Magnetic bead time resolution immunofluorescence quantitative detection H-FABP (heart-type fatty acid binding protein) kit
A time-resolved fluorescence, H-FABP technology, used in fluorescence/phosphorescence, measurement devices, analytical materials, etc., can solve the problems of long detection time, low sensitivity, poor stability, etc., to achieve simple and fast operation, high quantum yield, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In this embodiment, the magnetic bead time-resolved fluorescence immunoquantitative detection kit for H-FABP consists of reagent strips and H-FABP calibrator.
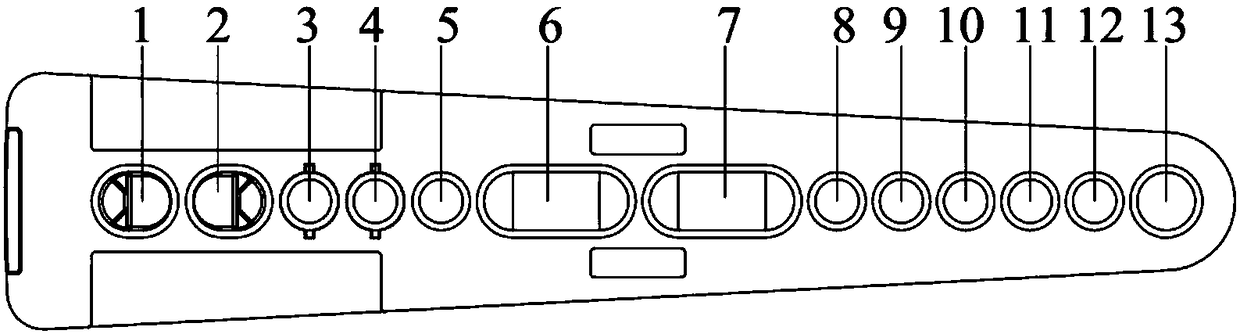
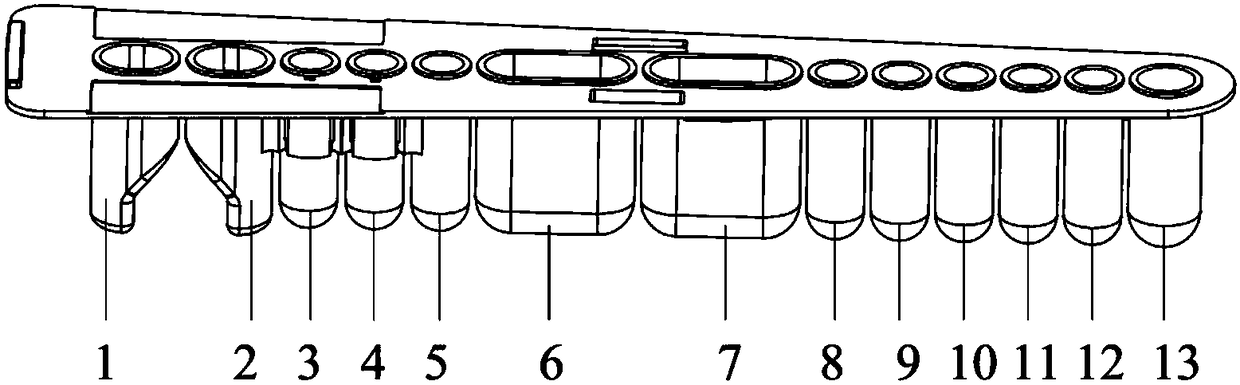
[0029]Wherein, the shape of the reagent strip is fan-shaped, and 1 to 13 holes are arranged sequentially from left to right. The 1st and 2nd holes are test holes, the 3rd and 4th holes are fluorescent marker holes, the 6th and 7th holes are cleaning solution holes, the 8th and 9th are sample diluent holes, the 12th are enhancement solution holes, the 5th and 10th holes are , 11,13 are preparatory holes. A magnet can be stored between the 1st and 2nd wells for magnetic separation experiments. The 1st and 2nd wells can store a liquid volume of 300 μl; the 3rd and 4th wells can be disassembled from the entire reagent strip to become independent components, which is convenient for fluorescent markers. Pack and store. The 3rd, 4th, and 5th wells can store a liquid volume of 400 μl; the 6th and 7th wells can store a...
