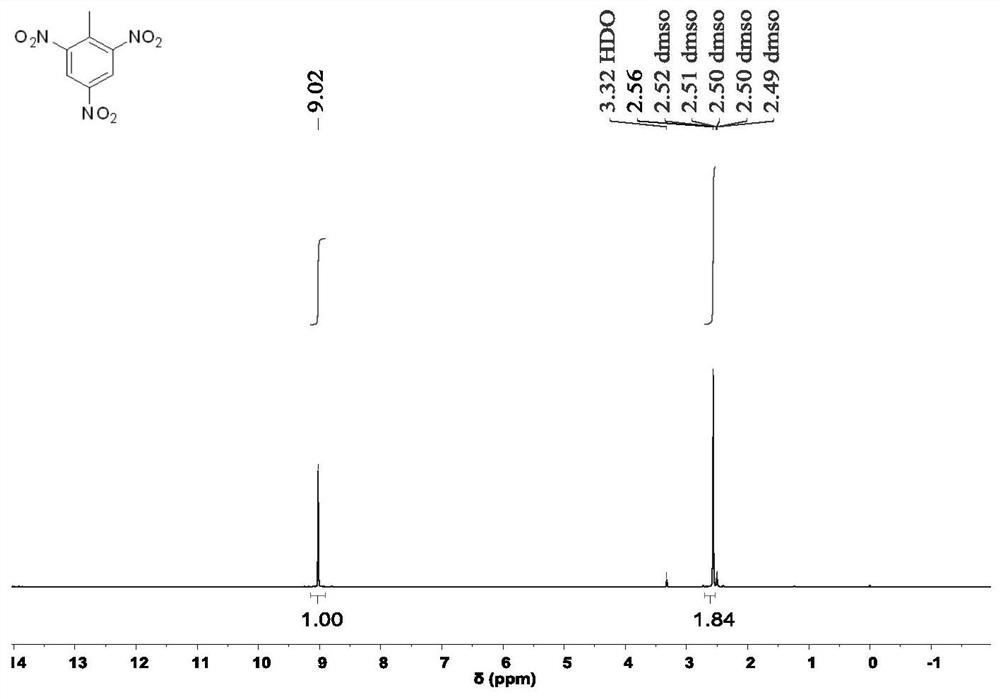
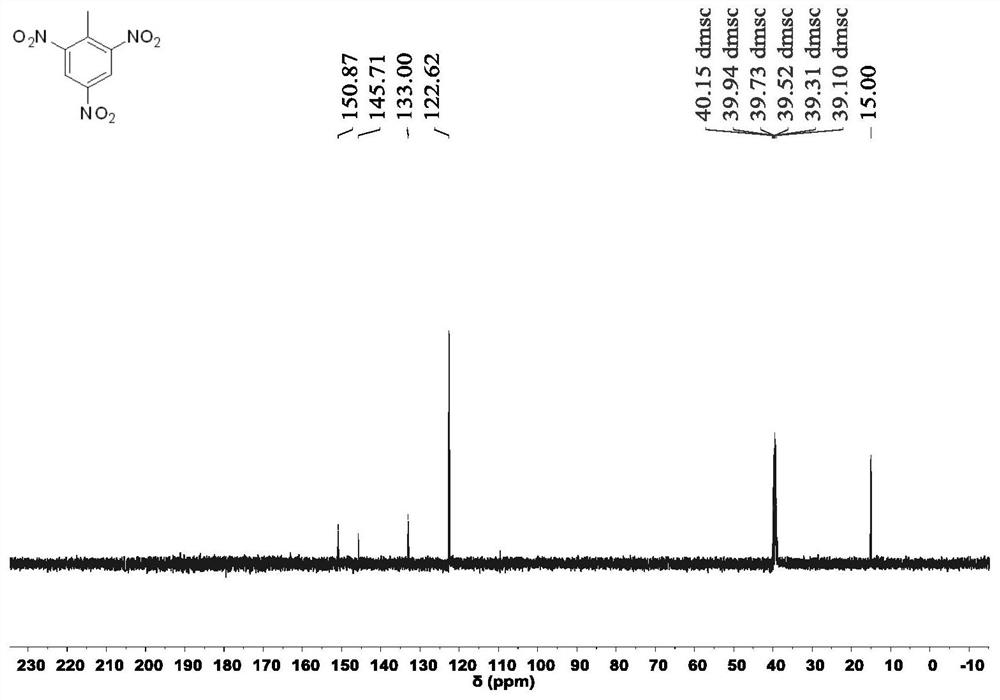
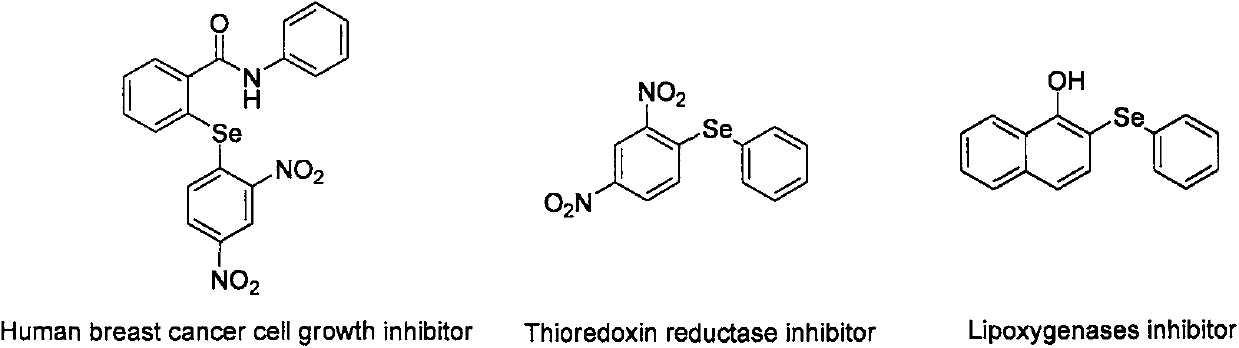
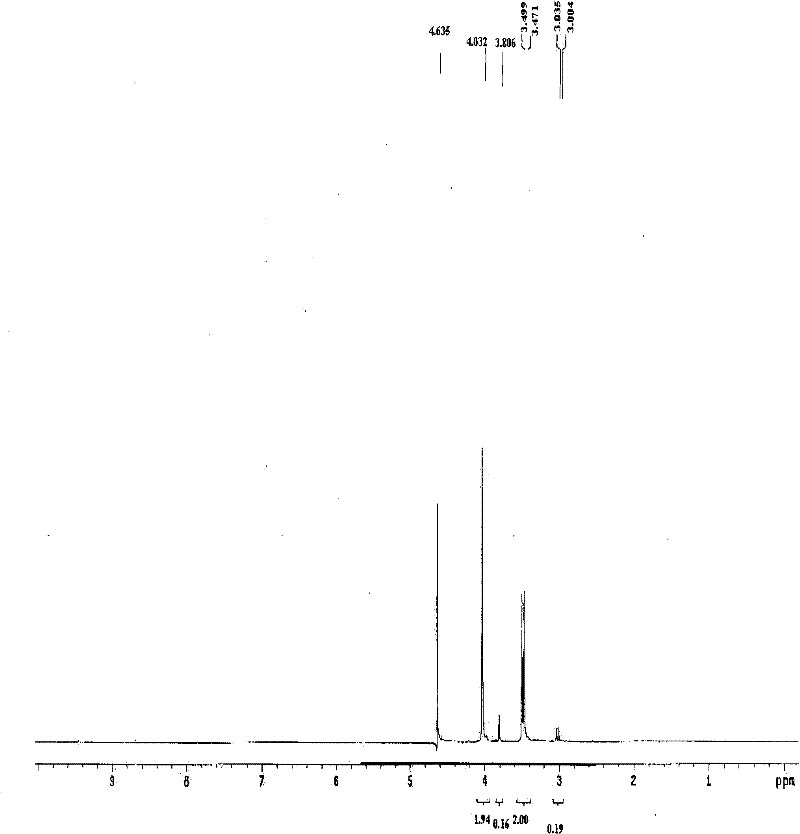
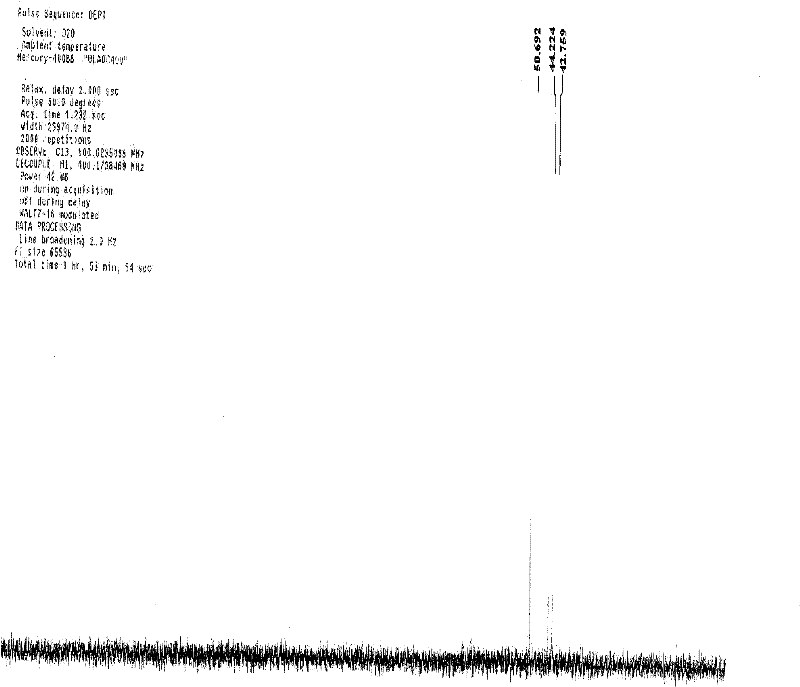
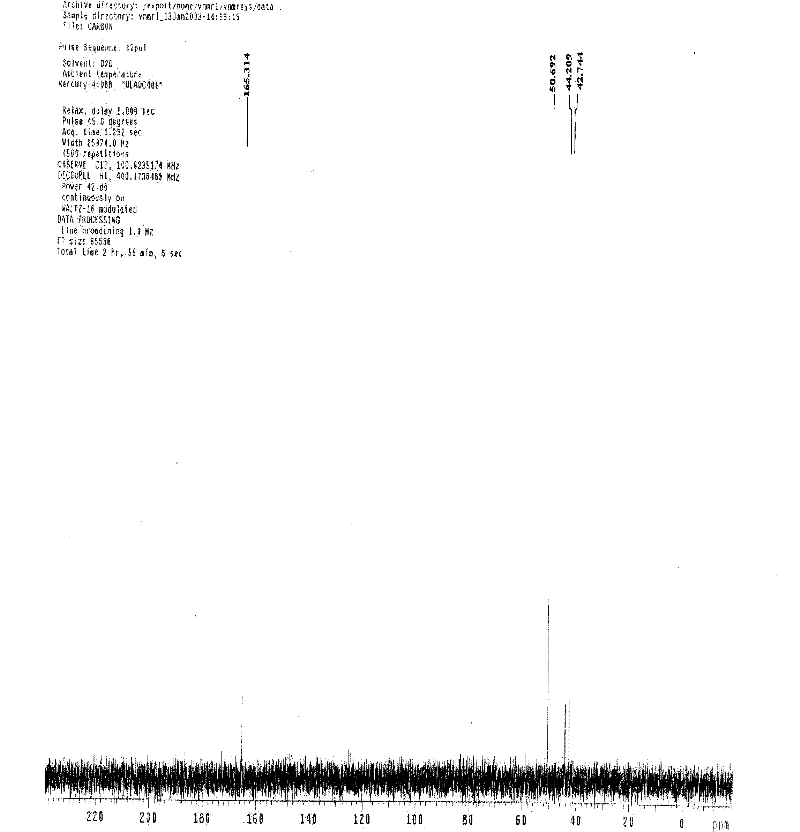
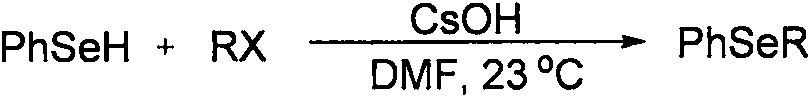
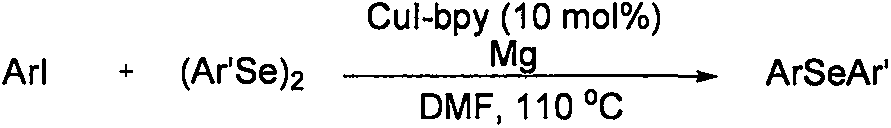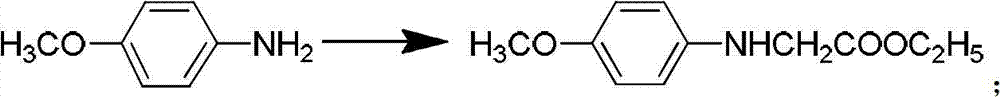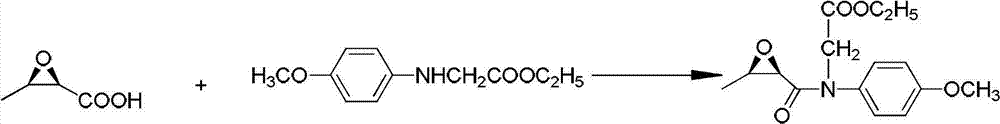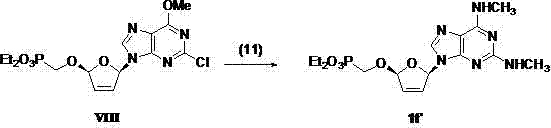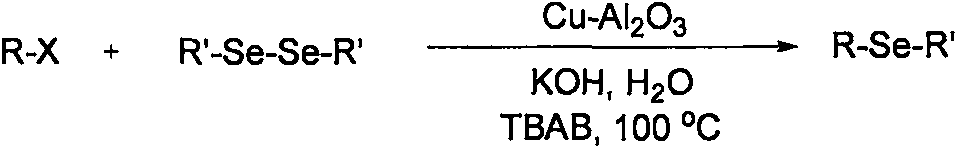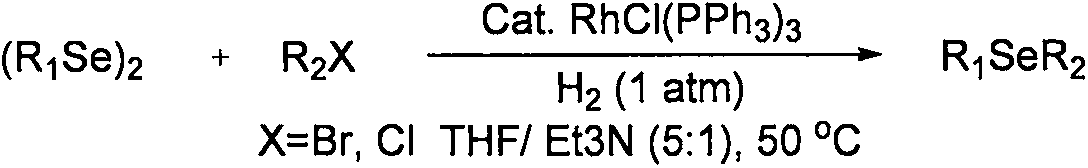Patents
Literature
41 results about "Oxidative decarboxylation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oxidative decarboxylation reactions are oxidation reactions in which a carboxylate group is removed, forming carbon dioxide. They often occur in biological systems: there are many examples in the citric acid cycle.
