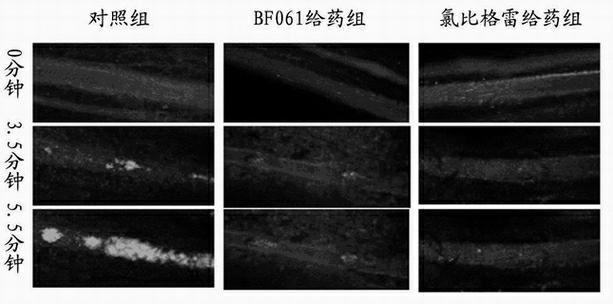
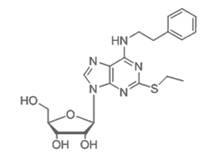
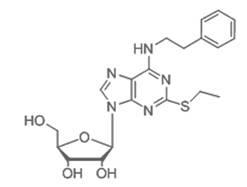
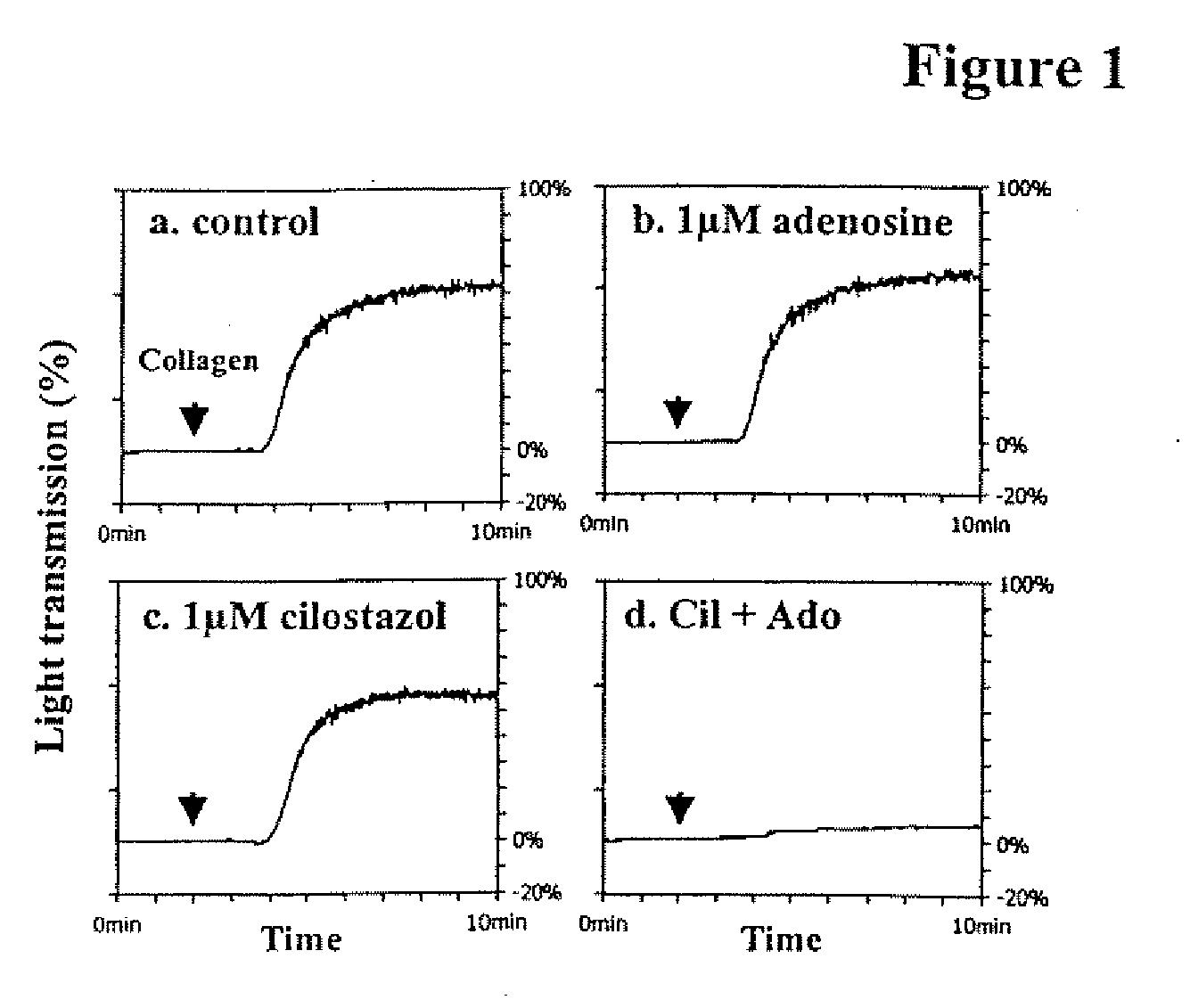
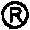Patents
Literature
76 results about "Cilostazol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
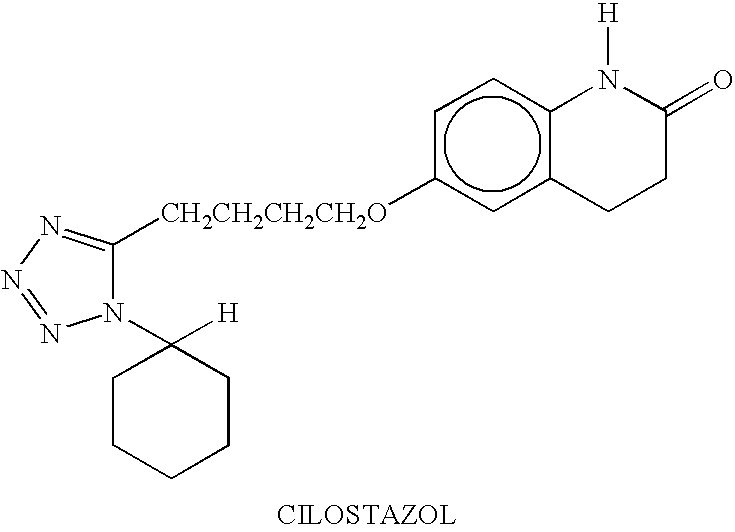
Cilostazol is used to improve the symptoms of a certain blood flow problem in the legs (intermittent claudication).
