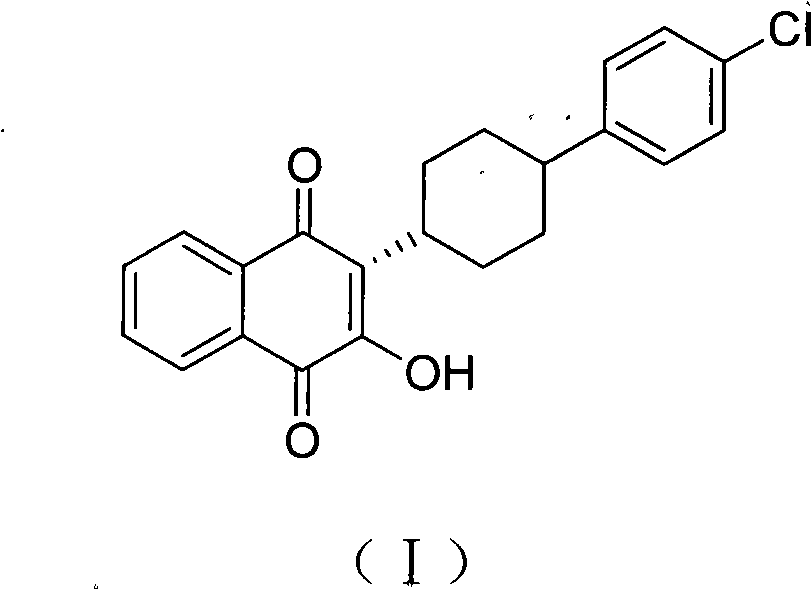
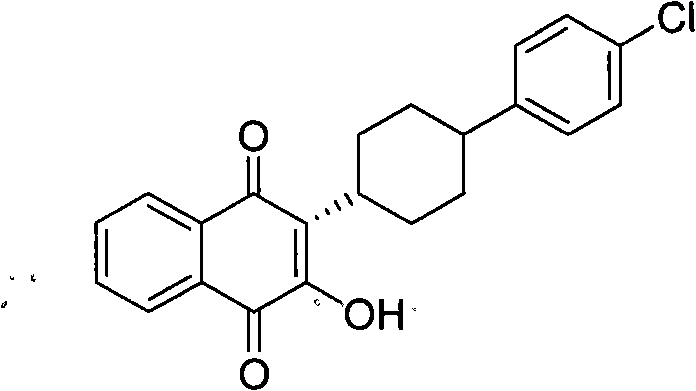
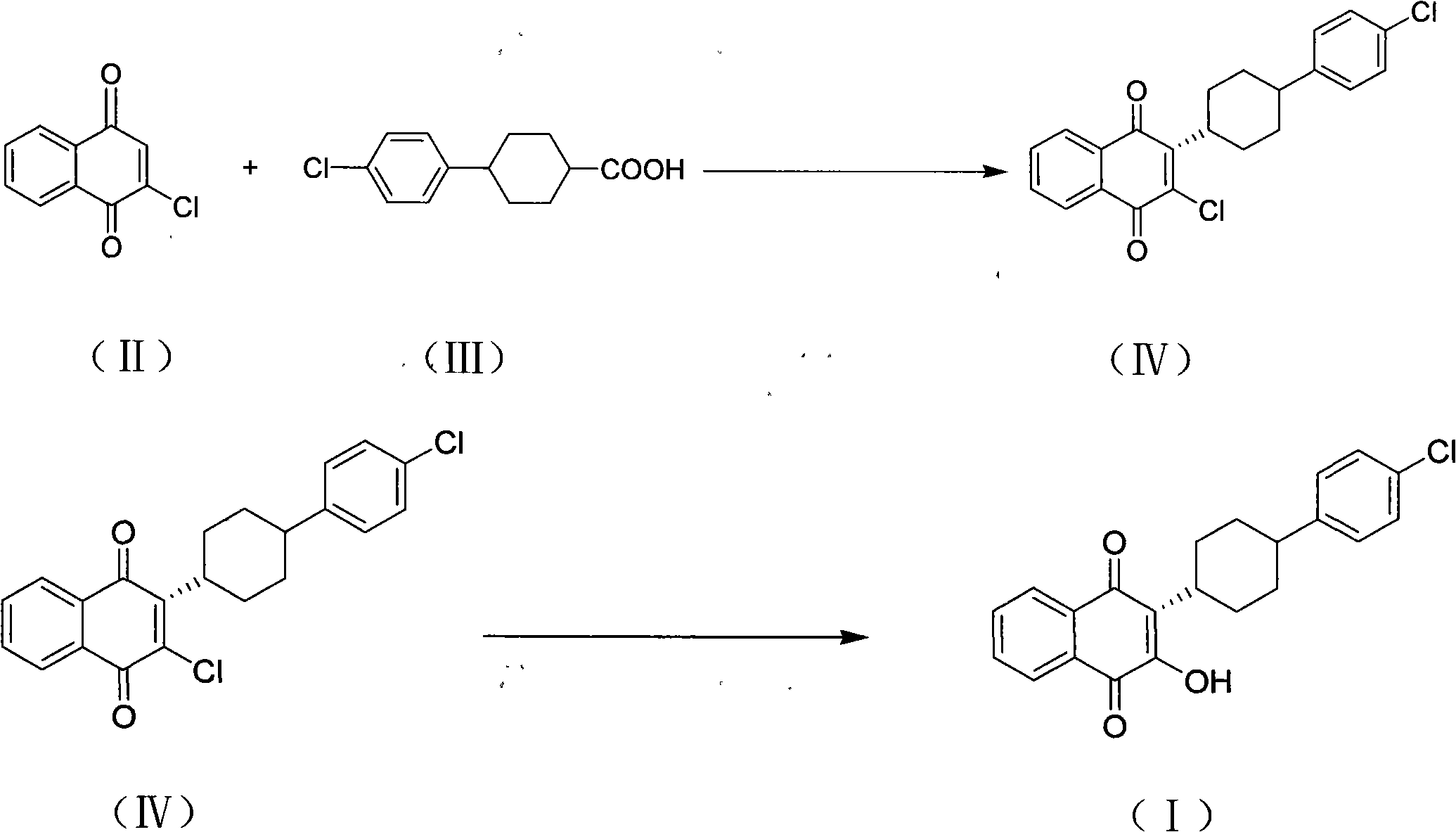
Method for preparing atovaquone
The technology of atovaquone and chlorophenyl is applied in the field of preparation of antimalarial drugs, can solve the problems of high industrial production cost, many side reactions, many impurities, etc., and achieves improved product quality, improved solubility, and improved conversion rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 4-(4-chlorophenyl)cyclohexyl-1-carboxylic acid (4.95g, 0.02mol) and 2-chloro-1,4-naphthoquinone (4g, 0.02mol), silver nitrate (2.1g, 0.012mol) Put it into a mixed solvent of acetonitrile (80ml) and dichloromethane (20ml), add dropwise 100ml aqueous solution of sodium persulfate (12.3g, 0.05mol) at 60°C, dropwise for 4.5 hours, and raise the temperature to 80°C after the addition Insulate the reaction for 3 hours, lower it to normal temperature, add 50ml of chloroform and stir for 10 minutes, let it stand for stratification, separate the water phase, concentrate the organic phase under reduced pressure, evaporate the solvent, add 80ml of acetonitrile to the residue to dissolve under reflux, and filter while it is hot. Crystallize at about 0°C, filter, and dry the filter cake to obtain 4 g of yellow powdery solid, which is (3S)-2-chloro-3-(4-(4-chlorophenyl)cyclohexyl)-1,4- Naphthalenedione was added to 60ml methanol and refluxed for 30 minutes, 15ml of 10% (weight) potas...