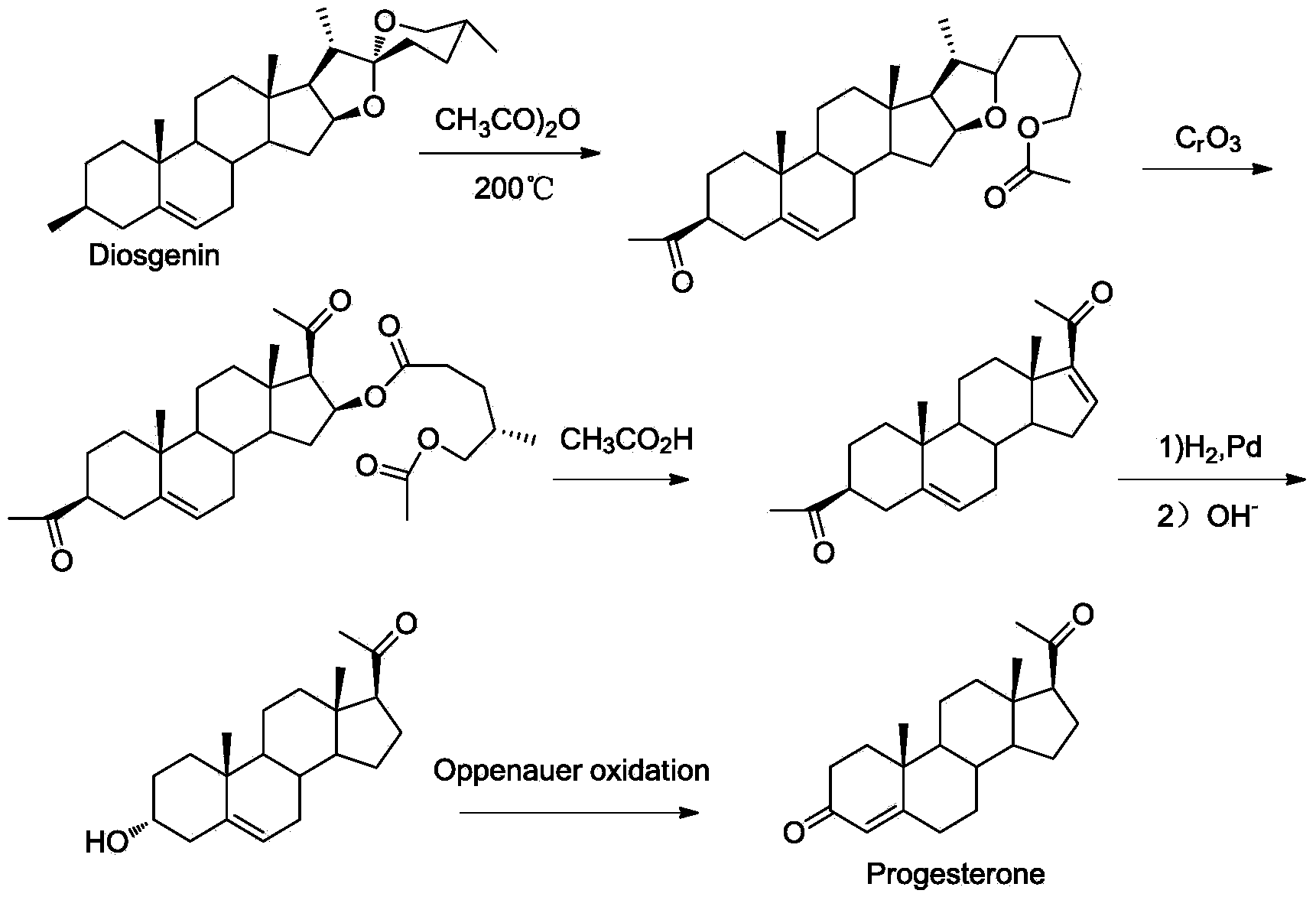
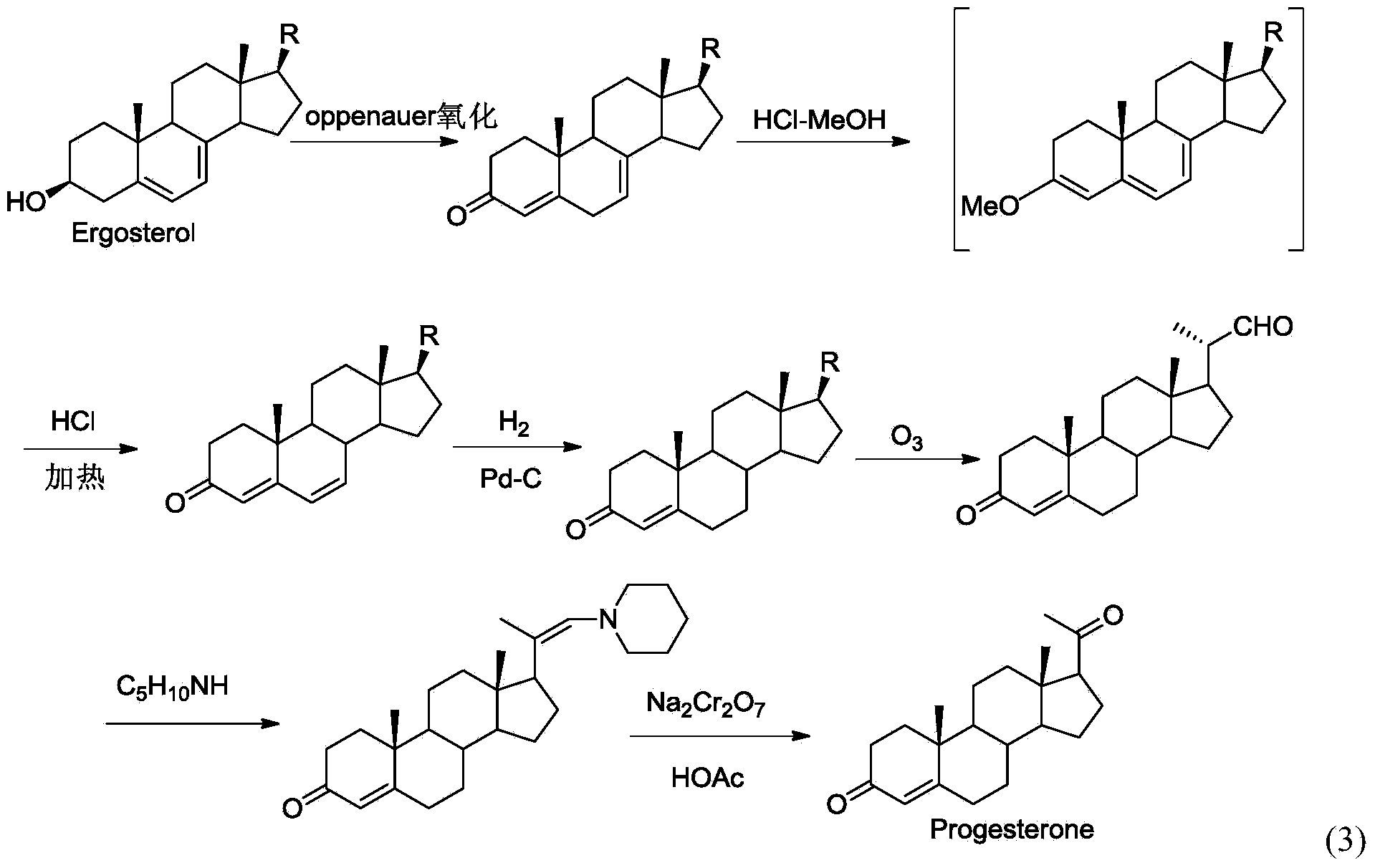
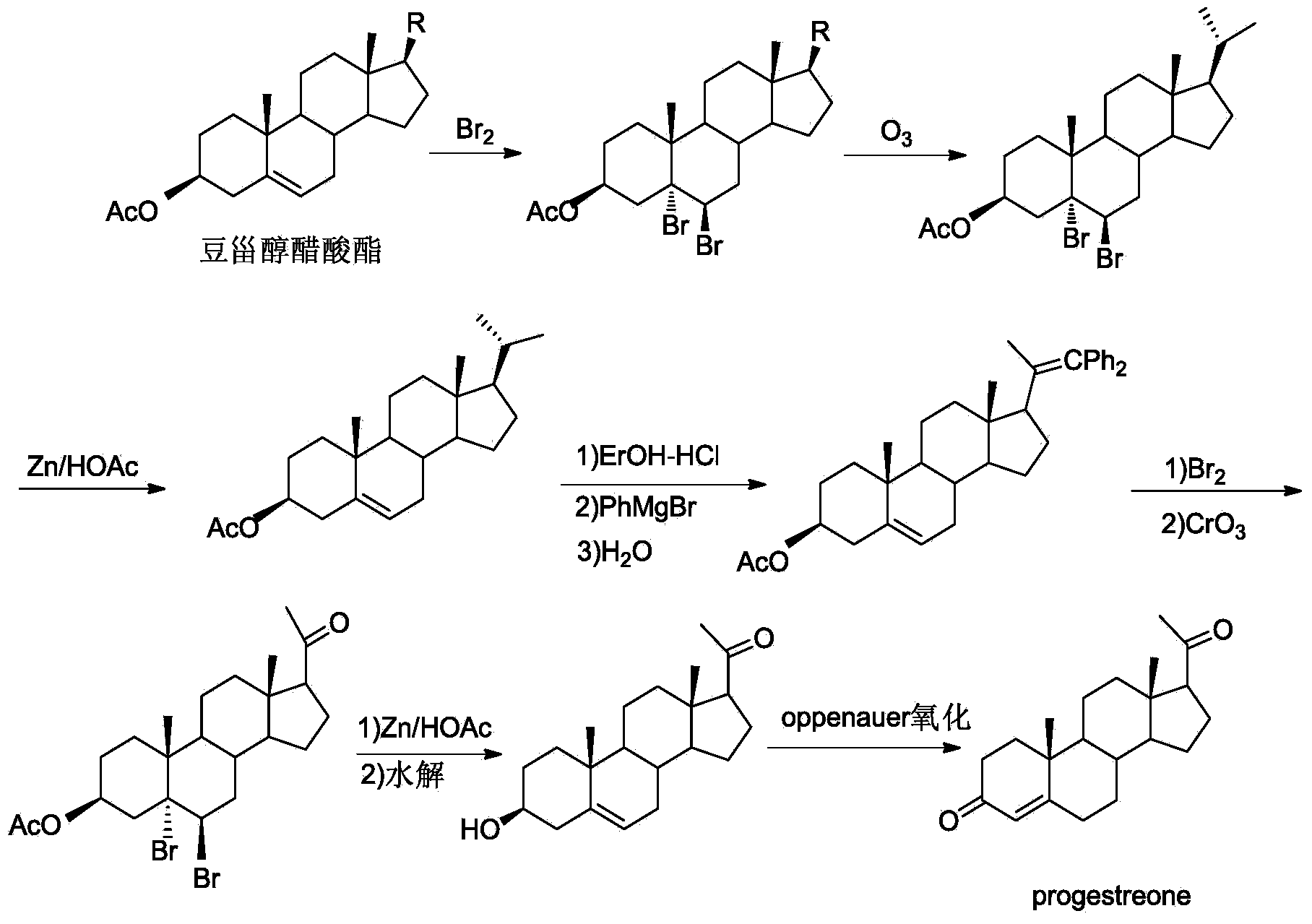
New technique for synthesizing progesterone
InactiveCN104109183ARaw materials are easy to getEasy to operateSteroidsN dimethylformamideN-Chlorosuccinimide
The invention discloses a new technique for synthesizing progesterone, which comprises the following steps: by using a phytosterin fermentation product (20S)-20-hydroxymethylpregna-4-ene-3-one as an initial raw material, oxidizing with a dimethyl sulfide / N-chlorosuccinimide mixture, and carrying out oxidative decarboxylation reaction in a DMF (N,N-dimethylformamide) solution of DBU (1,8-diazabicyclo(5.4.0)undec-7-ene), Cu(OAc)2.H2O and 2,2'-dipyridine to obtain the progesterone. The technique has the advantages of simple process, high yield (up to 80%), favorable product quality, accessible raw materials and low preparation cost.
Owner:HUBEI GEDIAN HUMANWELL PHARMACEUTICAL CO LTD
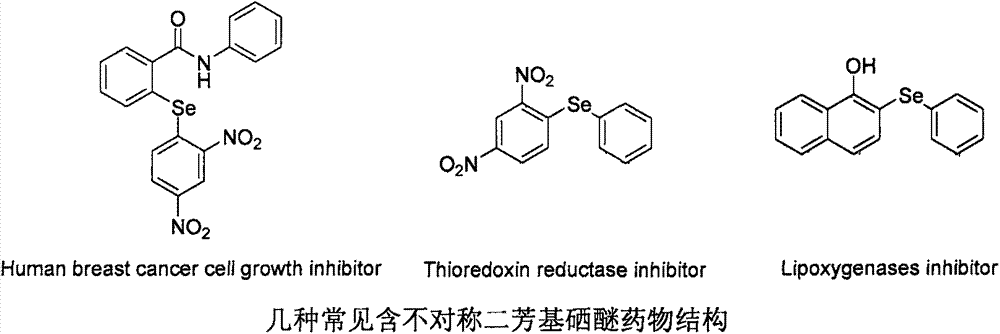
Method for synthesizing asymmetric diaryl selenide compound
The invention relates to a method for synthesizing an asymmetric diaryl selenide compound. The method comprises the following steps: taking aryl carboxylic acid and diaryl diselenides as reaction materials under oxygen conditions in an organic solvent, and carrying out an oxidative decarboxylation coupling reaction under the combined promotion action of a copper catalyst, a ligand and alkali, thereby obtaining the asymmetric diaryl selenide compound. The method is cheap and highly available in aryl carboxylic acid substrate and copper catalyst, wide in substrate range, simple in reaction conditions and high in product yield and purity, develops a novel synthetic route and method for the asymmetric diaryl selenide compound and has excellent application potential and research value.
Owner:WENZHOU MEDICAL UNIV
Method for improving fermentation yield of long-chain dicarboxylic acid
InactiveCN102808004AReduce unit consumptionImprove conversion rateMicroorganism based processesFermentationAlkaneBeta oxidation
The invention belongs to the technical field of chemical products, and relates to a method for improving the fermentation yield of long-chain dicarboxylic acid. According to the method, the long-chain dicarboxylic acid is produced by a microbiological fermentation method by taking C10 to C18 n-alkanes as raw materials; the method is characterized in that alpha oxidative decarboxylation inhibitor and beta-oxidation inhibitor are added in a fermentation production process to reduce the alpha-oxidative decarboxylation and beta-oxidation capacities of strains, wherein the alpha oxidative decarboxylation inhibitor is one or mixture of more of chlorpromazine hydrochloride, phenobarbital sodium, crylic acid and polyacrylic acid, and the concentration is 0.01 to 1mmol / L; and the beta-oxidation inhibitor is one or mixture of more of mercaptoacetic acid, sodium thioglycollate, ranolazine, ranolazine dihydrochloride and mildronate, and the concentration is 0.01 to 1 mmol / L. Compared with the prior art, the method has the advantages of high acid producing speed, weak alpha oxidative decarboxylation and beta-oxidation capacity, low unit consumption of alkane, and high fermentation alkane conversion rate, and is particularly suitable for preparing the long-chain dicarboxylic acid.
Owner:ZIBO GUANGTONG CHEM
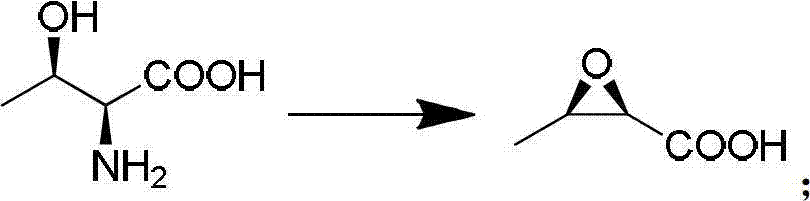
Synthetic method for penem and carbapenem antibiotic type key intermediate 4AA
InactiveCN102827199AEasy to operateAdvanced technologyGroup 4/14 element organic compoundsSNiAntibiotic Y
The invention relates to a synthetic method for a penem and carbapenem antibiotic type key intermediate 4AA, belonging to the technical field of medicine. The method comprises the following steps of: reacting L-threonine with sodium nitrite-hydrochloric acid to generate a diazo compound; performing an internal nucleophilic substitution reaction on the diazo compound under the action of sodium hydroxide; acidifying to obtain epoxy sodium butanoate; acidifying to obtain epoxy butyrate; reacting the epoxy butyrate with p-methoxyanilinoethyl acetate to obtain a condensation product; generating a quaternary ring compound from the condensation product under the actions of hexamethyldisilazane and lithium amide; and performing hydroxy protection, hydrolysis, oxidative decarboxylation and ozonization deprotection on the ring compound to obtain 4AA. The process has the advantages of readily-available raw material, mild reaction conditions, short reaction time, low pollution, high yield and the like, and is suitable for industrial production.
