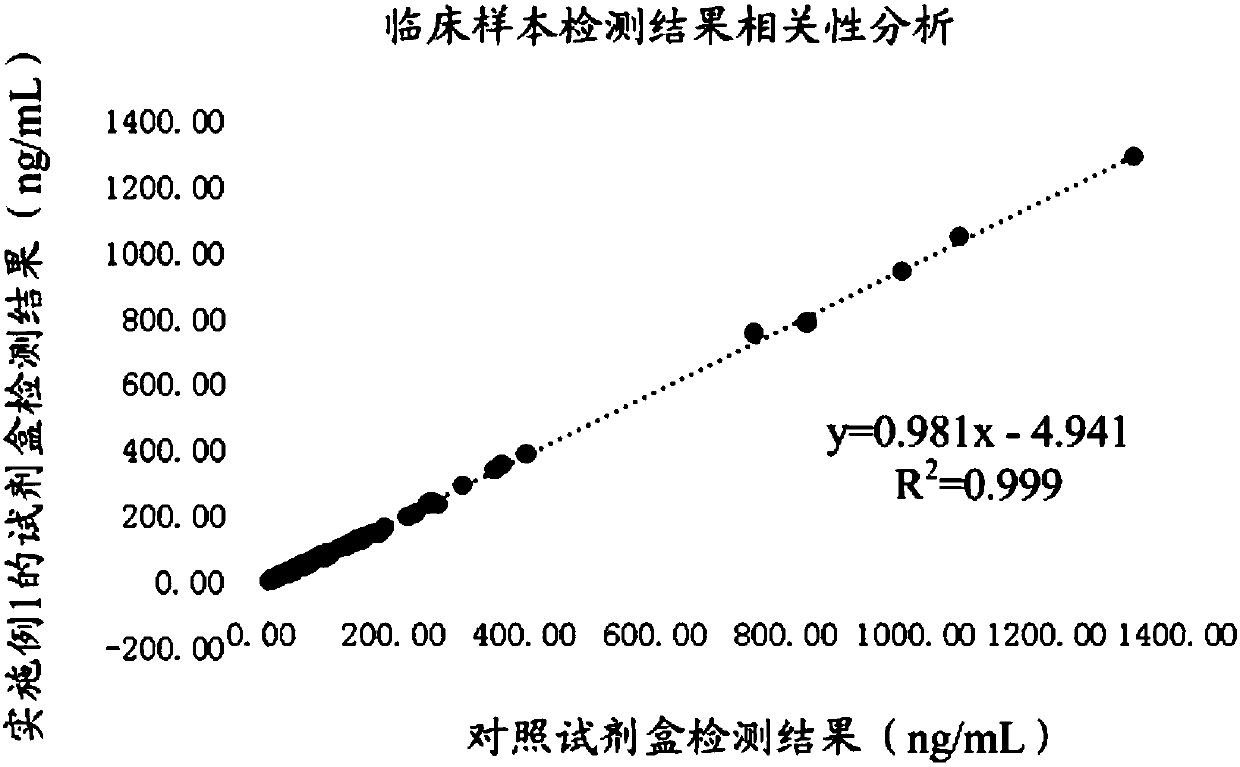
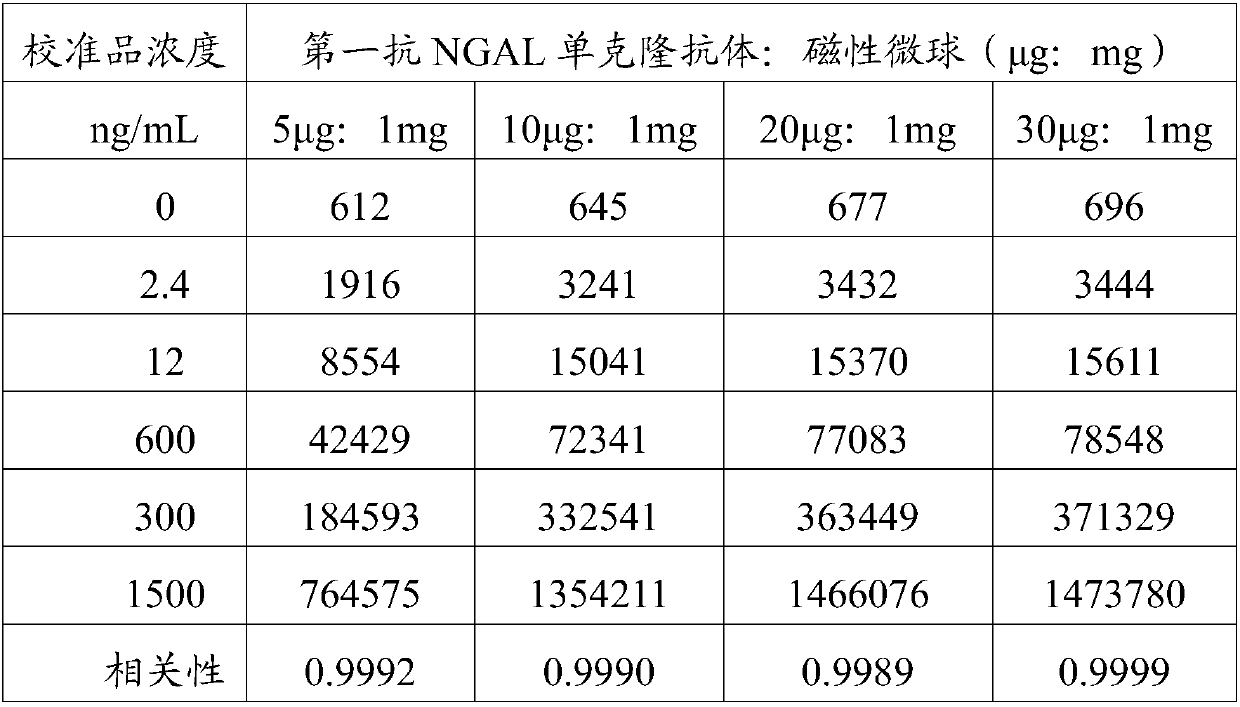
Detection kit for acute kidney injury
The invention relates to a detection kit for acute kidney injury. The detection kit comprises a first detection solution and a second detection solution, wherein the first detection solution containsa first anti-NGAL monoclonal antibody coated with magnetic particles, and the second detection solution contains an alkaline phosphatase-labeled second anti-NGAL monoclonal antibody. The first anti-NGAL monoclonal antibody and the second anti-NGAL monoclonal antibody are against different NGAL epitopes, respectively. The detection kit for acute kidney injury carries out detection with NGAL (neutrophil gelatinase-associated lipocalin) as a diagnosis and detection marker, can rapidly detect and diagnose acute kidney injury in an early stage of acute kidney injury, and has high sensitivity and specificity during detection.
Owner:FAPON BIOTECH INC +1
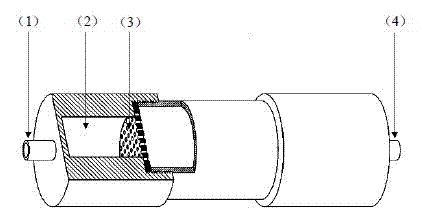
Double-barrelled accordance cylindrical flow-gas proportional counter tube
PendingCN106772535AReduce backgroundImproved energy resolutionRadiation intensity measurementImage resolutionEngineering
The present invention discloses a double-barrelled accordance cylindrical flow-gas proportional counter tube. The tube comprises a cylindrical tube body which is internally provided with an upper cavity and a lower cavity, an isolating membrane is configured to separate the upper cavity from the lower cavity, only obstructs [Alpha] particles and does not obstruct [Beta] particles, the isolating membrane is provided with an air vent hole, a sample room is arranged in the lower cavity, and an upper copper cavity and a lower copper cavity are respectively connected with ventilation interfaces; and the cylindrical tube body is earthed, the upper cavity and the lower cavity are internally provided with anode