Embodiment 2
[0027] 4-(4-chlorophenyl)cyclohexyl-1-carboxylic acid (4.95g, 0.02mol) and 2-chloro-1,4-naphthoquinone (4g, 0.02mol), silver nitrate (2.1g, 0.012mol) Put it into a mixed solvent of acetonitrile (80ml) and dichloromethane (20ml), add dropwise 100ml aqueous solution of potassium persulfate (14g, 0.05mol) at 60°C, add dropwise for 4.5 hours, and heat up to 80°C after the dropwise addition Reacted for 3 hours, and other operations were the same as in Example 1 to obtain 2.5 g of atovaquone with a yield of 33.1% and a purity (HPLC): 99.1%.
Embodiment 3
[0029] 4-(4-chlorophenyl)cyclohexyl-1-carboxylic acid (4.95g, 0.02mol) and 2-chloro-1,4-naphthoquinone (4g, 0.02mol), silver nitrate (2.1g, 0.012mol) Put it into a mixed solvent of acetonitrile (80ml) and dichloromethane (20ml), add dropwise 100ml aqueous solution of sodium percarbonate (16.3g, 0.05mol) at 60°C, dropwise for 4.5 hours, and raise the temperature to 80°C after the addition The reaction was incubated for 3 hours, and the remaining operations were the same as in Example 1 to obtain 1.9 g of atovaquone with a yield of 24.8% and a purity (HPLC): 98.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com