Owner:CHINA THREE GORGES UNIV
Novel preparation method of insensitive explosive TATB
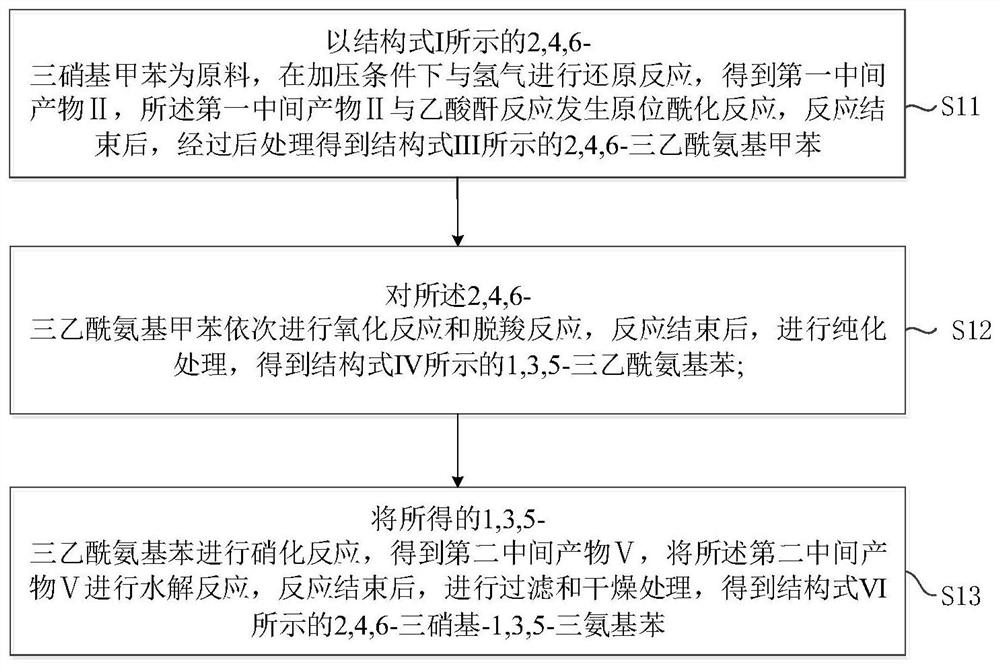
ActiveCN111995527AImprove solubilityThe synthesis process is matureOrganic compound preparationCarboxylic acid amides preparationChemical industryAcetic anhydride
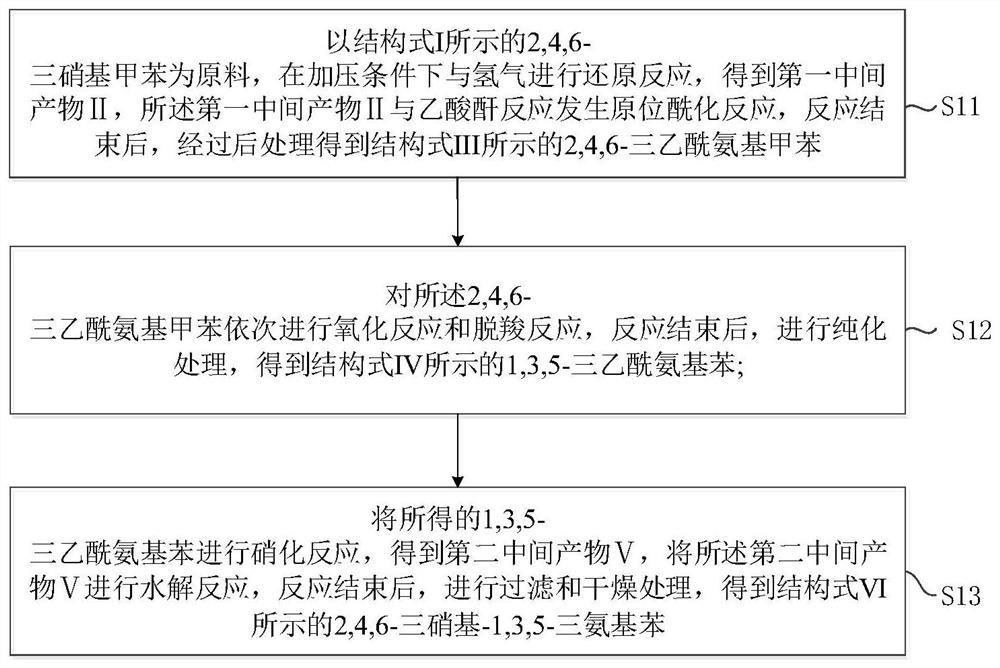
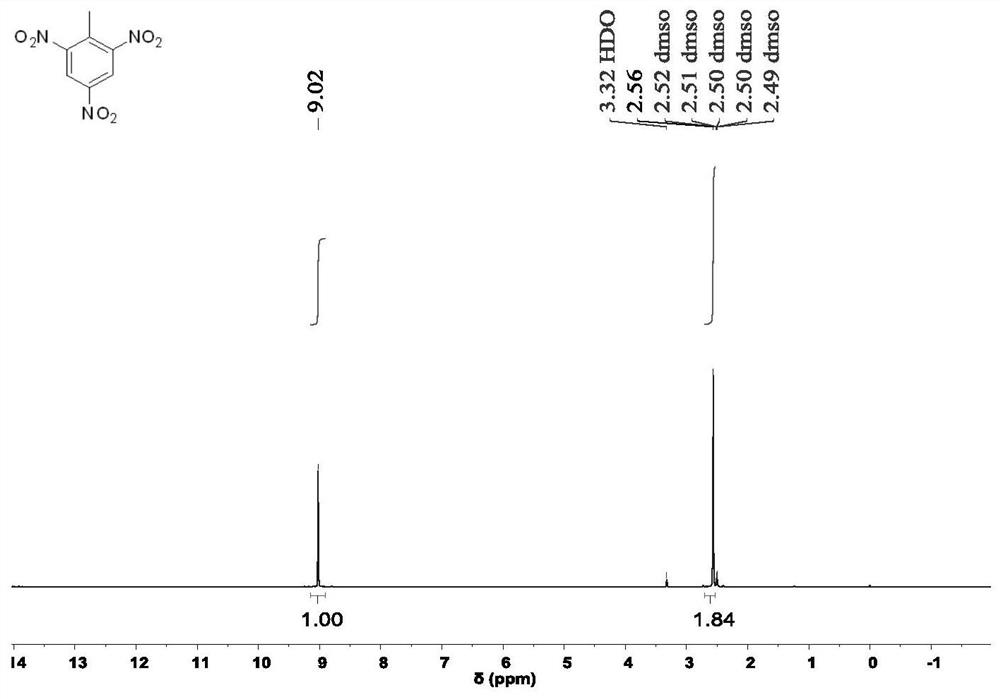
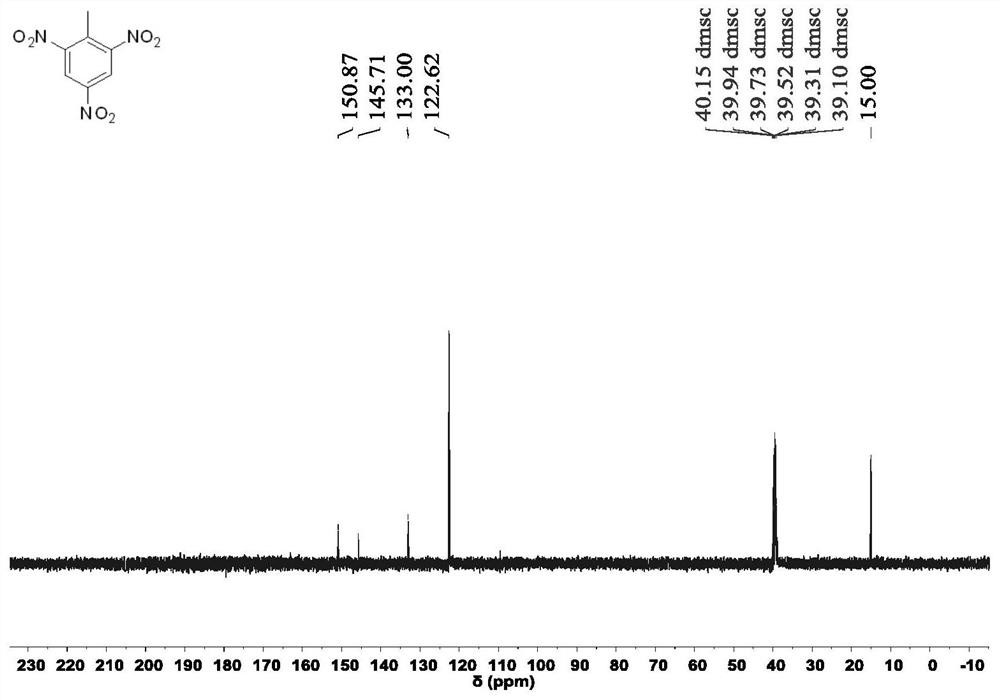
The invention provides a novel preparation method of an insensitive explosive TATB. The method comprises the following steps: 1, with 2,4,6-trinitrotoluene as a raw material, subjecting 2,4,6-trinitrotoluene to reacting with hydrogen and acetic anhydride in sequence to obtain 2,4,6-triacetylaminotoluene; 2, subjecting the obtained 2,4,6-triacetylaminotoluene o oxidation and a decarboxylation reaction to obtain 1,3,5-triacetylaminobenzene; and 3, carrying out nitrification and hydrolysis reactions on the obtained 1,3,5-triacetylaminobenzene to obtain 2,4,6-trinitro-1,3,5-triaminobenzene. According to the invention, the raw material used in the preparation method is the cheap 2,4,6-trinitrotoluene, and used reactants or catalysts are commonly used products in the chemical industry, so the preparation method has characteristics of low cost and usage of simple and easily available raw materials. In addition, the preparation method also has the characteristics of short synthesis steps, simple operation of each step, high yield, high reaction rate, easy separation and collection of intermediate and final products and the like, and is beneficial for realization of mass production of TATB.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Green synthetic method of beta-carboline compound
InactiveCN103864787AHigh yieldEasy to prepare and safe and environmentally friendlyOrganic chemistryPictet–Spengler reactionCarboxylic acid
The invention discloses a green synthetic method of a beta-carboline compound. The green synthetic method of the beta-carboline compound is technically characterized by comprising the following steps: carrying out Pictet-Spengler reaction on L-tryptophan to prepare tetrahydro-beta-carboline-3-carboxylic acid; and decarboxylating and oxidizing the tetrahydro-beta-carboline-3-carboxylic acid under the effect of an oxidizing decarboxylating agent hydrogen peroxide to prepare the beta-carboline compound. The invention discloses specific synthetic steps and main reaction equations in the preparation process. The method disclosed by the invention has the advantages of simple preparation and safety and environmental-friendliness, and the yield of the beta-carboline compound is high, so that the demand on green chemistry is satisfied.
Owner:HENAN NORMAL UNIV
Method for production of 1,5-pentanediamine by chemical decarboxylation of L-lysine and separation and extraction method
ActiveCN110143882ALow costMild reaction conditionsAmino compound purification/separationOrganic compound preparationReducing agentRaw material
The invention provides a method for production of 1,5-pentanediamine by chemical decarboxylation of L-lysine and a separation and extraction method. The method of the invention comprises the followingsteps: (1) dissolving L-lysine or L-lysine hydrochloride in a citric acid-disodium hydrogen phosphate buffer solution; (2) dropwise adding an oxidizer solution into the buffer solution obtained in the Step (1), and reacting at 10-75 DEG C for 20-30 min; and (3) adding transition metal salt into a reaction solution obtained in the Step (2), and simultaneously adding a reducing agent and mixing intensely and reacting for 20-120 min so as to obtain a 1,5-pentanediamine solution. According to the invention, L-lysine or its hydrochloride is used as a raw material to undergo an oxidative decarboxylation reaction and a cyan reduction reaction to obtain 1,5-pentanediamine. The reaction has mild conditions, is easy to operate and has good repeatability. By using cation exchange resin to contact with the 1,5-pentanediamine solution, the method of the invention is low-cost and environmentally-friendly in comparison with existing 1,5-pentanediamine separation methods.
Owner:郑州中科新兴产业技术研究院
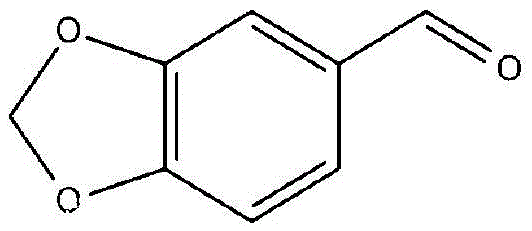
Synthetic method of 3, 4-methylene dioxybenzaldehyde
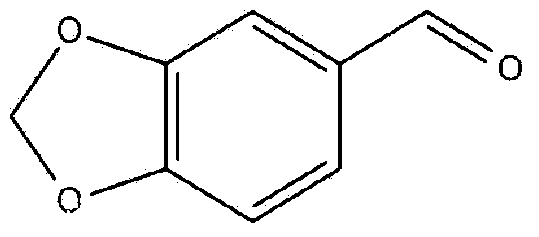
The invention discloses a synthetic method of 3, 4-methylene dioxybenzaldehyde and relates to the field of chemical organic synthesis, and particularly relates to a synthetic method in which an intermediate generated in a process of preparing 3, 4-methylene dioxybenzaldehyde by taking catechol methylene ether as a raw material is oxidized by suing a nitryl-free oxidizing agent to generate the final product. According to the synthetic method of 3, 4-methylene dioxybenzaldehyde, oxidative decarboxylation is carried out on the intermediate piperonyl mandelic acid (prepared from catechol methylene ether) as an initial reactant in the presence of at least one catalyst selected from metal chloride Lewis acid and at least one oxidizing agent selected from oxygen and peroxides in an acidic environment so as to synthesize 3, 4-methylene dioxybenzaldehyde. The synthetic method provided by the invention is simple in technical step, thorough in conversion of the raw materials, high in finished product yield and short in technical time, and the product does not contain color-changing impurities and is stable in technical quality.