wires, and the upper cavity and the lower cavity respectively output mutually independent pulse signals at the normal work state. The problems are solved that a traditional flow-gas proportional counter tube is high in housing background and bad in resolution ratio, and [Alpha] and [Beta] perform mutual crosstalk when the total [Alpha] activity and the total [Beta] activity are measured at the same time.
Owner:上海新漫传感科技有限公司
Magnetic micro particle immunofluorescence kit for quantitatively assaying classical swine fever virus antibody
InactiveCN108152509AImprove responseImproving immunogenicityBiological testingAntigenPositive control
The invention discloses a magnetic micro particle immunofluorescence kit for quantitatively assaying classical swine fever virus antibody, which consists of classical swine fever E2 antigen immunomagnetic beads, fluorescent substance-labeled goat anti-pig antibody, detergent, enhancement solution, negative control serum and positive control serum. Compared with the RT-PCR (reverse transcription-polymerase chain reaction) method and the ELISA (enzyme-linked immunosorbent assay) method, the magnetic micro particle immunofluorescence kit for quantitatively assaying classical swine fever virus antibody which is disclosed by the invention is easy to operate and suitable for assaying different numbers of samples, and has high assay speed (a result can be obtained in about 20 minutes); and compared with the colloidal gold immunochromatographic strip, the kit has the advantages of higher sensitivity, wider linear range, capability of realizing accurate quantification and the like. The assay result of the kit can accurately reflect the change law of anti-CSFV (anti-classical swine fever virus) serum IgG antibody of the body in the process of vaccination and provide scientific and reasonabletechnical support for pig farmers in preventing and controlling CSF (classical swine fever).