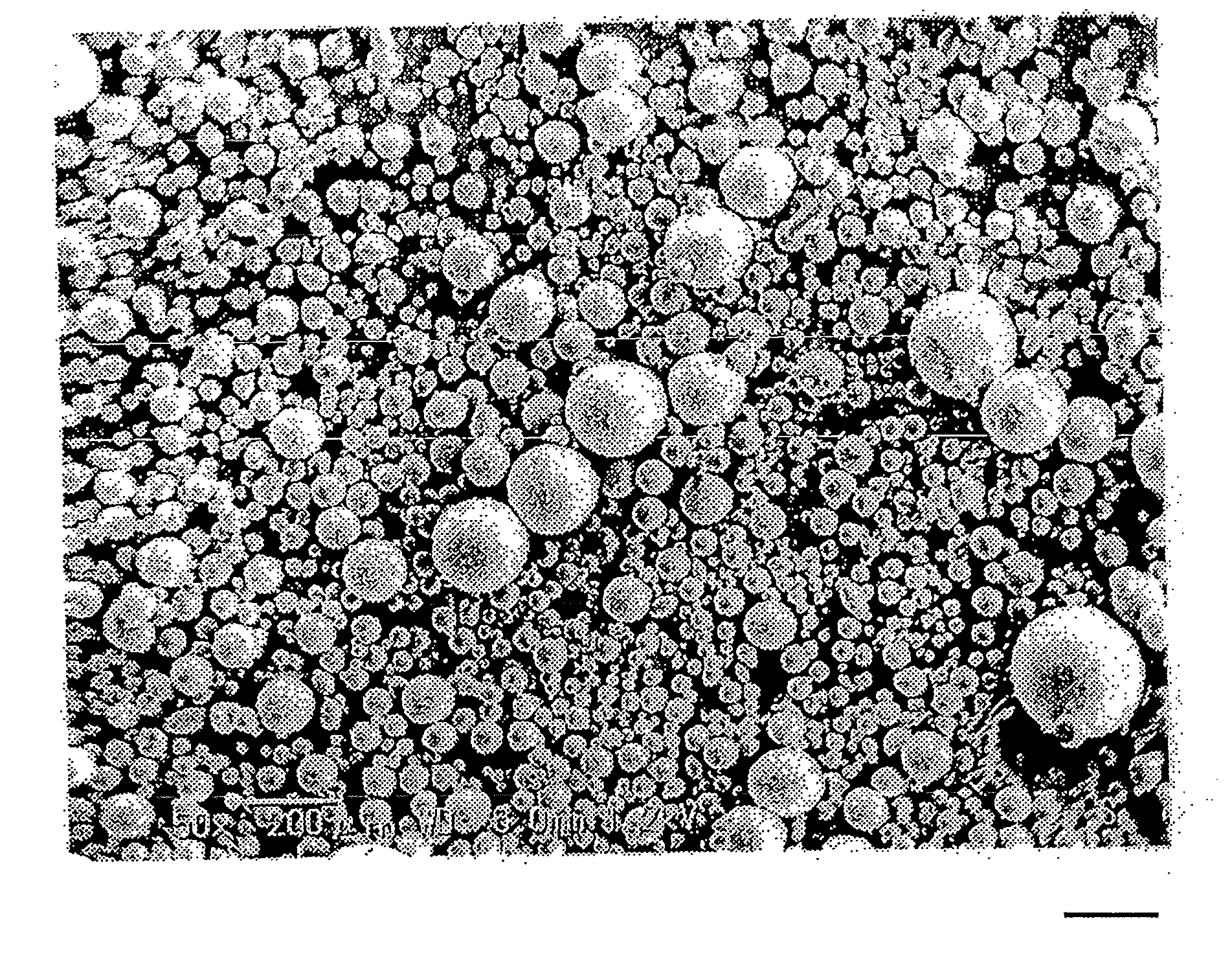
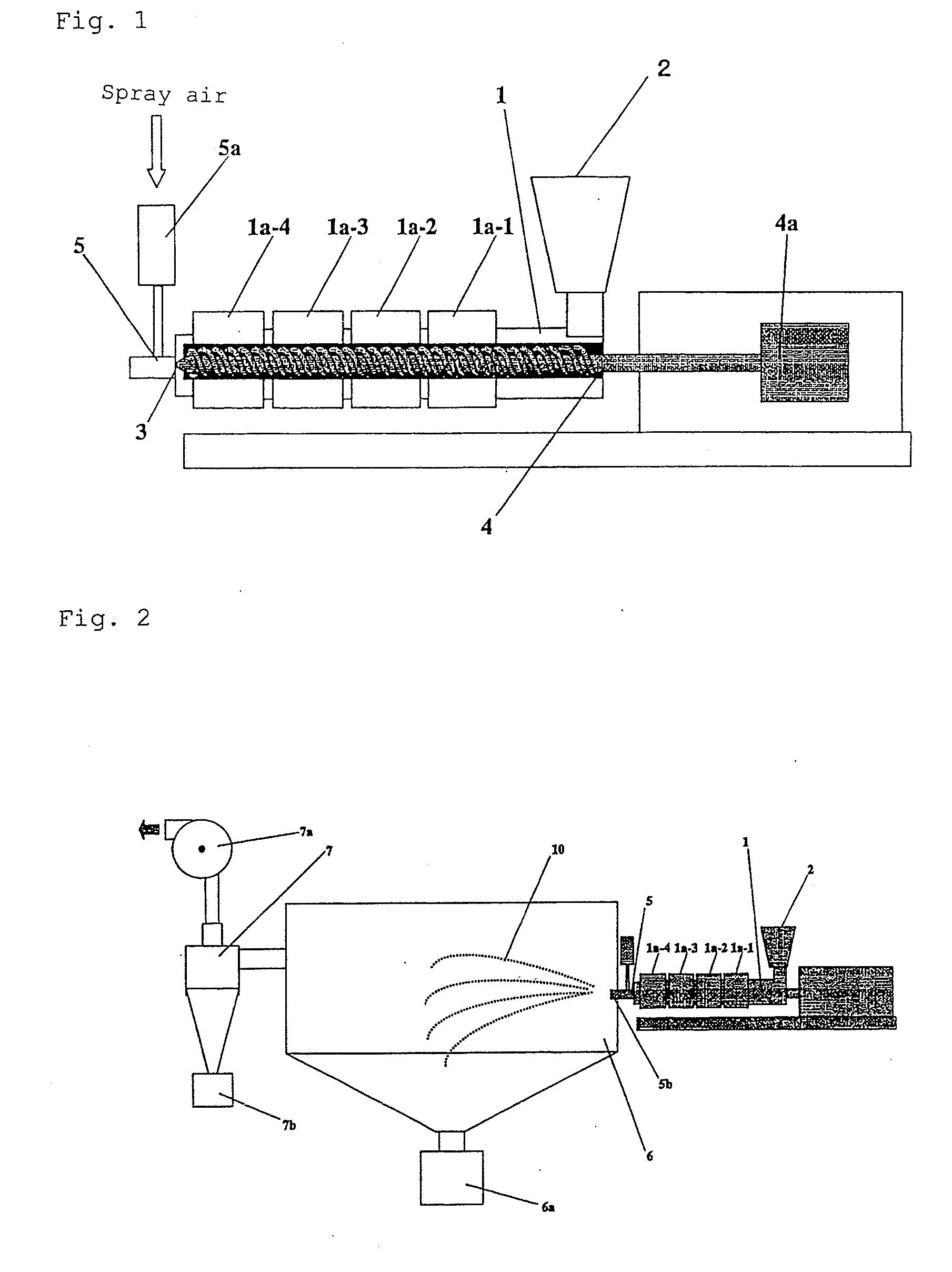
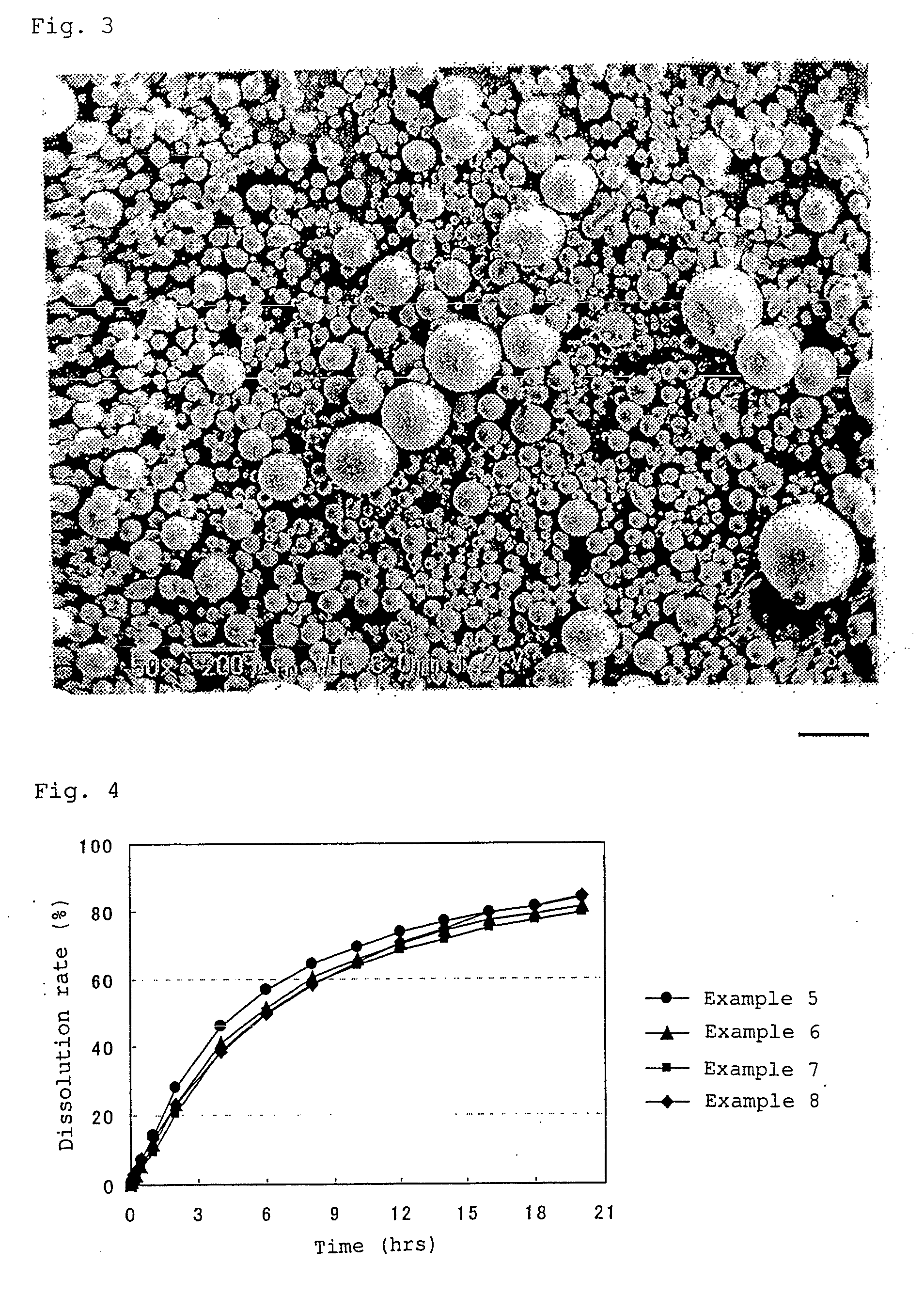
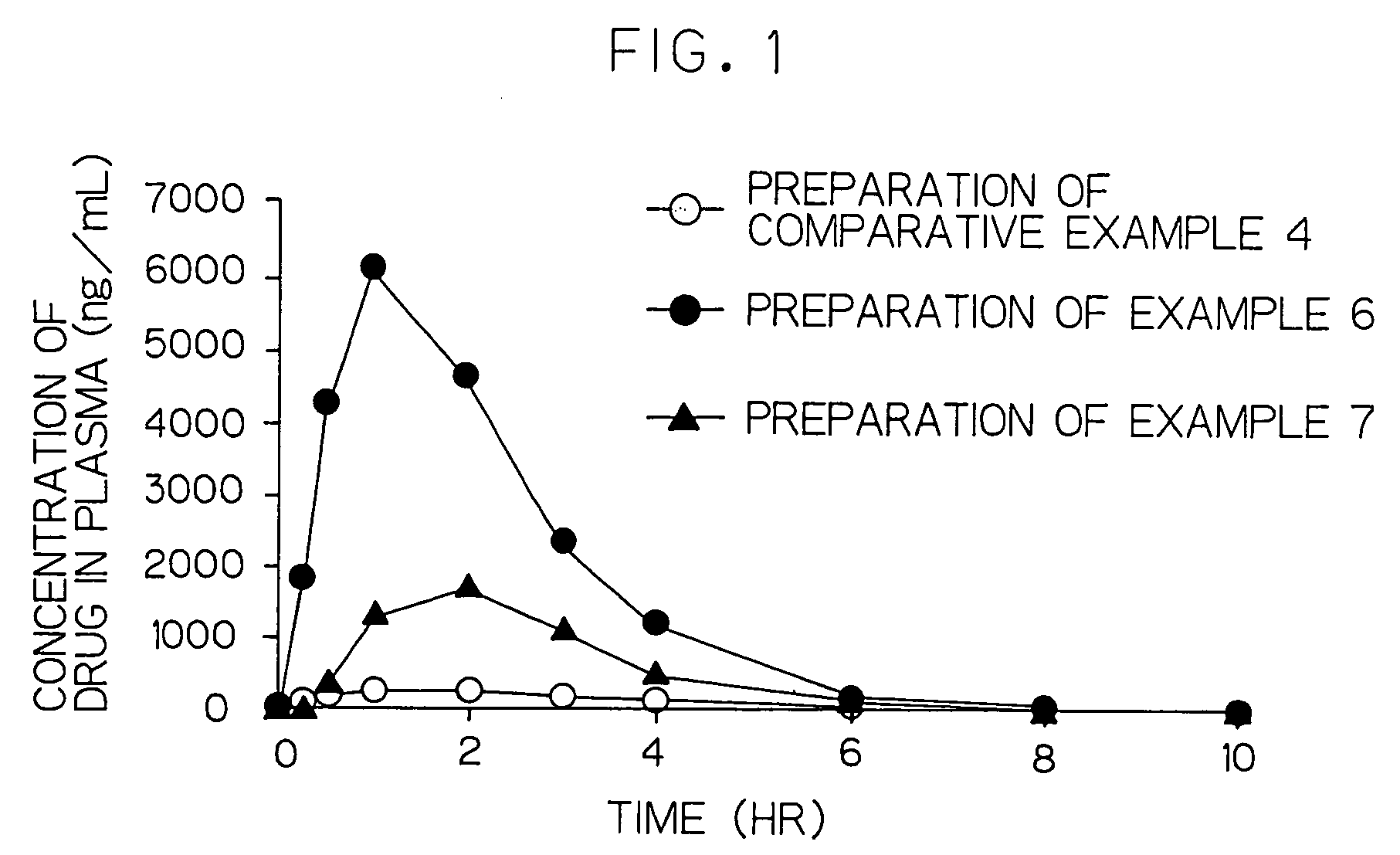
Method of producing drug-containing wax matrix particles, extruder to be used in the method and sustained-release preparation containing cilostazol