Owner:成都建中香料香精有限公司
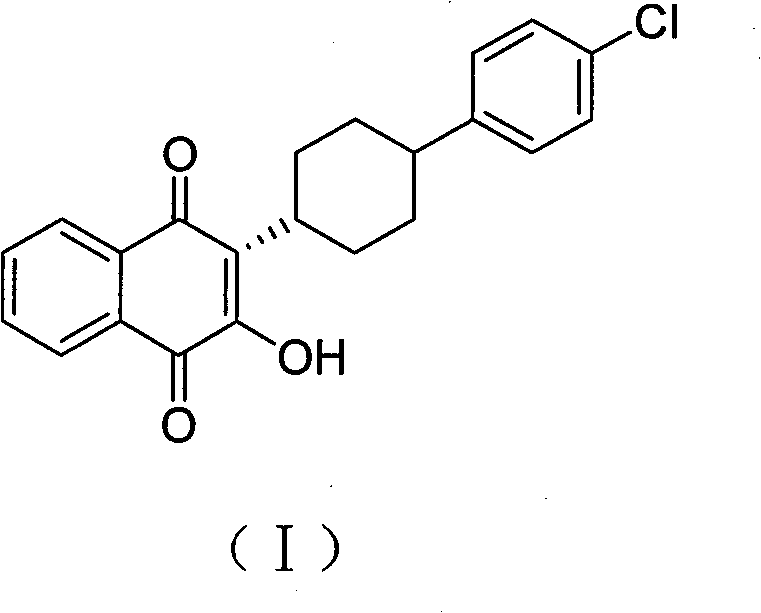
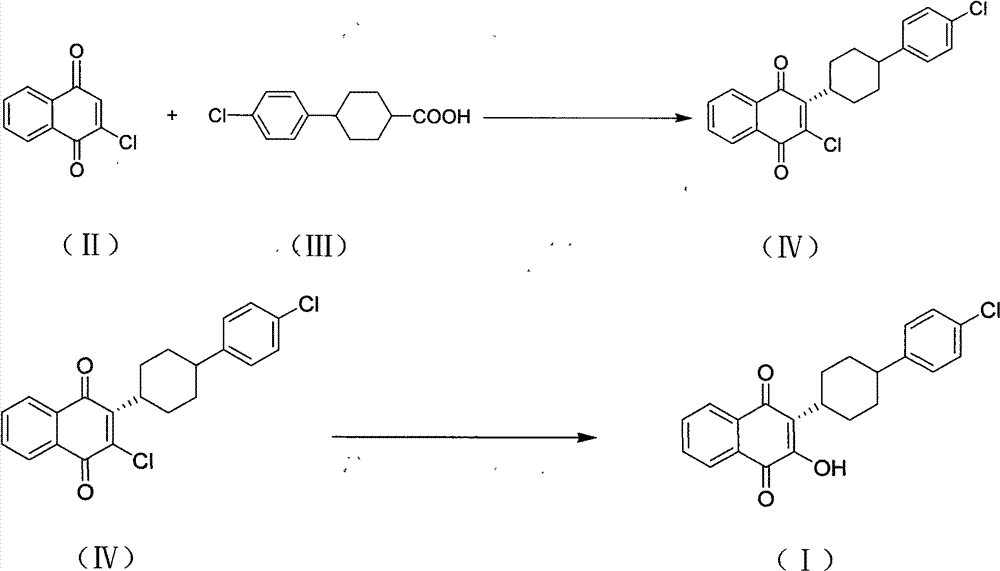
Method for preparing atovaquone
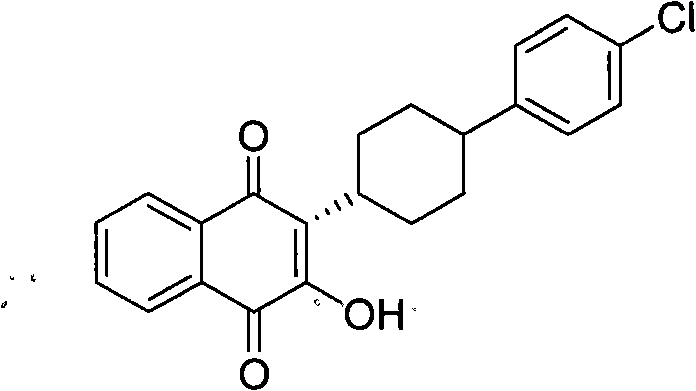
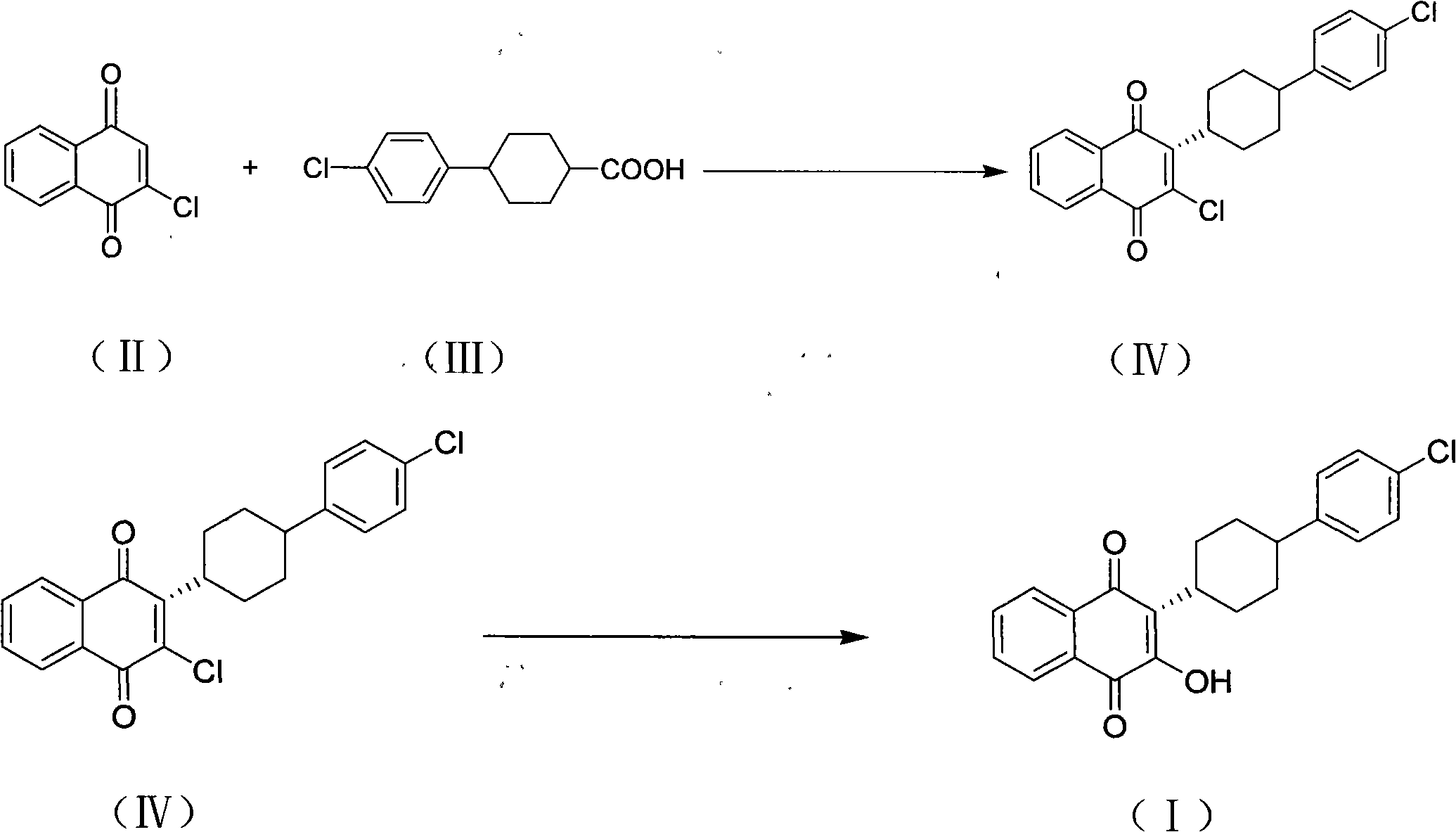
InactiveCN101774901AImprove dissolutionReduce generationOrganic compound preparationQuinone preparationPotassium persulfateAtovaquone
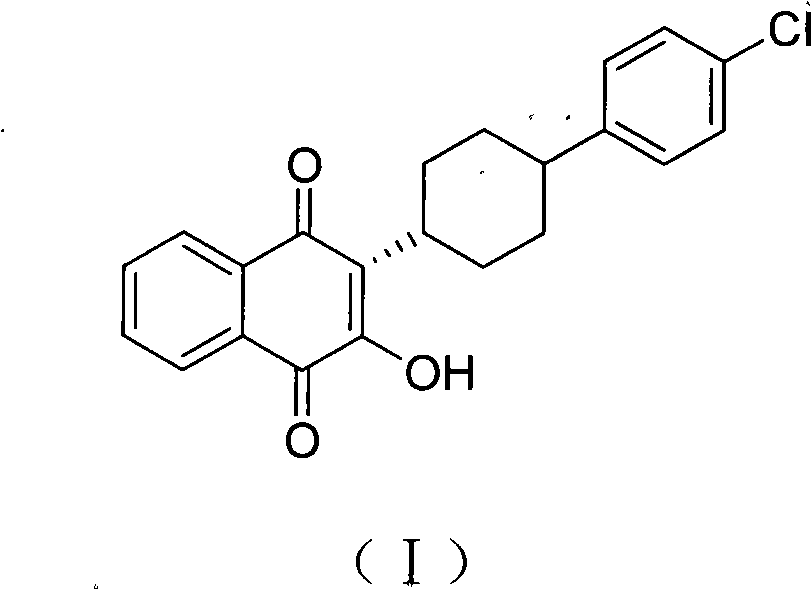
The invention discloses a method for preparing atovaquone, which comprises the following steps: taking 4-(4-chlorophenyl)-cyclohexyl-1-methanoic acid and 2-chlorine-1, 4-naphthoquinone as raw materials, generating (3S)-2-chlorine-3-(4-(4-chlorophenyl) cyclohexyl)-1, 4-naphthalenedione by oxidative decarboxylation through a peroxide in the action of a catalyst of silver nitrate, and then obtaining the atovaquone through hydrolysis with alkaline. The method is characterized in that the mixed solvent of acetonitrile and choromethane is used as the solvent for the oxidative decarboxylation and the peroxide is one of the four substances, i.e., sodium persulfate, potassium persulfate, sodium percarbonate and potassium peroxycarbonate. The improved preparation method considerably improves the dissolution of the raw materials in the solvent, raises the rate of conversion, increases the yield, reduces impurities, and significantly enhances the product quality. Therefore, the method is more suitable for industrial production.
Owner:WUHAN TITON BIOTECH
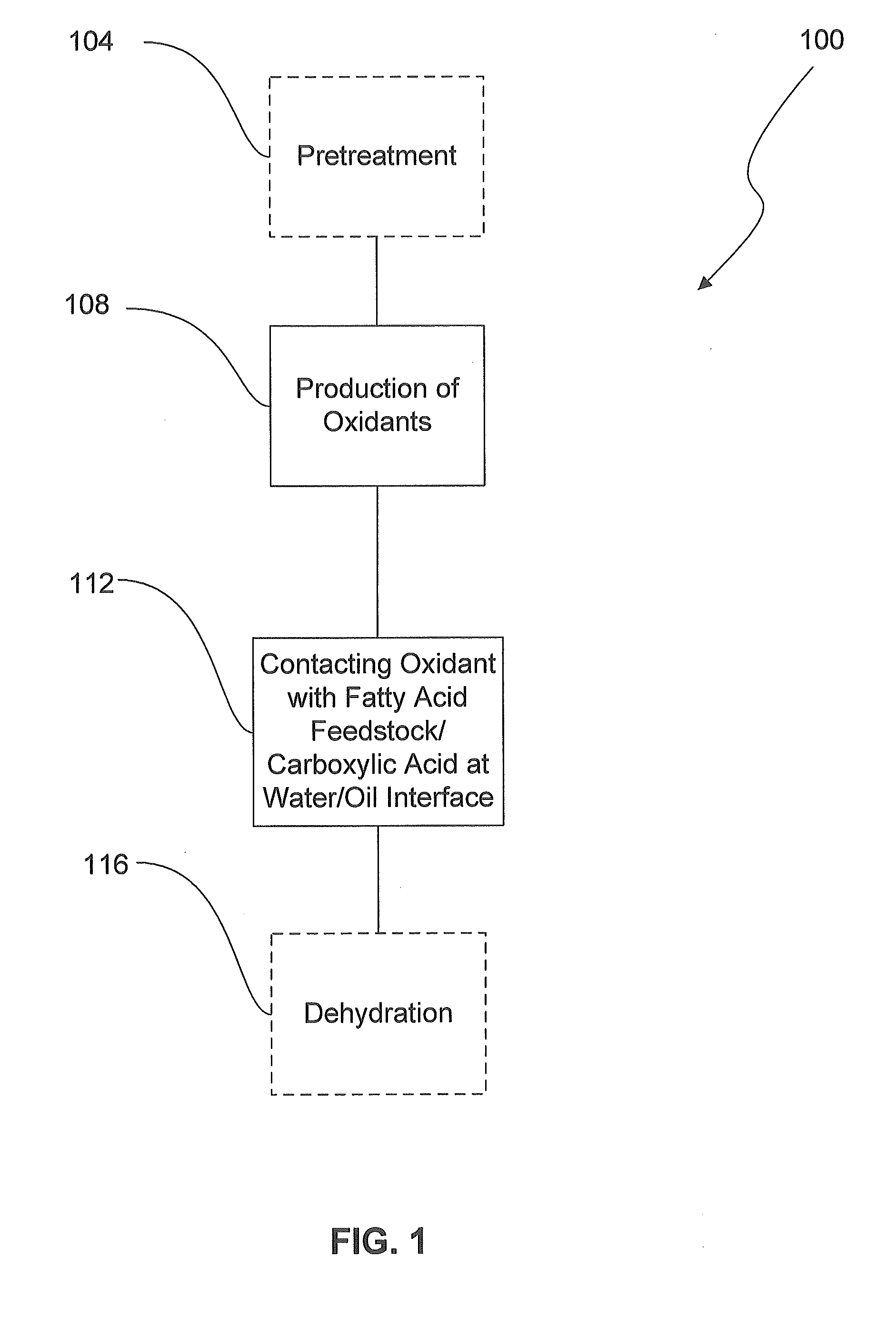
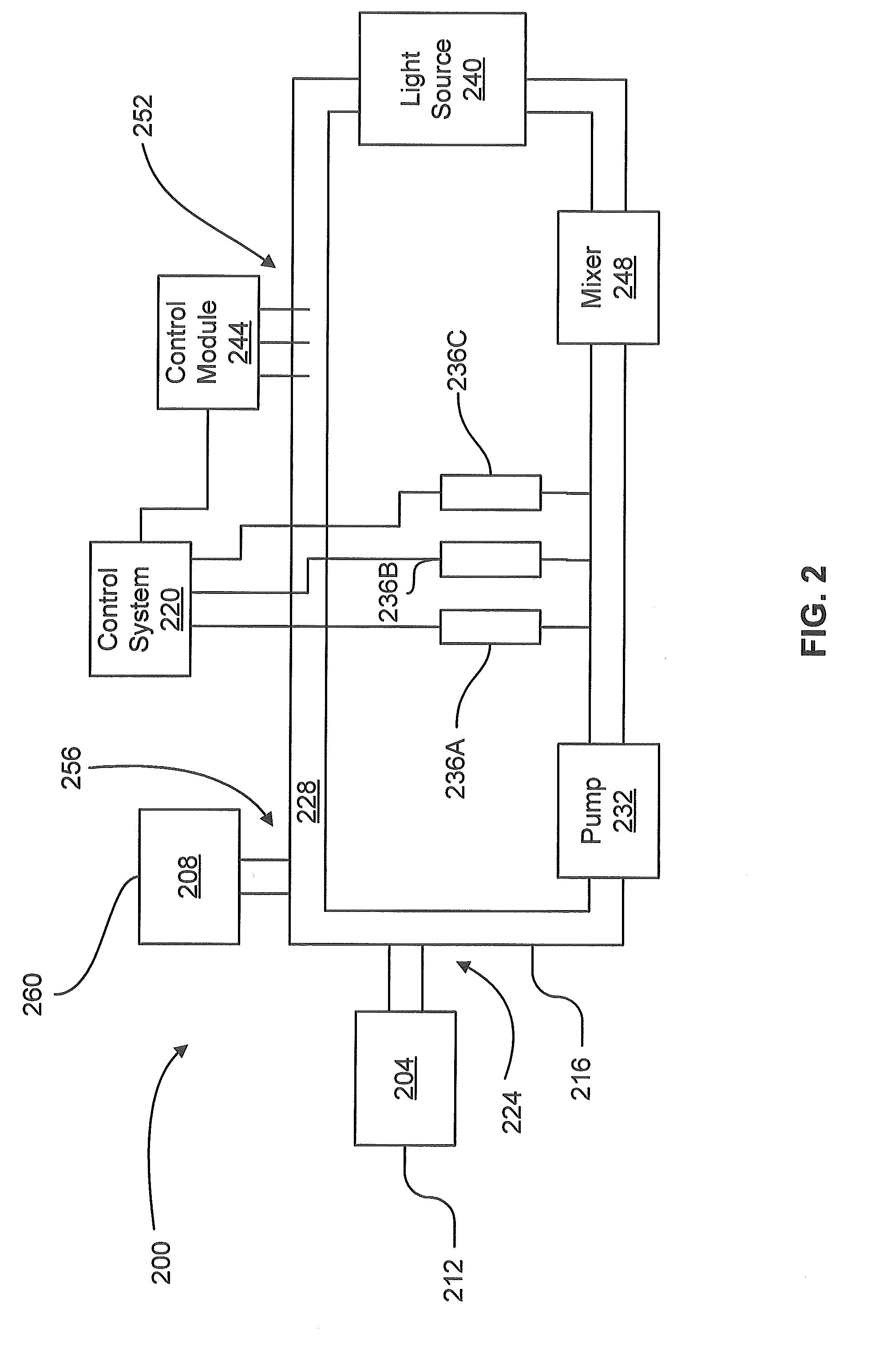
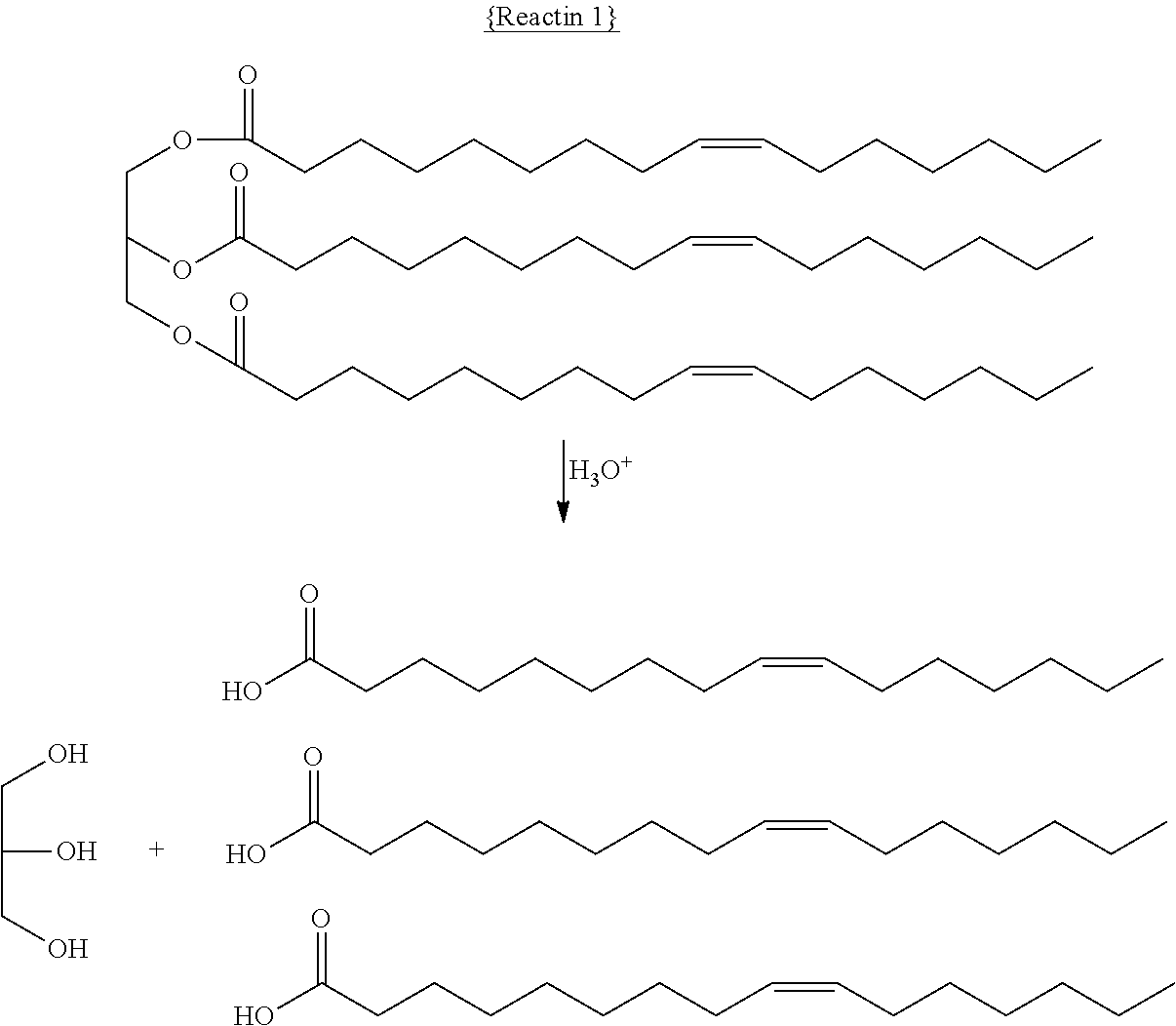
Method and System For the Selective Oxidative Decarboxylation of Fatty Acids
ActiveUS20120209049A1Avoid contactFatty acid chemical modificationLiquid hydrocarbon mixture productionFenton reagentRoom temperature
Selective, radically initiated oxidative decarboxylation may produce low viscosity renewable fuels from biologically derived fats and oils. Fatty acids and triglycerides may be decarboxylated using oxidants at a water / oil interface. The oxidants may be produced using photo-Fenton reagents. The reaction advantageously can be carried out at room temperature and pressure and has fewer unwanted byproducts than traditional decarboxylation techniques.