Owner:GUANGZHOU BIOKEY HEALTH TECH CO LTD
POCT full-automatic chemiluminiscence device based on active magnetic separation technology
ActiveCN110749742ARealize dual-channel detectionImprove detection efficiencyMaterial analysisTemperature controlEmergency treatment
The invention relates to the field of full-automatic chemiluminescence, especially to a POCT full-automatic chemiluminiscence device based on active magnetic separation technology. The POCT full-automatic chemiluminiscence device comprises a mounting frame, a sample adding arm, a constant-temperature control bin, a TIP head unloading frame and a magnetic separation component are mounted on the mounting frame, the constant-temperature control bin comprises a first reaction bin and a second reaction bin, the magnetic separation component comprises a first magnetic separation component and a second magnetic separation component, the first magnetic separation component is correspondingly arranged above the first reaction bin, and the second magnetic separation component is correspondingly arranged above the second reaction bin. The first reaction bin and the second reaction bin which are independent of each other are adopted, the first reaction bin and the second reaction bin can operate independently, dual-channel detection is achieved, the detection efficiency can be improved, and emergency treatment requirements are met. According to the active magnetic separation technology of themagnetic separation component, magnetic beads are directly attracted to the bottom of a magnetic separation sleeve through cooperation of the disposable magnetic separation sleeve and a magnetic bar,the active magnetic separation effect is achieved, the cleaning efficiency is improved, and the background of the system is reduced.
Owner:成都宜乐芯生物科技有限公司
Detection, localization and staging of tumors using labeled activated lymphocytes directed to a tumor specific epitope
InactiveUS20060171883A1Bind more effectivelyGood effectDisease diagnosisRadioactive preparation formsAbnormal tissue growthCell specific