InactiveUS20090047357A1Easy to produceSolve low usagePowder deliveryBiocideSpray nozzleSustained-Release Preparations
The present invention aims to provide a method for producing, by a simple method, drug-containing wax matrix granules, particularly drug-containing wax matrix granules having an average particle diameter of 1 mm or lower, while avoiding liquid blockage due to the recrystallization of a molten drug during the period from a melting step to a spray step.Drug-containing wax matrix granules having at least one wax and at least one drug are produced by the following steps (i) and (ii): (i) supplying the at least one drug and the at least one wax to an extruder in which the temperature of a barrel and the temperature of a die are adjusted to be higher than the melting point of the at least one wax; and (ii) while melting and kneading the at least one drug and the at least one wax in the extruder to give a molten kneaded drug and wax, spraying the molten kneaded drug and wax into an atmosphere having a temperature lower than the melting point of the wax from a spray nozzle directly mounted onto a die provided at a top end of the barrel of the extruder, thereby forming the mixture into granules.
Owner:OTSUKA PHARM CO LTD
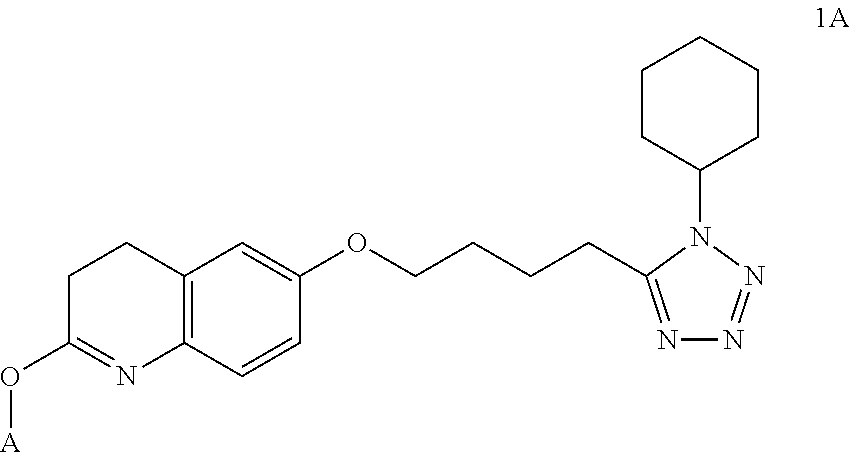
Nitrate derivatives of cilostazol for the treatment of vascular and metabolic diseases
InactiveUS20110230520A1Superior vasodilating propertyBiocideOrganic chemistryPharmacologyLuetic disease
Nitrate derivatives of cilostazol are described. They have superior properties and clinical advantages compared to cilostazol in the treatment of vascular and metabolic diseases.