Owner:UNIVERSITY OF VERMONT
Curing agent, preparation method thereof and application of curing agent in preparation of polyacrylate pressure-sensitive adhesive
PendingCN114478975AHigh molecular weightReduce dosageMacromolecular adhesive additivesEster polymer adhesivesAdhesive cementPolymer science
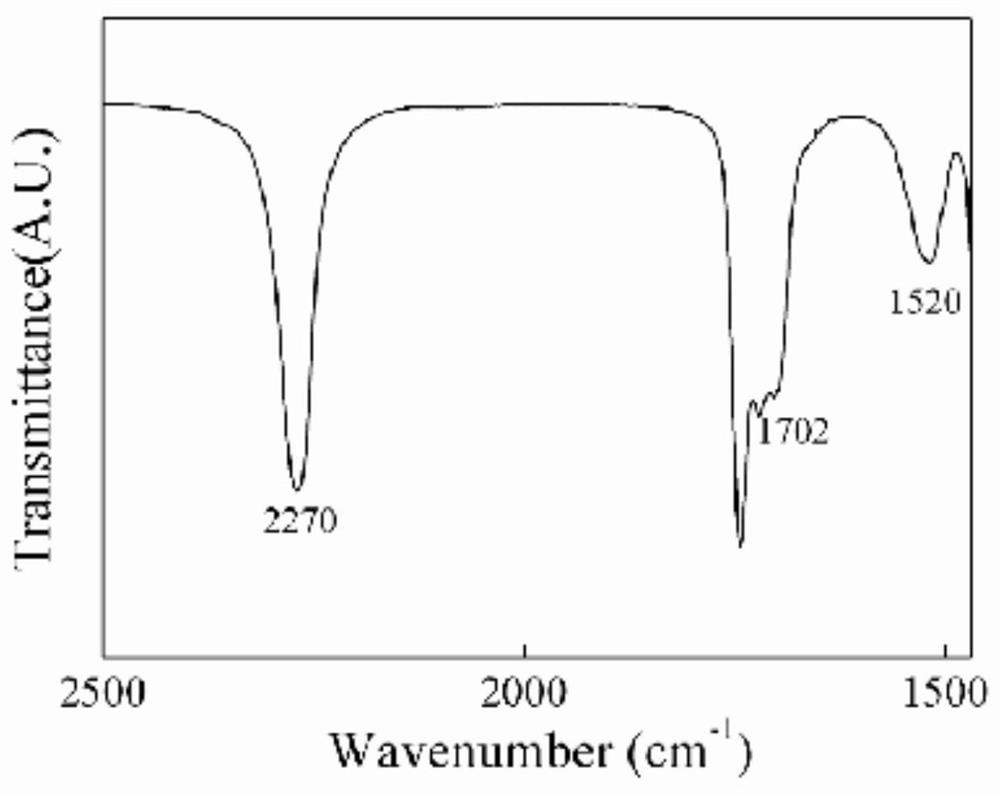
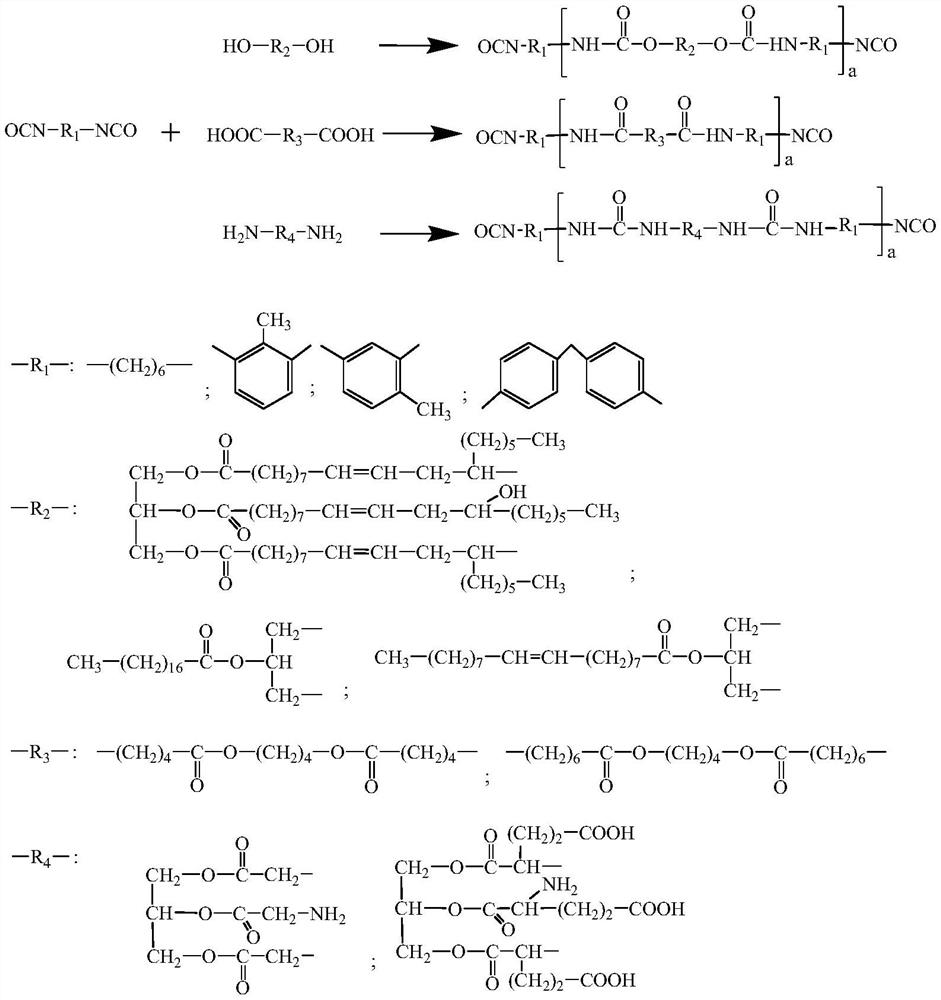
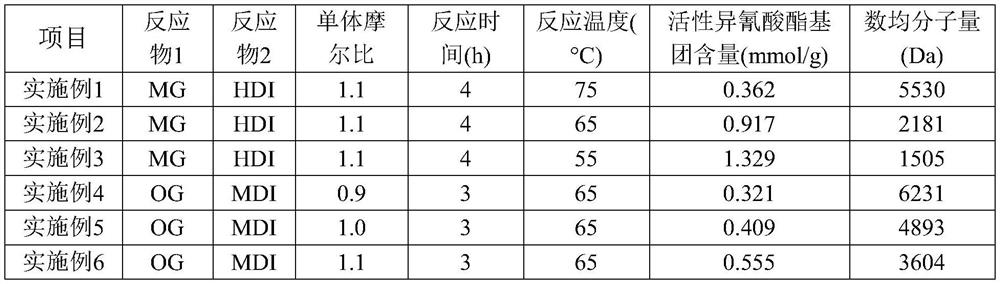
The invention relates to a curing agent, a preparation method of the curing agent and application of the curing agent in preparation of polyacrylate pressure-sensitive adhesives, the curing agent is a diisocyanate-terminated oligomer obtained by reaction of a compound containing reactive hydrogen atoms and diisocyanate, the curing agent provided by the invention contains a plurality of hydrolyzable groups, according to the present invention, the curing agent is hydrolyzed, the hydrolyzed substance is easily degraded through oxidation decarboxylation and other modes, and then the hydrolyzed substance is introduced into the adhesive, such that the prepared pressure-sensitive adhesive has characteristics of high curing agent corresponding component content and many hydrolysis groups so as to achieve good final biodegradability;
Owner:NINGBO SOKEN CHEM
The synthetic method of 3,4-methylenedioxybenzaldehyde
The invention discloses a synthetic method of 3, 4-methylene dioxybenzaldehyde and relates to the field of chemical organic synthesis, and particularly relates to a synthetic method in which an intermediate generated in a process of preparing 3, 4-methylene dioxybenzaldehyde by taking catechol methylene ether as a raw material is oxidized by suing a nitryl-free oxidizing agent to generate the final product. According to the synthetic method of 3, 4-methylene dioxybenzaldehyde, oxidative decarboxylation is carried out on the intermediate piperonyl mandelic acid (prepared from catechol methylene ether) as an initial reactant in the presence of at least one catalyst selected from metal chloride Lewis acid and at least one oxidizing agent selected from oxygen and peroxides in an acidic environment so as to synthesize 3, 4-methylene dioxybenzaldehyde. The synthetic method provided by the invention is simple in technical step, thorough in conversion of the raw materials, high in finished product yield and short in technical time, and the product does not contain color-changing impurities and is stable in technical quality.
Owner:成都建中香料香精有限公司
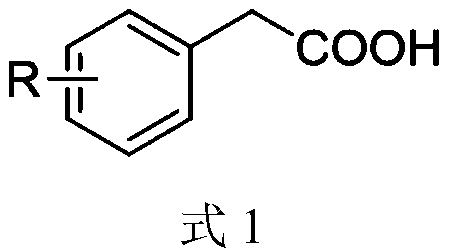
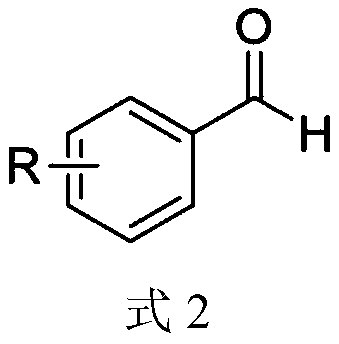
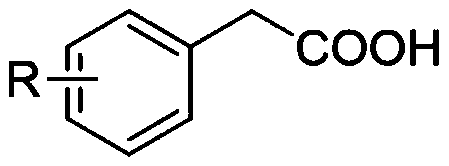
Synthesis method of aryl aldehyde compound
ActiveCN110963900ALow costMild reaction conditionsOrganic compound preparationCarbonyl compound preparationArylPtru catalyst
The invention discloses a synthesis method of an aryl aldehyde compound. The method comprises the following step: carrying out an oxidative decarboxylation reaction on an aryl acetic acid compound andan organic alkali under the catalytic action of a visible light catalyst to obtain the aryl aldehyde compound. According to the method, the aryl aldehyde compound is generated through one-step oxidative decarboxylation of aryl acetic acid at room temperature under visible light in an open system, so that the method is simple to operate, mild in reaction condition, cheap and easily available in raw materials and catalysts and high in reaction yield, and is an environment-friendly synthesis method.
Owner:HUAIHUA UNIV
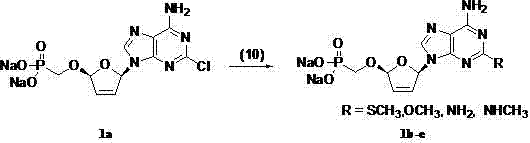
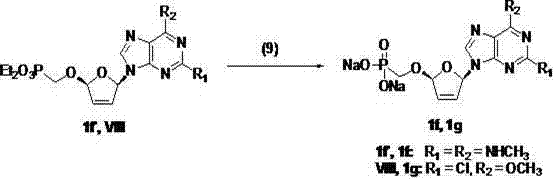
(2R,5R)-5-phosphoryl methoxy-2-(2-substituted adenine-9-yl)-2,5-dihydrofuran nucleoside analog as well as preparation method and application thereof
InactiveCN103788160AImprove stabilityExcellent resistance to nucleolytic enzyme decompositionOrganic active ingredientsSugar derivativesFuranPurine
The invention discloses a novel beta-furan phosphonate purine nucleoside analog, and in particular relates to a (2R,5R)-5-phosphoryl methoxy-2-(2-substituted adenine-9-yl)-2,5-dihydrofuran nucleoside analog as well as a preparation method and an application thereof, which belongs to the field of nucleoside chemicals and pharmaceutical chemistry. The analog has a structure as shown in the formula (1) in the specification, wherein R1 represents C1, NH2, OCH3, SCH3, NHCH3 or NHNH2; R2 represents NH2, OCH3 and NHCH3; R' represents H, Na or K. Chlorinated sugar with high activity is formed through chlorination of 3.5-di-O-p-methyl benzoyl-2-deoxidation-D-ribose glucoside, the chlorinated sugar is subsequently reacted with alkali, and a novel beta-D-dihydrofuran phosphonate adenine nucleoside analog is formed through selective oxidation, decarboxylation, addition, elimination, functional group conversion and ethyl removal. The analog has anti-virus activity and good development prospect.