A Disclosed are methods for detecting and localizing a cell-specific antigen in a mammal, such as a human subject, comprising exposing peripheral blood mononuclear cells (PBMCs) of the mammal to an immunogenic peptide epitope of the antigen, under conditions for antigen-specific activation of T lymphocytes in the PBMCs, thereby producing antigen-specific T lymphocytes that at least bind to the cell-specific antigen. Labeled antigen-specific T lymphocytes are administered to the mammal, typically with-out IL-2, either intraperitoneally or intravenously. The distribution of these cells in the mammal is determined by imaging, thereby detecting and localizing cell-specific antigen in the mammal. Exposing PBMCs to the immunogenic peptide typically involves a cell-free peptide preparation and interleukin-2 (IL-2), but no additional cells such as antigen presenting cells (APC) separately pulsed with antigen. The antigen-specific T lymphocytes typically are cytolytic for cells expressing the cell-specific antigen and may comprise CD4+, CD8+, and / or CD45RO+ memory T cells.
Owner:PHILLIPS CATHERINE A DR +1
Confining liquid for liquid chip, confining method and application
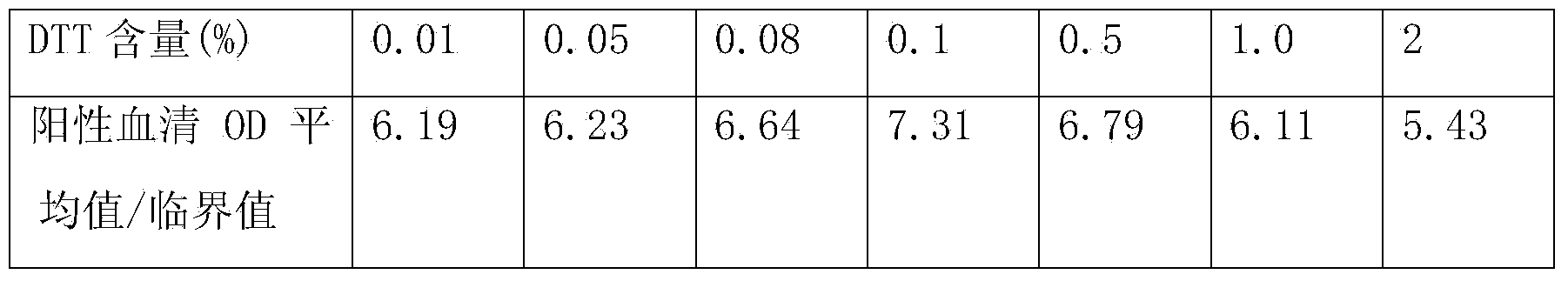
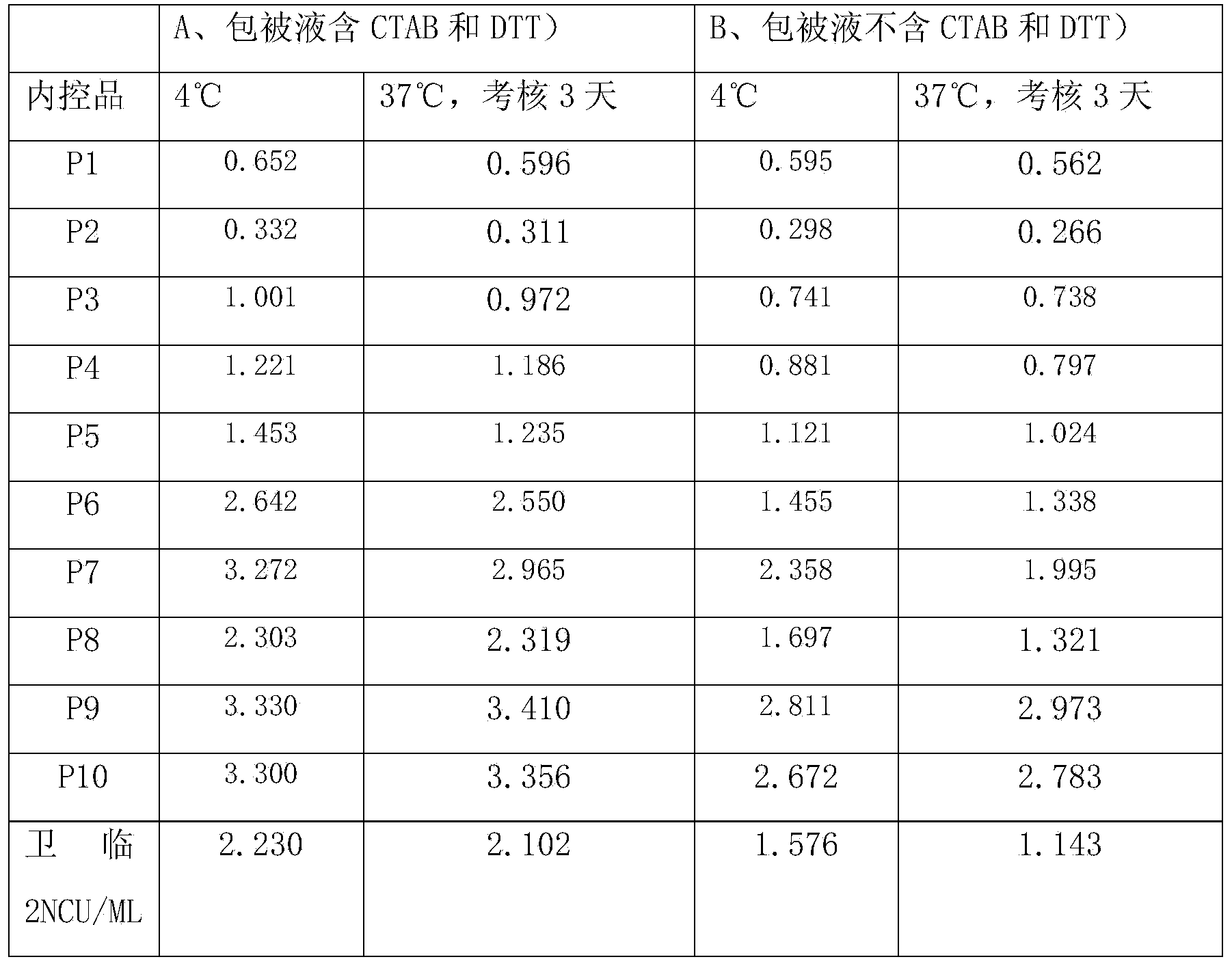
PendingCN110836965AReduce non-specific bindingReduce backgroundBiological testingProteinogenic amino acidActive agent
The invention provides confining liquid for a liquid chip. The confining liquid comprises a phosphate buffer solution, protein, amino acid, a surfactant and a preservative. According to the confiningliquid, a confining link of coating antibody magnetic beads is added in the traditional liquid chip magnetic bead coating process, and the amino acid and the protein in the confining liquid can be well combined with gaps in the magnetic beads, so that the nonspecific binding of serum protein is reduced, the background of the reaction is reduced, and the sensitivity and precision of the reaction are improved.