Owner:CARDIOLYNX
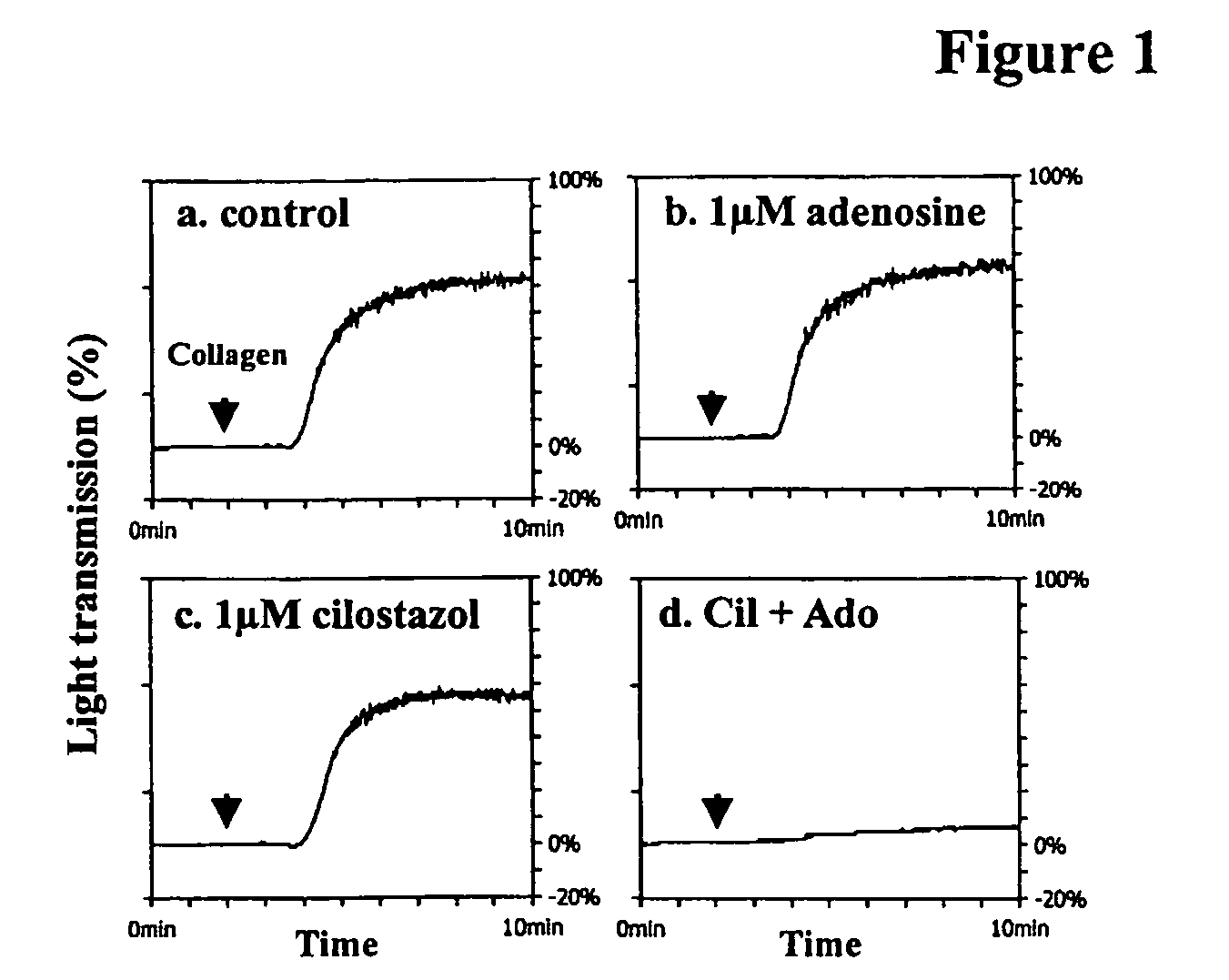