Owner:ZHENGZHOU UNIV
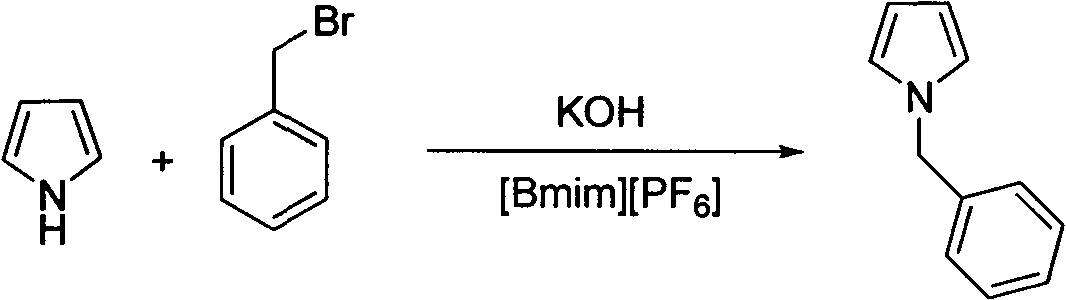
Preparation method of N-substituted-1H-pyrrole
The invention relates to a method for preparing N-substituted-1H-pyrrole. N-substituted- 4-benzoyloxyproline used as a raw material is subjected to oxidative decarboxylation aromatization under the action of an oxidizer in a system using metal as a catalyst and amine as a ligand, thereby generating the target compound N-substituted-1H-pyrrole. The method provided by the invention has the advantages of cheap and accessible raw material, novel method, high efficiency and the like.
Owner:EAST CHINA UNIV OF SCI & TECH
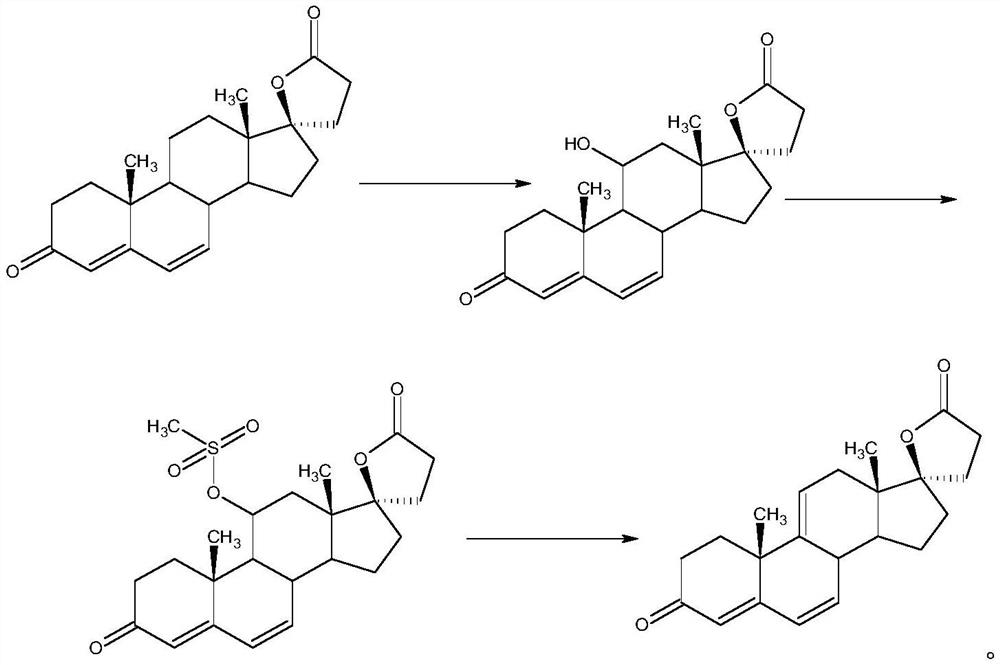
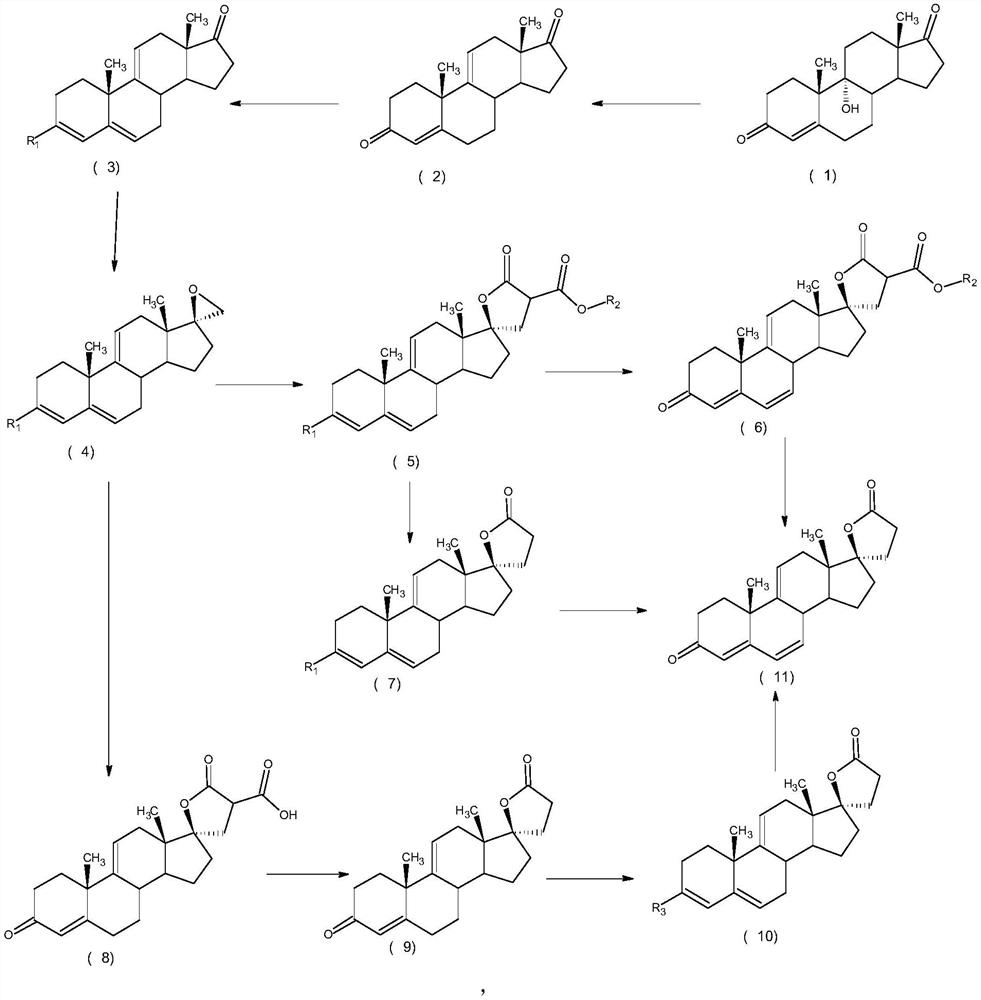
Efficient preparation method of delta<9, 11>-canrenone
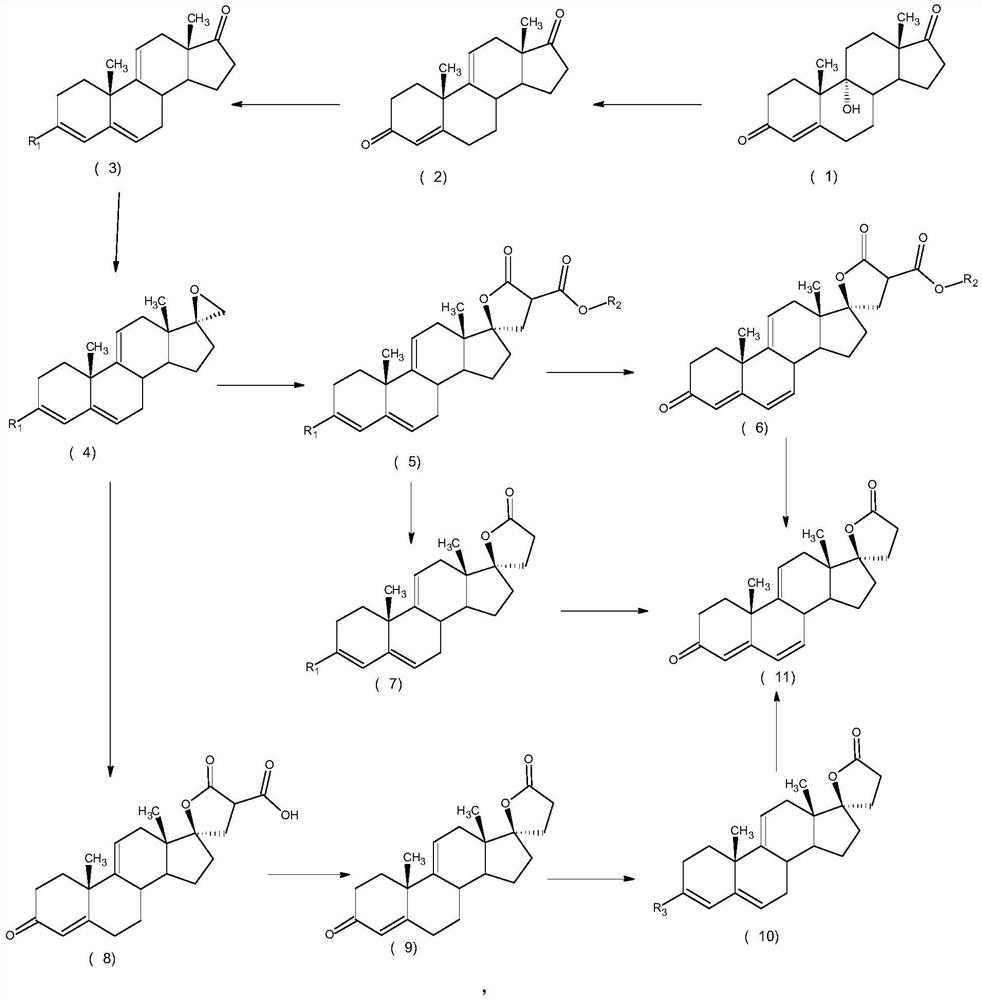
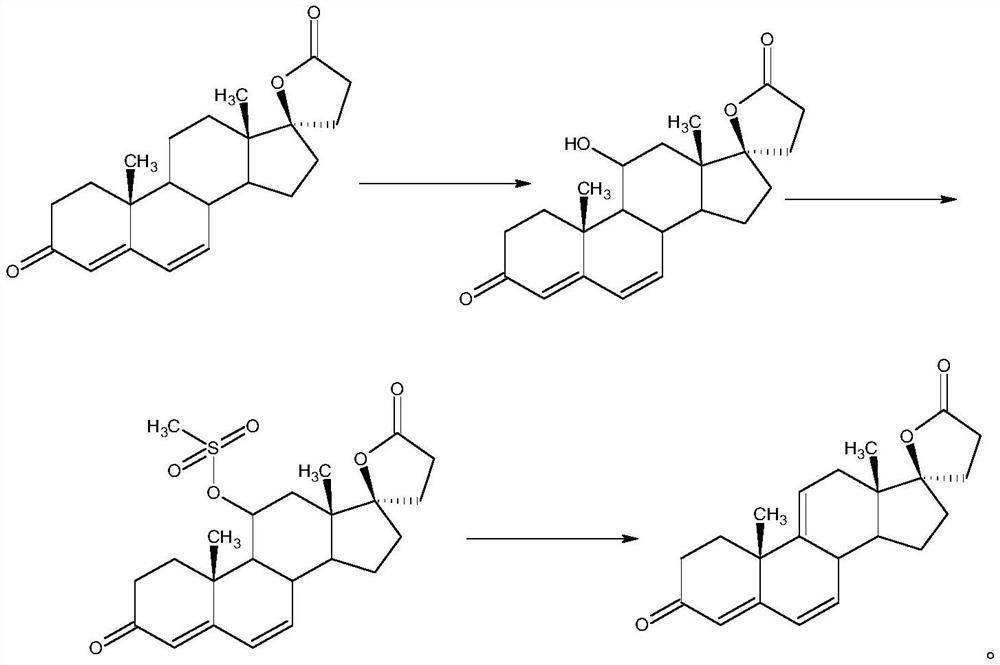
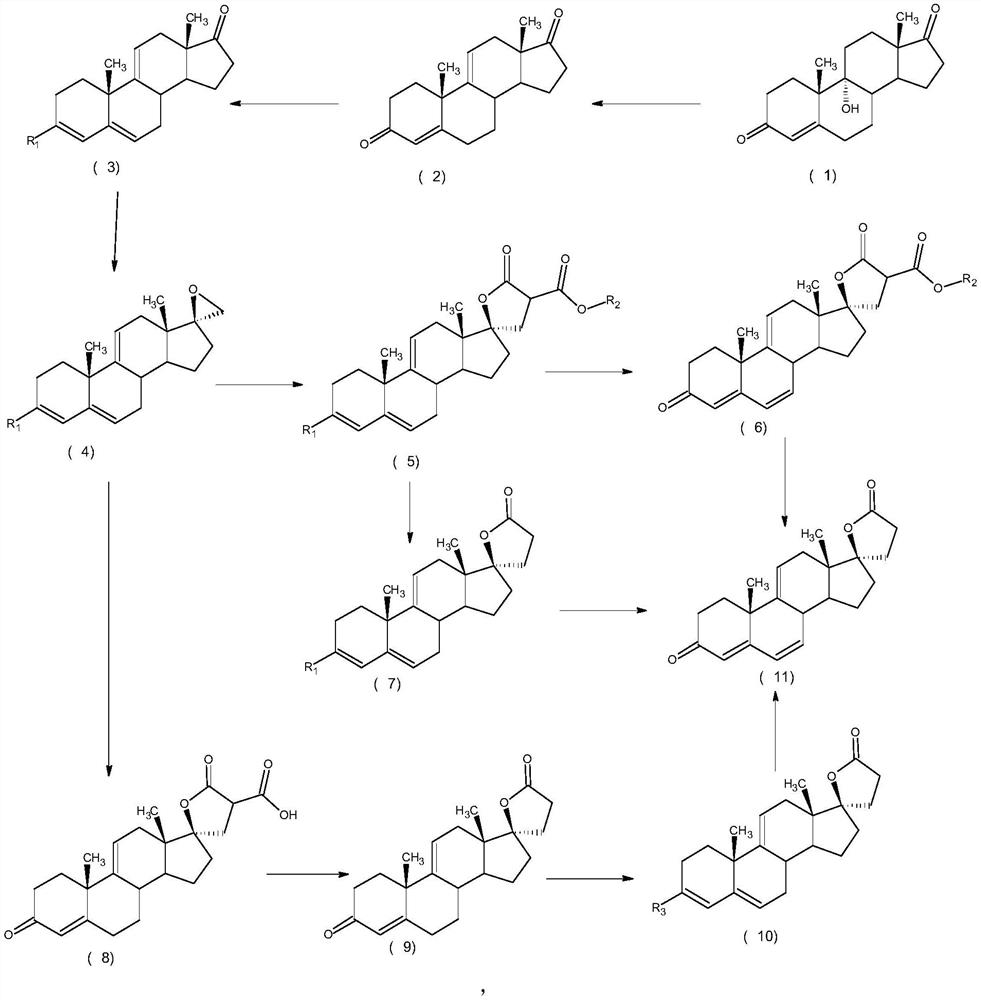
ActiveCN112062805AReduce process stepsEasy to purifyLactone steroidsDouble bondPharmaceutical Substances
The invention discloses an efficient preparation method of delta<9, 11>-canrenone, and belongs to the technical field of preparation of intermediates of medicines. The method comprises the following steps of: by taking 9 alpha-hydroxyl-4-androstenedione as a raw material, firstly removing 9-site hydroxyl through dehydration reaction to generate delta<9, 11> double bonds, then protecting 3-site carbonyl, then performing epoxidation on 17-site carbonyl, condensing with malonic acid diester to form a lactone ring, and performing oxidative decarboxylation or decarboxylation oxidation reaction to obtain delta<9, 11>-canrenone. According to the method, the raw materials are cheap and easy to obtain, the cost is low, reaction products in all steps are easy to purify, the total mass yield of the final product is higher than 80%, and the method is high in operability, extremely high in commercial competitiveness, suitable for industrial large-scale production and good in economic benefit.