Owner:北京协和洛克生物技术有限责任公司
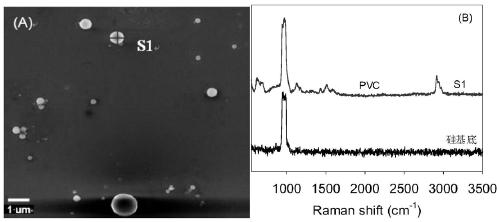
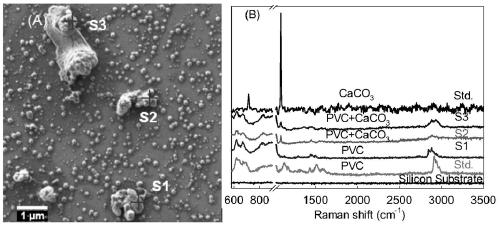
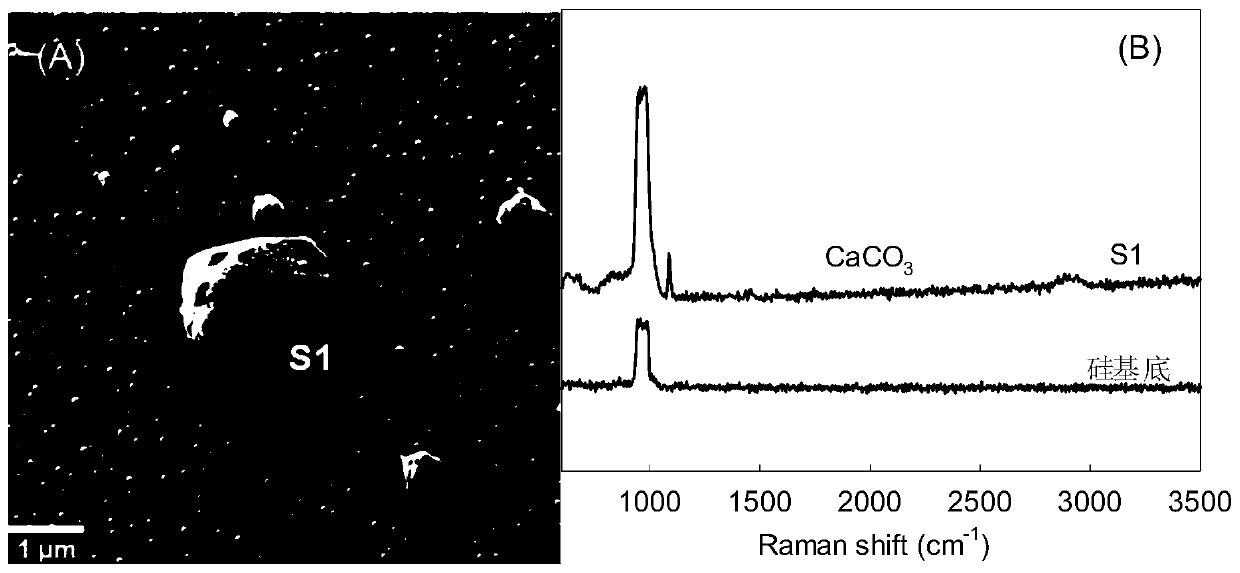
Method for identifying nano plastic particles in aqueous solution based on scanning electron microscope-Raman technology
InactiveCN111122634AQuality improvementImprove spatial resolutionMaterial analysis using wave/particle radiationRaman scatteringImaging analysisNanoparticle
The invention belongs to the field of nano plastic identification. The invention discloses a method for identifying nano plastic particles in an aqueous solution based on a scanning electron microscope-Raman technology. Targeted recognition and in-situ identification of nano plastic particles are realized by setting and optimizing parameters of a scanning electron microscope and a white-light-containing confocal microscopic Raman spectrum combination instrument; the method comprises the following steps: uniformly dripping a to-be-detected aqueous solution on a clean silicon wafer at room temperature after carrying out graded filtration on the to-be-detected aqueous solution through 10 [mu]m and 1 [mu]m polyethersulfone filter membranes in sequence; naturally air-drying a sample, placing the sample on a sample table in a vacuum cavity of a scanning electron microscope, setting corresponding test parameters to obtain a scanning electron microscope image, automatically transmitting the detection sample to a Raman spectrum, keeping the detection sample at the same position, setting proper test parameters to obtain a Raman image, and carrying out image analysis and judgment. Compared with a traditional detection method, the method has the advantages that the morphology of the nanoparticles in the solution can be obtained while ensuring that a nano plastic sample is not damaged, andmeanwhile, whether the nanoparticles contain plastic components or not is identified.
Owner:TONGJI UNIV
CRISPR-Cas9-based nucleic acid detection system and application thereof
ActiveCN110777159AQuick checkReduce backgroundMicrobiological testing/measurementStable introduction of DNACell freeNucleic acid detection
The invention provides a CRISPR-Cas9-based nucleic acid detection system and application thereof. A protein expression regulating and control system comprises Cas9 protein, RsgRNA and a cell-free protein expression system; and the RsgRNA comprises antisense nucleic acid and sgRNA according to a direction of 5' to 3'. The nucleic acid detection system provided by the invention combines a CRIPSR / Cas9 nucleic acid identification regulating and control system with a gene loop, regulates and controls the expression of protease by the RsgRNA, converts a nucleic acid signal into generation of the protease, and realizes rapid detection of the nucleic acid by detecting the protease.