Embodiment 2
[0059] The preparation method of the H-FABP calibrator is: dilute the H-FABP antigen to 0, 1, 5, 10, 50, 100 , 160ng / ml. The making and detection of embodiment 2 reagent strips
[0060] The semi-finished reagent strips in this example are assembled through the following procedures: 50 μL of H-FABP immunomagnetic beads, 200 μL of europium-labeled H-FABP antibody, 2000 μL of washing solution, 2000 μL of Washing solution, 400 μL enhancement solution, and then seal with a film sealing machine. The sealing film is coated with product information identification that can be scanned and recognized by an automatic fluorescence detection analyzer, including enterprise standard curve, batch, production date, and expiration date. The finished reagent strips, the packaged H-FABP calibrator and other accessories are assembled into a kit.
[0061] Sample testing:
[0062] ①Add sample:
[0063] Put the sample or calibrator to be tested into the loading system of the automatic instrument, ...
Embodiment 3
[0074] The comparison of embodiment 3 and commercially available kit
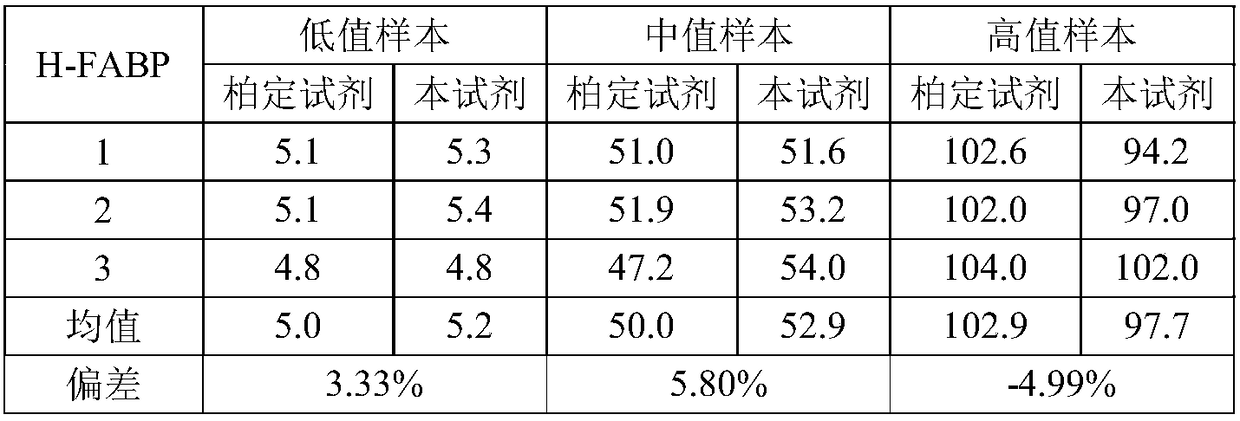
[0075] Adopt commercially available heart-type fatty acid-binding protein assay kit (immunoturbidimetric method) (Padding Bioengineering (Beijing) Co., Ltd.) and the method detection sample in embodiment 1 (in the described sample, the concentration range of H-FABP is 2.5 ~160ng / mL) was repeated three times to verify the correctness of the detection results of the kit of the present invention, and the results are shown in Table 1 below.
[0076] Table 1 Sample test results
[0077]
[0078] Compared with commercially available reagents, the deviation of this reagent is less than ±10%, and the detection result of this reagent is accurate and reliable.
[0079] The commercially available heart-type fatty acid binding protein assay kit (immunoturbidimetric method) (Padin Bioengineering (Beijing) Co., Ltd.) and the reagents in Example 1 were used to detect 200 clinical samples, and the test results are show...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com