Owner:ZHEJIANG SHENZHOU PHARMA
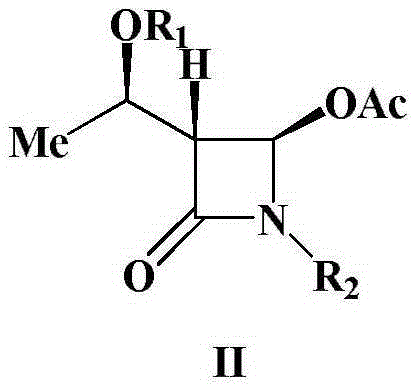
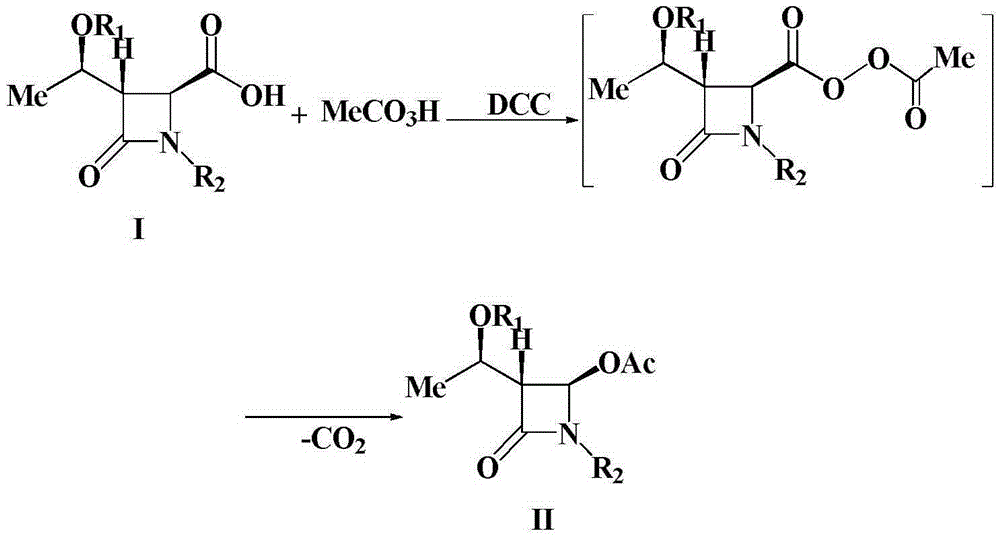
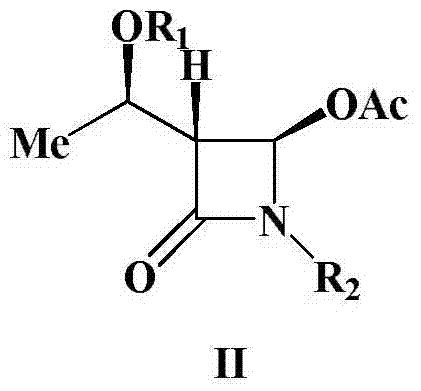
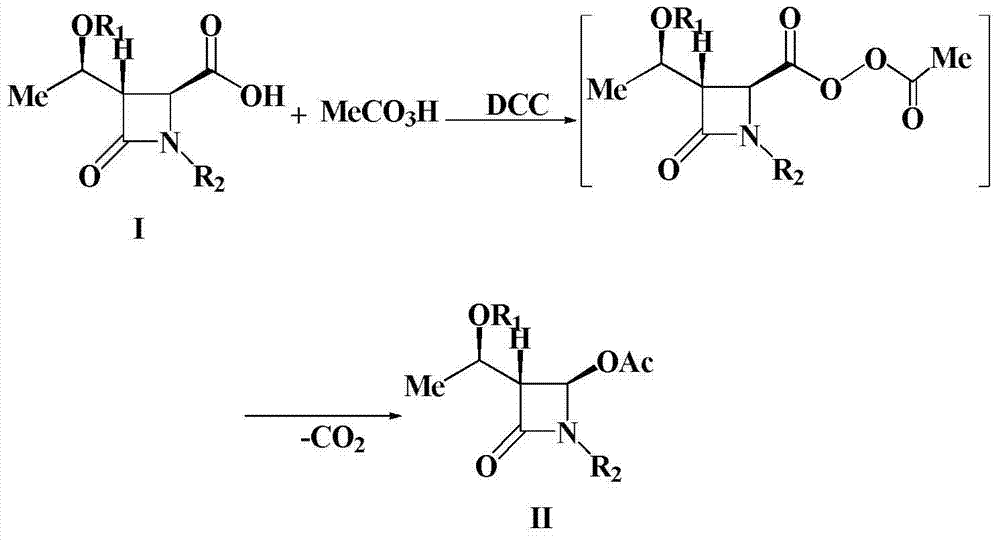
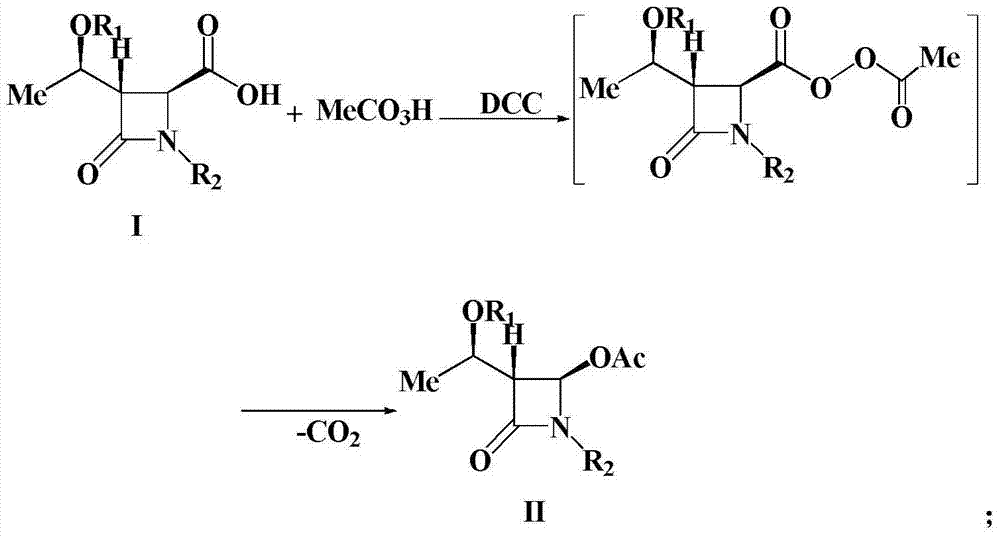
A kind of preparation method of 4-acetoxy-2-azetidinone compound
InactiveCN103539813BResidue reductionEmission reductionOrganic chemistry4-carboxy-2-azetidinone2-butanone
The invention relates to a preparation method of 4-acetyloxy-2-azetidinone compounds, and is used for solving the problem that the large-scale industrial production cannot be favorably realized because of defects such as severe reaction condition, complex treatment process, high cost, serious environment pollution and the like in the prior art. The method provided by the invention comprises the step of carrying out oxidization deacidification in the existence of a dehydrating agent N, N'-dicyclohexylcarbodiimide and an oxidizing agent peroxyacetic acid by taking 4-carboxyl-2-azetidinone compounds I as raw materials to obtain the 4-acetyloxy-2-azetidinone compounds II. A heavy metal oxidizing agent or catalyst is prevented from being used in the oxidization deacidification process, so that the residue and discharge of heavy metals and environmental pollution caused by the heavy metals are greatly reduced. Byproducts generated in a reaction process can be conveniently recycled, so that the production cost is reduced to the great extent, and the preparation method is more excellent on the aspect of economical efficiency. The method is mild in reaction condition, simple and convenient to operate and suitable for large-scale production; the 4-acetyloxy-2-azetidinone compounds are easy to separate and purify and high in yield.
Owner:TAIZHOU VOCATIONAL & TECHN COLLEGE +1
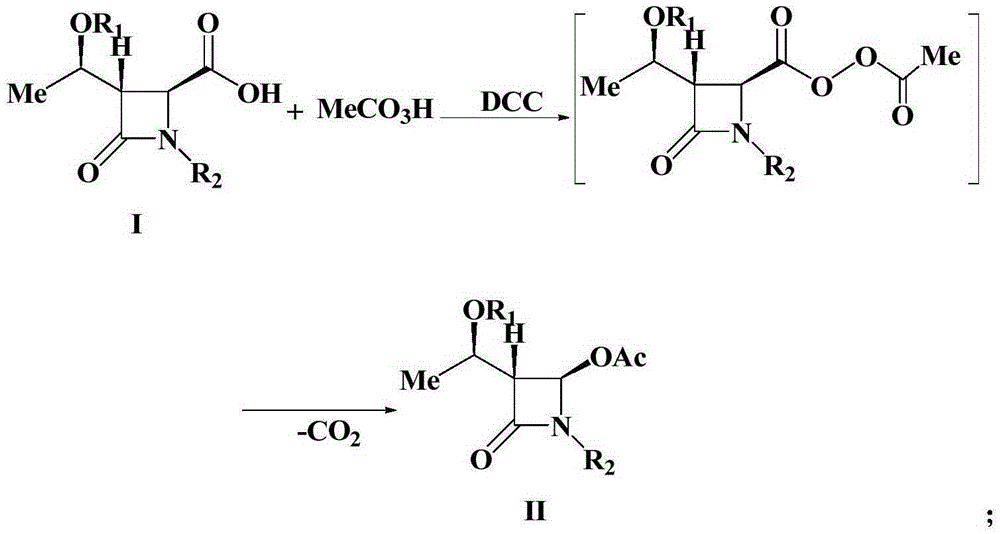
Preparation method of 4-acetyloxy-2-azetidinone compounds
InactiveCN103539813AResidue reductionEmission reductionGroup 4/14 element organic compounds4-carboxy-2-azetidinoneOxidizing agent
The invention relates to a preparation method of 4-acetyloxy-2-azetidinone compounds, and is used for solving the problem that the large-scale industrial production cannot be favorably realized because of defects such as severe reaction condition, complex treatment process, high cost, serious environment pollution and the like in the prior art. The method provided by the invention comprises the step of carrying out oxidization deacidification in the existence of a dehydrating agent N, N'-dicyclohexylcarbodiimide and an oxidizing agent peroxyacetic acid by taking 4-carboxyl-2-azetidinone compounds I as raw materials to obtain the 4-acetyloxy-2-azetidinone compounds II. A heavy metal oxidizing agent or catalyst is prevented from being used in the oxidization deacidification process, so that the residue and discharge of heavy metals and environmental pollution caused by the heavy metals are greatly reduced. Byproducts generated in a reaction process can be conveniently recycled, so that the production cost is reduced to the great extent, and the preparation method is more excellent on the aspect of economical efficiency. The method is mild in reaction condition, simple and convenient to operate and suitable for large-scale production; the 4-acetyloxy-2-azetidinone compounds are easy to separate and purify and high in yield.
Owner:TAIZHOU VOCATIONAL & TECHN COLLEGE +1
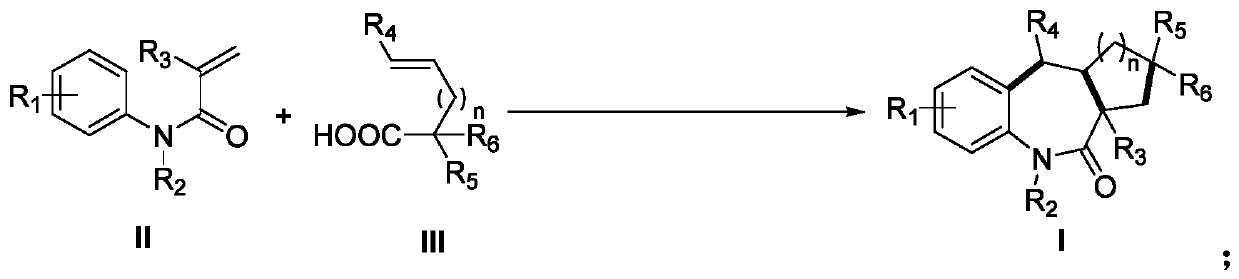
Preparation method of benzoazepine compounds
ActiveCN111018784AMild reaction conditionsEasy to operateOrganic chemistryArylCombinatorial chemistry
The invention discloses a preparation method of benzoazepine compounds. The method comprises the following steps: reacting under a metal-free catalytic reaction condition, a N-aryl acrylamide compoundand a 4-alkenyl alkyl carboxylic acid compound are subjected to oxidation decarboxylation / cyclization to prepare a series of benzoazepine compounds. The method has advantages of mild reaction condition, simple operation, economy, green property, low cost and the like, and a convenient and effective approach is provided for the synthesis of benzoazepine compounds.
Owner:NANCHANG HANGKONG UNIVERSITY
Preparation method of glyphosate polymer
ActiveCN101974159AGood effectThe amount of release is controllableHydrogen SulfateProguanil Hydrochloride
The invention provides a preparation method of glyphosate polymer, in particular to a N-(Phosphonomethyl)iminodiaceticacid catalytic polycondensation method. The preparation method comprises the following steps of: mixing and conditioning proguanil hydrochloride, sulfuric acid and a catalyst into slurry, wherein the catalyst is a compound selected from sodium bisulfate, potassium bisulfate, ammonium hydrogen sulfate and tripolyphosphate; heating the slurry for carrying out an oxidative decarboxylation reaction until no bubble is generated; and adding one of catalysts and uniformly mix with the slurry after the oxidative decarboxylation reaction and heating the uniformly mixed slurry for carrying out a polycondensation reaction until the reaction is finished. The glyphosate polymer of the invention is used for weeding and has the advantages of high efficiency, durability and slow release effect.
Owner:SHENZHEN NOPOSION AGROCHEM
A kind of preparation method of insensitive explosive tatb
ActiveCN111995527BEasy to separate and collectEase of mass productionOrganic compound preparationCarboxylic acid amides preparationChemical industryAcetic anhydride
The invention provides a preparation method of insensitive explosive TATB. The method comprises: step 1: using 2,4,6-trinitrotoluene as a raw material, successively reacting with hydrogen and acetic anhydride to obtain 2,4,6-triacetamidotoluene; step 2: converting the obtained 2,4 ,6-triacetamidotoluene is oxidized and decarboxylated to obtain 1,3,5-triacetamidobenzene; step 3: the resulting 1,3,5-triacetamidobenzene is nitrated and hydrolyzed to obtain 2, 4,6‑Trinitro‑1,3,5‑triaminobenzene. Since the raw material used in the preparation method is cheap 2,4,6-trinitrotoluene, and the reactants or catalysts used are common products in the chemical industry, the preparation method has the advantages of low cost and simple raw materials. Easy to get features. In addition, the preparation method also has the characteristics of short synthesis steps, simple operation of each step, high yield, fast reaction rate, and easy separation and collection of intermediate and final products, which is conducive to the realization of mass production of TATB.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
A kind of synthetic method of aryl selenium heterocyclic compound
The invention relates to a method for synthesizing an arylseleno-heterocyclic compound. The method comprises the following step: in an organic solvent, under an oxygen condition, by taking heterocyclic aromatic carboxylic acid and diaryl diselenide ether as reaction raw materials, and under the cooperative promotion action of a copper catalyst, a ligand and alkali, performing oxidative decarboxylation coupling reaction, thereby obtaining the arylseleno-heterocyclic compound. By adopting the method, the substrate, namely the heterocyclic aromatic carboxylic acid, and the copper catalyst are cheap and easy to obtain, the substrate range is wide, reaction conditions are simple, the yield and the purity of the product are high, novel synthesis routes and methods are developed for arylseleno-heterocyclic compounds, and the method has good application potentials and study values.