Owner:SUZHOU DIYINAN BIOTECHNOLOGY CO LTD
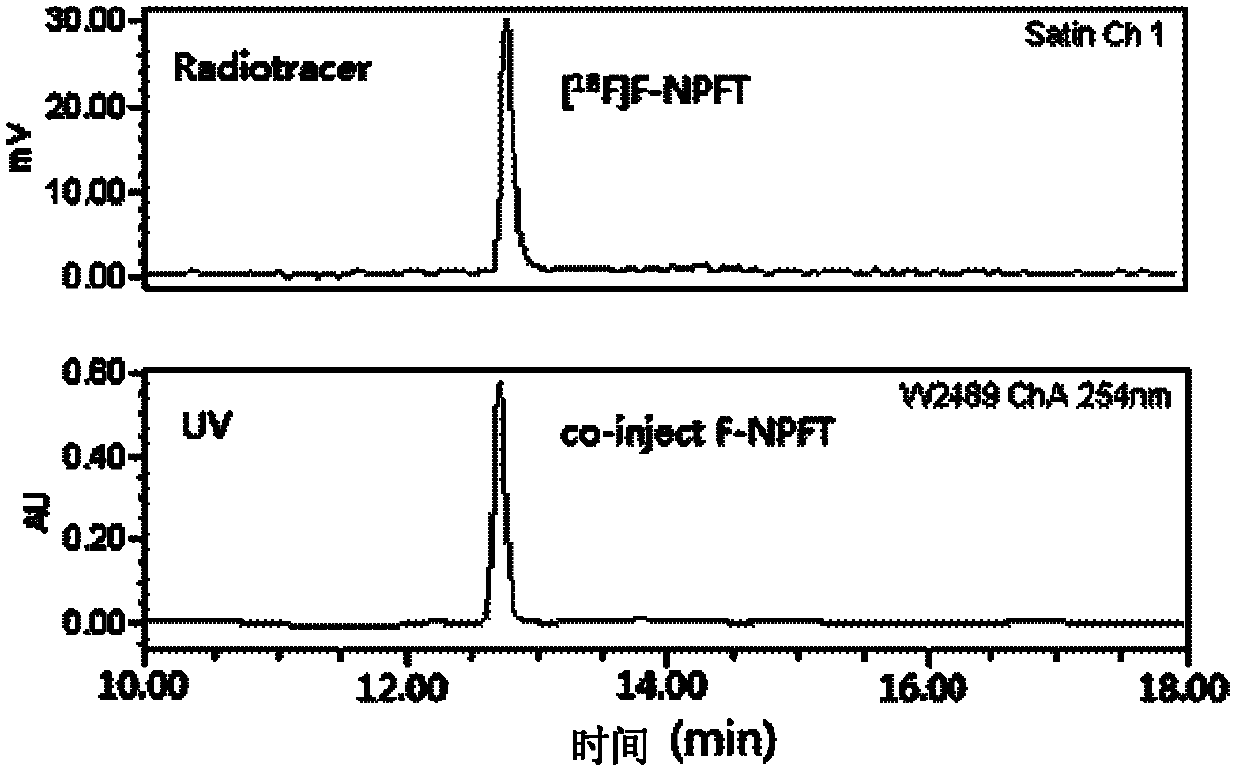
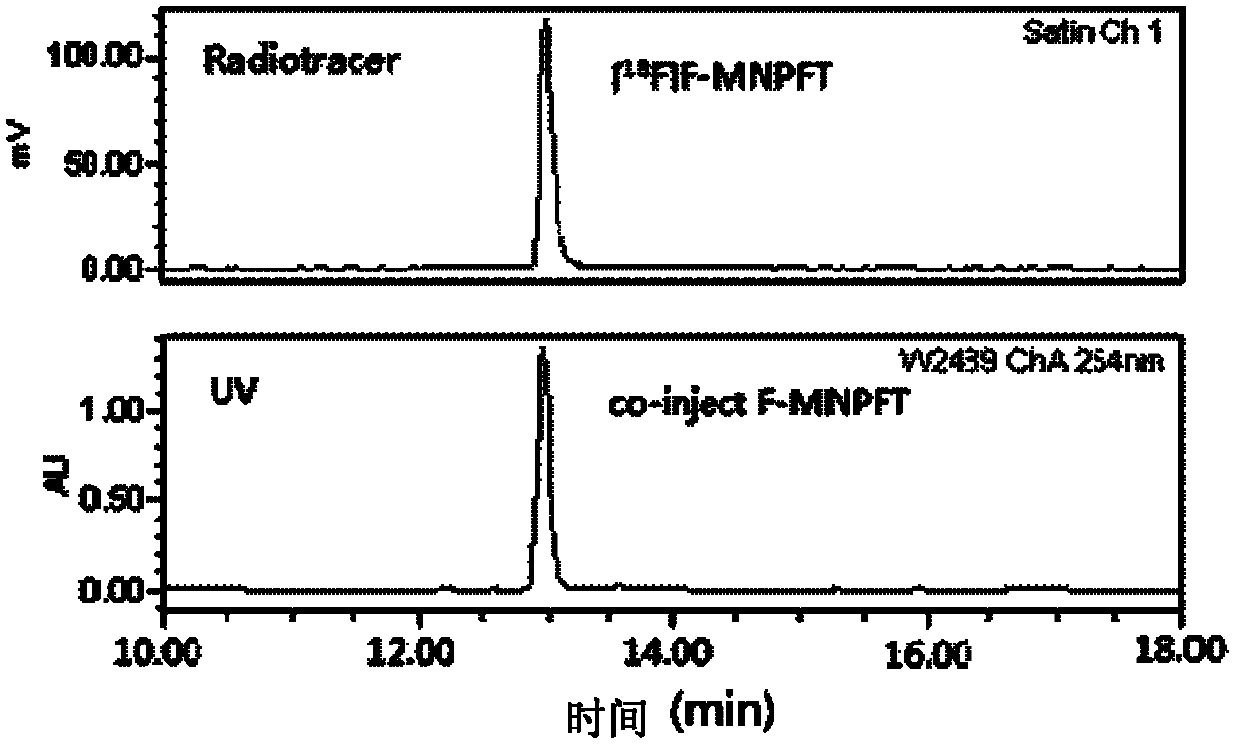
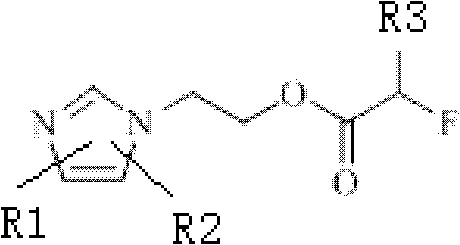
18/19F-ester nitroimidazole compound, preparation method thereof and application as hypoxic tissue developing agent
InactiveCN102603647AImprove metabolic propertiesWell targeted uptakeOrganic chemistryRadioactive preparation carriersNitroimidazoleTumor target
The invention relates to an 18 / 19F-ester nitroimidazole compound, preparation method thereof and application as a hypoxic tissue developing agent. The invention provides F-ester nitroimidazole compounds as shown in a general formula, wherein an 18F-ester nitroimidazole compound is a radioactive compound. According to an S180 tumor-bearing mice experiment, the 18F-ester nitroimidazole compound has the characteristics of good tumor targeted intake and low liver background level, and is a potential positron emission tomography (PET) hypoxic tissue developing agent.
Owner:BEIJING NORMAL UNIVERSITY
Magnetic bead time resolution fluorescence immunoassay quantitative determination cTnI (cardiac troponin I) kit
InactiveCN108333370AIncrease binding areaLonger storage time and more stableBiological testingMagnetic beadFluorescence
The invention discloses a magnetic bead time resolution fluorescence immunoassay quantitative determination cTnI kit. The cTnI kit comprises an immunomagnetic bead coating a cTnI monoclonal antibody,a cTnI standardized product solution, an europium-marked cTnI monoclonal antibody solution, washing liquid and enhancement liquid. The immunomagnetic bead coating the cTnI monoclonal antibody is a covalent conjugate of a superpara magnetic bead modified by a functional