Pharmaceutical composition containing cilostazol and preparation method thereof
A technology of cilostazol and composition, which is applied in the field of pharmaceutical composition containing cilostazol and its preparation, can solve the problems of low-cost cilostazol preparation bioavailability, etc., and achieve low production cost and excellent preparation process. Simple, easy-to-operate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
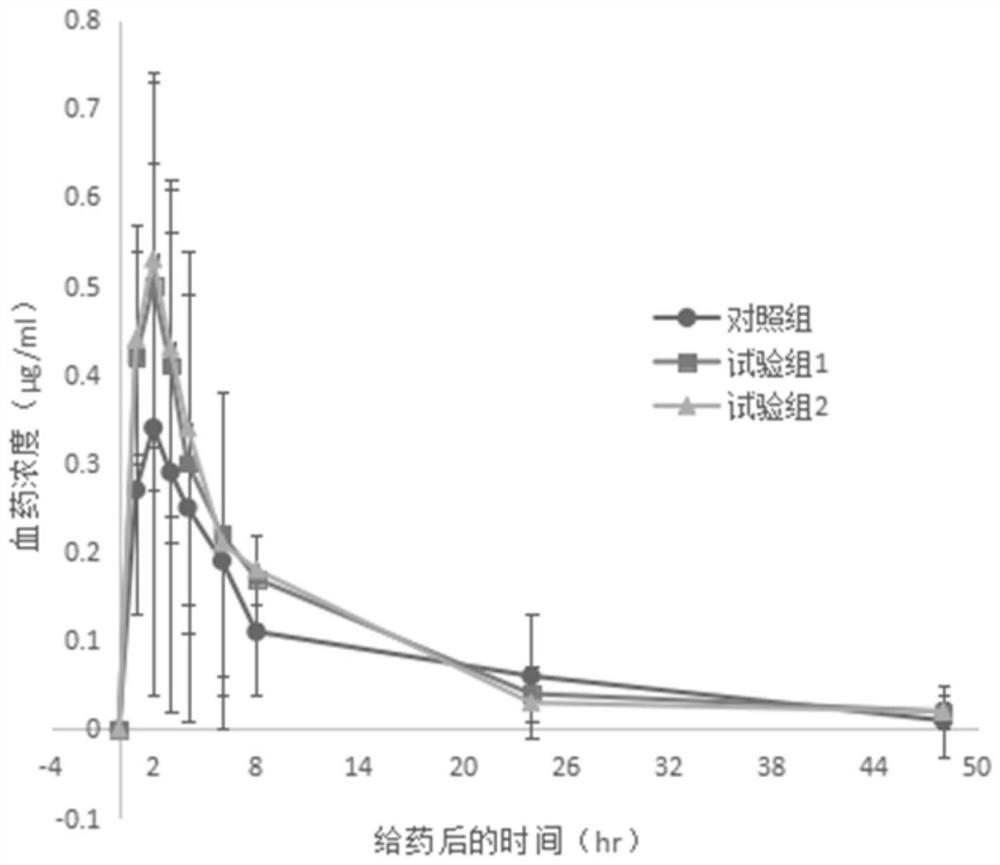
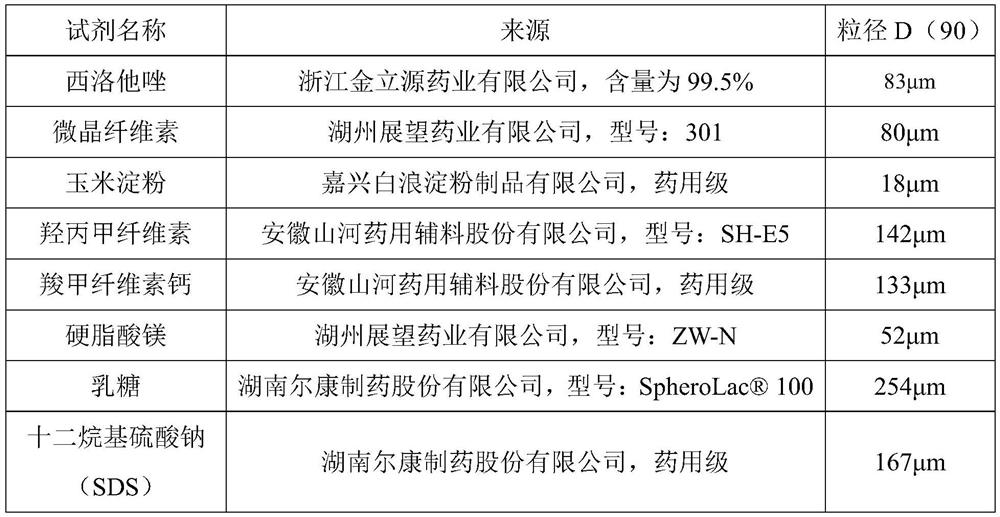
[0049] The present embodiment provides a kind of pharmaceutical composition containing cilostazol, which is cilostazol tablet, and each tablet contains cilostazol 50mg, and 1000 tablets are made according to the following prescription: granule composition 53.3g (made by cilostazol Hexazol 50g, hypromellose 1.8g, SDS1.5g), microcrystalline cellulose 27g, corn starch 23.7g, carmellose calcium (internal addition) 5g, carmellose calcium (external addition) 5g, Magnesium stearate 1g. The preparation method sequentially comprises: a preparation step of the granule composition, a mixing step, a soft material preparation step, a granulation step, a drying step, a granulation step, a total mixing step, and a tabletting step.
[0050] Wherein, the preparation step of the granule composition is to weigh and mix cilostazol, SDS, and hypromellose according to the prescription quantity, and pulverize them with a jet mill until the particle diameter D(90) is 40 μm to obtain the granule compo...
Embodiment 2
[0052] This embodiment provides a pharmaceutical composition containing cilostazol, its prescription and preparation method are basically the same as in Example 1, the only difference is that in the preparation method of the granular composition, cilostazol, SDS, hydroxypropyl The mixture of methylcellulose was co-pulverized with a jet mill until the particle diameter D(90) was 20 μm to obtain a granular composition.
Embodiment 3
[0054] This embodiment provides a pharmaceutical composition containing cilostazol, its prescription and preparation method are basically the same as in Example 1, the only difference is that in the preparation method of the granular composition, cilostazol, SDS, hydroxypropyl The mixture of methyl cellulose was co-pulverized with a jet mill until the particle diameter D(90) was 10 μm to obtain a granular composition.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com