Owner:WENZHOU MEDICAL UNIV
Preparation method of glyphosate polymer
ActiveCN101974159BGood effectThe amount of release is controllableHydrogen SulfateProguanil Hydrochloride
The invention provides a preparation method of glyphosate polymer, in particular to a N-(Phosphonomethyl)iminodiaceticacid catalytic polycondensation method. The preparation method comprises the following steps of: mixing and conditioning proguanil hydrochloride, sulfuric acid and a catalyst into slurry, wherein the catalyst is a compound selected from sodium bisulfate, potassium bisulfate, ammonium hydrogen sulfate and tripolyphosphate; heating the slurry for carrying out an oxidative decarboxylation reaction until no bubble is generated; and adding one of catalysts and uniformly mix with the slurry after the oxidative decarboxylation reaction and heating the uniformly mixed slurry for carrying out a polycondensation reaction until the reaction is finished. The glyphosate polymer of the invention is used for weeding and has the advantages of high efficiency, durability and slow release effect.
Owner:SHENZHEN NOPOSION AGROCHEM
A kind of synthetic method of aryl methyl selenide compound
The invention relates to a synthetic method of an aryl methyl selenide compound. In an organic solvent, under the oxygen condition, aryl carboxylic acid and dimethyldiselenide are used as reaction raw materials to obtain the aryl methyl selenide compound through oxidative decarboxylation coupling reaction under the joint promoting effect of a copper catalyst, a ligand and alkaline. The aryl carboxylic acid and the copper catalyst adopted in the method are cheap and easy to obtain, a substance range is wide, the reaction condition is simple, the product yield and purity are high, a new synthetic route and method is developed for the aryl methyl selenide compound, and the aryl methyl selenide compound has good utilization potential and a research value.
Owner:WENZHOU MEDICAL UNIV
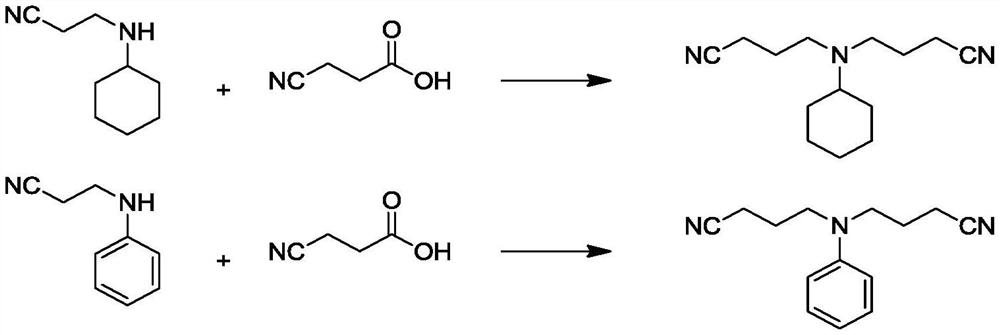
Preparation method of dicyanoethyl tertiary amine
ActiveCN114591200ANo need for post-treatment deacidificationIncrease reaction rateCarboxylic acid nitrile preparationOrganic compound preparationPtru catalystPropanoic acid
The invention provides a preparation method of dicyanoethyl tertiary amine. The preparation method comprises the following steps: carrying out catalytic reaction on mono-nitrile ethyl secondary amine and 3-cyanopropionic acid to obtain dicyanoethyl tertiary amine mother liquor; and carrying out post-treatment on the dicyanoethyl tertiary amine mother liquor to obtain a dicyanoethyl tertiary amine product. Wherein a Fe-containing catalyst is adopted in the catalytic reaction. The 3-cyanopropionic acid provided by the invention is a biomass raw material continuously obtained by oxidative decarboxylation of glutamic acid, is an acidic catalyst, and does not need a post-treatment deacidification link; the yield of 3-cyanopropionic acid catalyzed by the Fe-containing catalyst can reach 98%, the reaction rate is high, and the yield is high.
Owner:WANHUA CHEM GRP CO LTD
Method for preparing vanillin
InactiveCN104072350AReduce productionEasy to useOrganic compound preparationCarbonyl compound preparation by condensationGlyoxylic acidExtractive distillation
The invention discloses a method for preparing vanillin and relates to the technical field of chemical engineering. The method comprises the following steps of mixing wood phenols, alkali, glyoxylic acid and sulfuric acid to carry out condensation, carrying out extractive distillation, adding air, water and alkali, carrying out oxidative decarboxylation reaction, adding an acid for acidifying treatment, then adding toluene, concentrating for extracting, distilling the mixture extracted by concentrating to filter the residue off, putting the mixture and methanol into a crystallization vessel and crystallizing, and putting the crystallized mixture into a centrifugal device to separate out methanol mother liquor, drying the mixture subjected to centrifugal separation to obtain a vanillin product, packaging and warehousing. The method disclosed by the invention has the beneficial effects of simpleness and convenience in preparation, less yield of waste acid, less investment in equipment, high purity and convenience in operation and the prepared vanillin has a good application effect and is safe and reliable.
Owner:安徽佑骏商品混凝土有限公司
A high-efficiency δ 9,11 -The preparation method of canrenone
The invention discloses a high-efficiency Δ 9,11 A preparation method of canrenone belongs to the technical field of preparation of intermediates of medicines. The method is based on 9α-hydroxy-4-androstenedione as a raw material, and the 9-position hydroxyl group is first eliminated by dehydration reaction to generate Δ 9,11 Double bond, then protect the 3-position carbonyl group, then epoxidize the 17-position carbonyl group, and condense with malonic acid diester to form a lactone ring, and obtain Δ after oxidative decarboxylation or decarboxylation oxidation reaction 9,11 ‑Canrenone, the raw materials of the method of the present invention are cheap and easy to obtain, the cost is low, the reaction products involved in each step are easy to purify, and the total mass yield of the final product is higher than 80%. The method of the present invention has strong operability and extremely high commercial Competitive, suitable for industrialized large-scale production, and has good economic benefits.
Owner:ZHEJIANG SHENZHOU PHARMA
Method for preparing 1, 3, 5-trinitrobenzene
ActiveCN113149843ASimple processReduce operating costsOrganic chemistryOrganic compound preparationSodium chlorateNitrobenzene
The invention relates to a method for preparing 1, 3, 5-trinitrobenzene, and belongs to the technical field of organic intermediates. The method comprises the following steps: respectively preparing a TNT alcoholic solution and a sodium chlorite aqueous solution, adjusting the pH value of the TNT alcoholic solution with hydrochloric acid, heating to a reaction temperature, dropwise adding the sodium chlorite aqueous solution into the TNT alcoholic solution, reacting at a constant temperature, and finally filtering, washing and recrystallizing to obtain the 1, 3, 5-trinitrobenzene. Sodium chlorite is adopted as an oxidizing agent, so that oxidation and decarboxylation reactions are completed in one step, the process is simplified, and the operation cost is reduced; the energy consumption required by the reaction is low, and the product purity is high; the reaction process is safer, and the reaction yield is higher.
Owner:ZHONGBEI UNIV
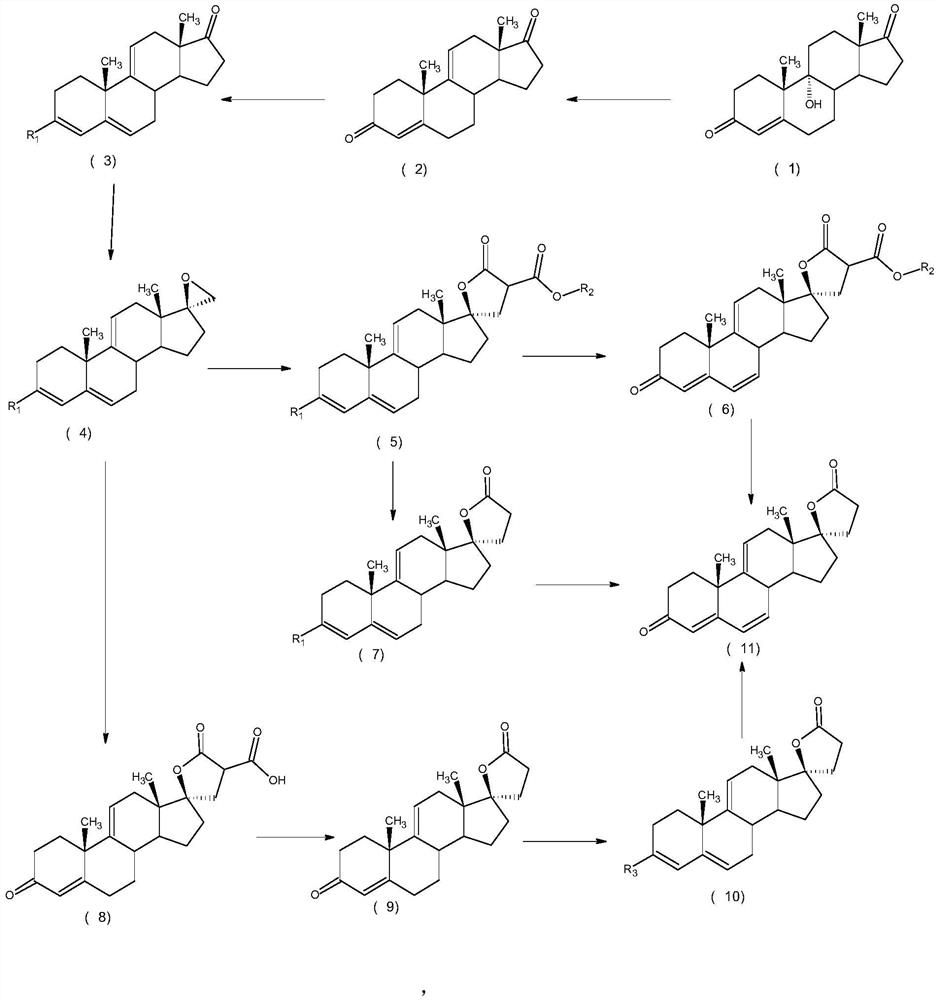
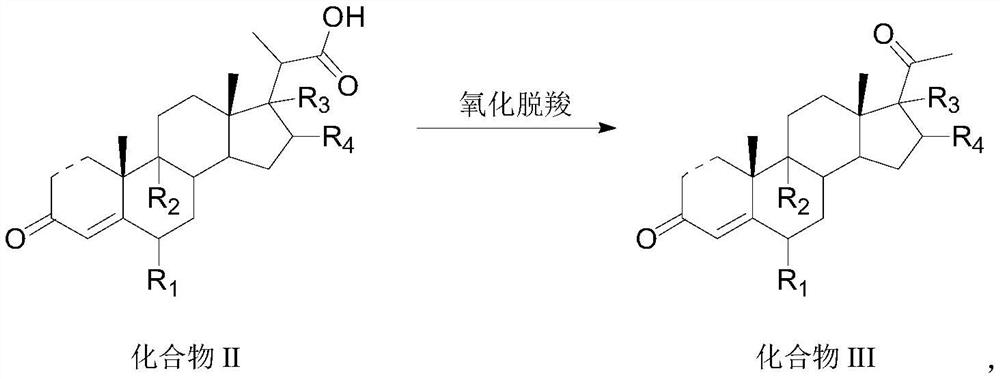
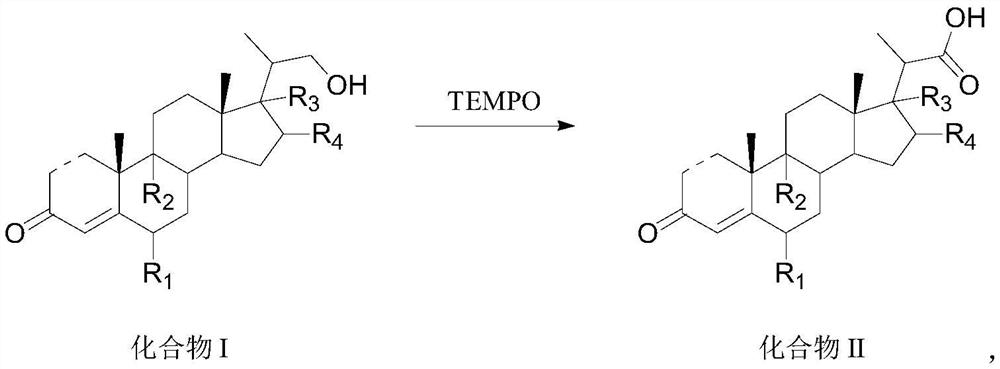
Preparation method and application of steroid intermediate
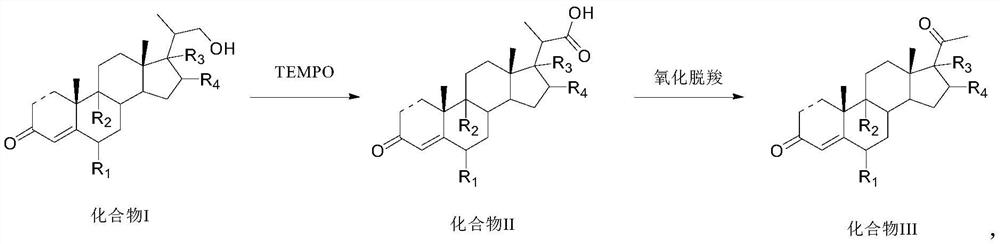
PendingCN114315946AReduce usageRaise the reaction temperatureSteroidsChemical synthesisPtru catalyst
The invention provides a preparation method and application of a steroid compound intermediate, and relates to the technical field of chemical synthesis. Comprising the following steps: (a) carrying out oxidation reaction on a compound I under the action of a TEMPO catalyst, pypocholoride and an alkali reagent to obtain a compound II; (b) the compound II is subjected to an oxidative decarboxylation reaction under the action of a Schiff base metal complex, an oxidizing agent and organic alkali, and a compound III is obtained. According to the method, a brand new synthesis route is adopted, the reaction time is shortened, the reaction temperature is increased, a reagent with high toxicity and high odor in an original route is avoided, meanwhile, an oxidizing agent with high cost is also avoided, the reaction condition is milder and easy to control, and the production applicability is improved.
Owner:TIANJIN PHARMA GROUP CORP
Method for preparing atovaquone
InactiveCN101774901BImprove dissolutionReduce generationOrganic compound preparationQuinone preparationPotassium persulfateAtovaquone
The invention discloses a method for preparing atovaquone, which comprises the following steps: taking 4-(4-chlorophenyl)-cyclohexyl-1-methanoic acid and 2-chlorine-1, 4-naphthoquinone as raw materials, generating (3S)-2-chlorine-3-(4-(4-chlorophenyl) cyclohexyl)-1, 4-naphthalenedione by oxidative decarboxylation through a peroxide in the action of a catalyst of silver nitrate, and then obtaining the atovaquone through hydrolysis with alkaline. The method is characterized in that the mixed solvent of acetonitrile and choromethane is used as the solvent for the oxidative decarboxylation and the peroxide is one of the four substances, i.e., sodium persulfate, potassium persulfate, sodium percarbonate and potassium peroxycarbonate. The improved preparation method considerably improves the dissolution of the raw materials in the solvent, raises the rate of conversion, increases the yield, reduces impurities, and significantly enhances the product quality. Therefore, the method is more suitable for industrial production.
Owner:WUHAN TITON BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com