group and with the diameter being 1-3 microns and the cTnI monoclonal antibody. The kit has the high sensibility, the sensibilityof cTnI is 0.05ng / mL, and a blood serum (plasma) does not need to be diluted; the determination time is short, and a report can be resulted within 30 minutes; the demanding amount of the sample is less, and only 100 microliters are needed for one-time sample loading; and the kit is equipped with a full-automatic time resolution immune analysis meter, operation is easy, and labor is saved.
Owner:GUANGZHOU BIOKEY HEALTH TECH CO LTD
Fluorescent quantitative PCR method for detecting vibrio parahaemolyticus causing acute hepatic pancreatic necrosis
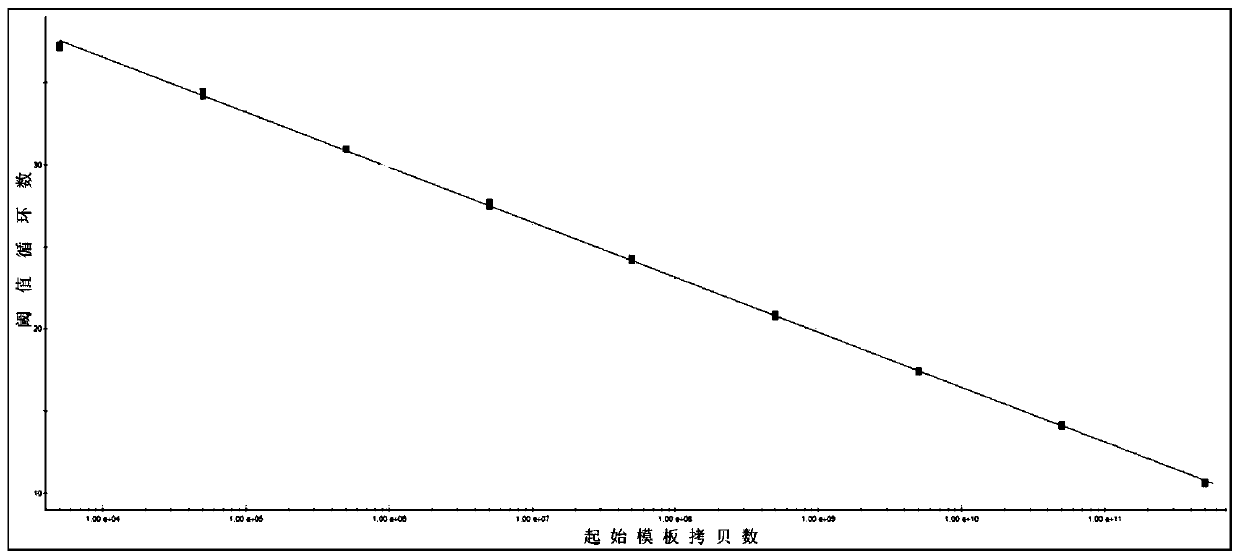
PendingCN110106264AImprove stabilityReduce backgroundMicrobiological testing/measurementMicroorganism based processesVibrio parahaemolyticusBiology
The invention discloses a fluorescent quantitative PCR method for detecting vibrio parahaemolyticus causing acute hepatic pancreatic necrosis. The method specifically comprises the following steps of(1) taking the PirAVp gene of VpAHPND as a detection target gene and designing a specific primer and a TaqMan-MGB probe; (2) extracting the DNA of the VpAHPND, constructing a recombinant plasmid, preparing a standard substance, and storing at -20 DEG C for use; (3) performing a quantitative PCR, and establishing a standard curve and a standard equation between the logarithm of the initial templatecopy number and the threshold cycle number; (4) detecting the to-be-detected sample with the primer and the probe in the step (1),and based on the measured threshold cycle number, according to the standard curve, calculating the copy number of the VpAHPND in the to-be-detected sample. The Fluorescent quantitative PCR method for detecting the VpAHPND for non-diagnostic purposes established by theinvention has the advantages of high sensitivity, strong specificity, good reproducibility, rapid quantification and the like, and can be used for clinically detecting the VpAHPND of shrimp samples and water samples.
Owner:GUANGXI ACADEMY OF FISHERY SCI
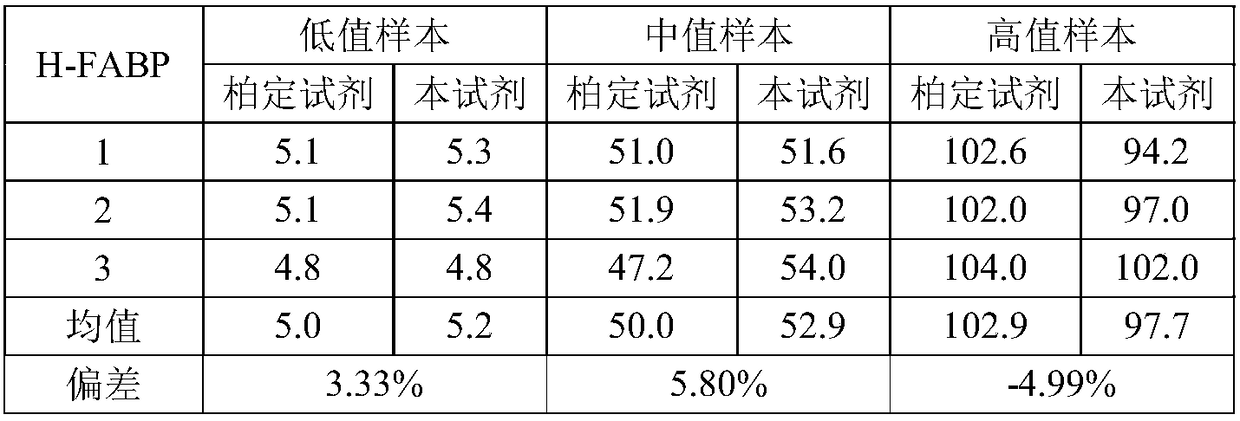
Magnetic bead time resolution immunofluorescence quantitative detection H-FABP (heart-type fatty acid binding protein) kit
InactiveCN108181287AIncrease binding areaLonger storage time and more stableFluorescence/phosphorescenceFluorescenceMagnetic bead
The invention discloses a magnetic bead time resolution immunofluorescence quantitative detection H-FABP (heart-type fatty acid binding protein) kit. The kit comprises an H-FABP monoclonal antibody-coated immunomagnetic bead, an H-FABP calibration product solution, a europium-marked H-FABP monoclonal antibody solution, cleaning liquid and an enhancement solution. The H-FABP monoclonal antibody-coated immunomagnetic bead is super paramagnetic bead with functional group modification and the diameter of 1 to 3 [mu]m and an H-FABP monoclonal antibody covalent conjugate. The kit is high in sensitivity, the sensitivity of H-FABP is 2 ng / mL, and a serum (plasma) sample does not need to be diluted; the detection time is short and a report can be output within 30 minutes; the demand quantity of samples is small and one-time sample loading only needs 50 [mu]L; the matched fully-automatic time resolution immunoanalyzer is simple in operation and labor-saving.
Owner:GUANGZHOU BIOKEY HEALTH TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com