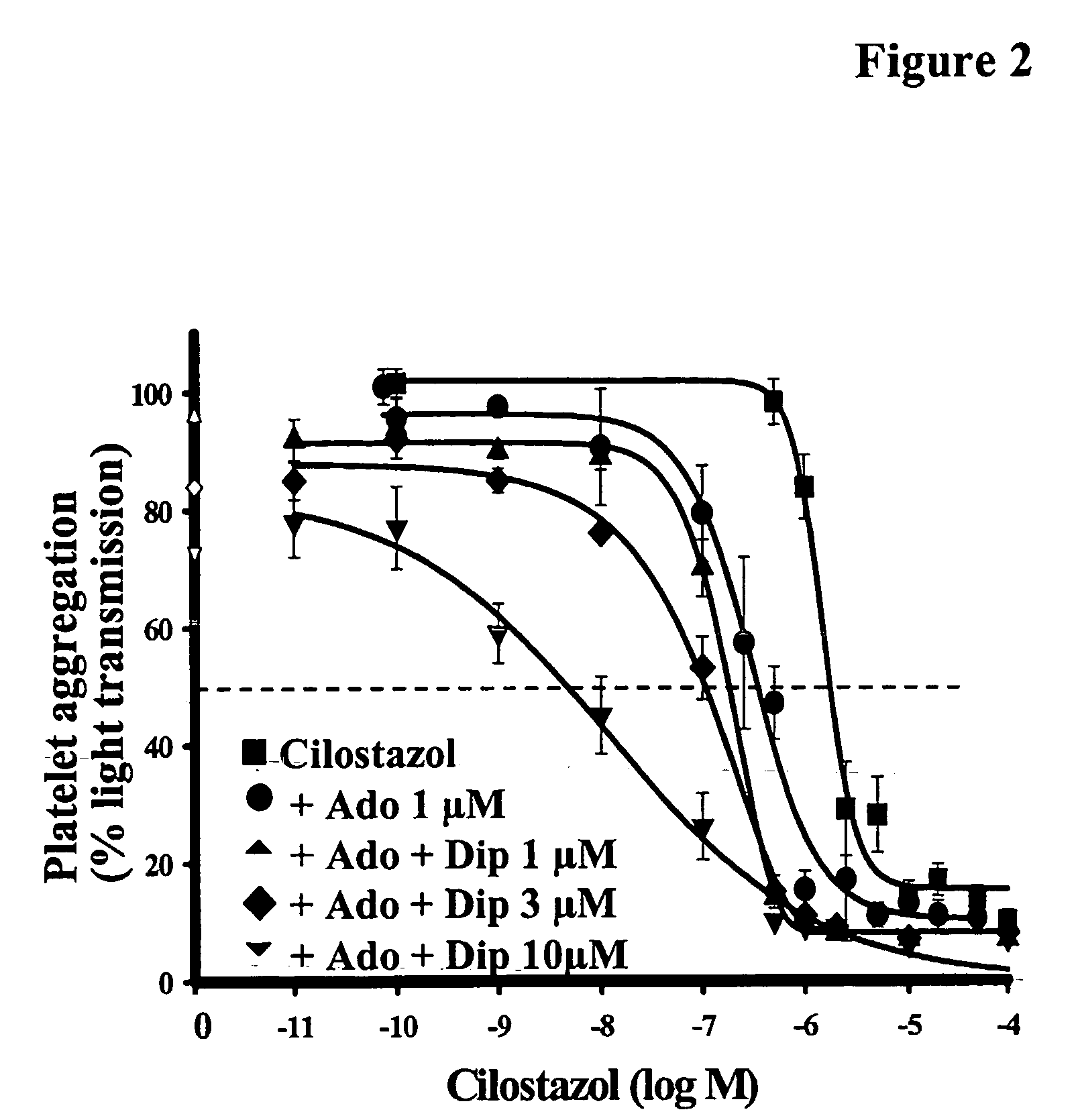
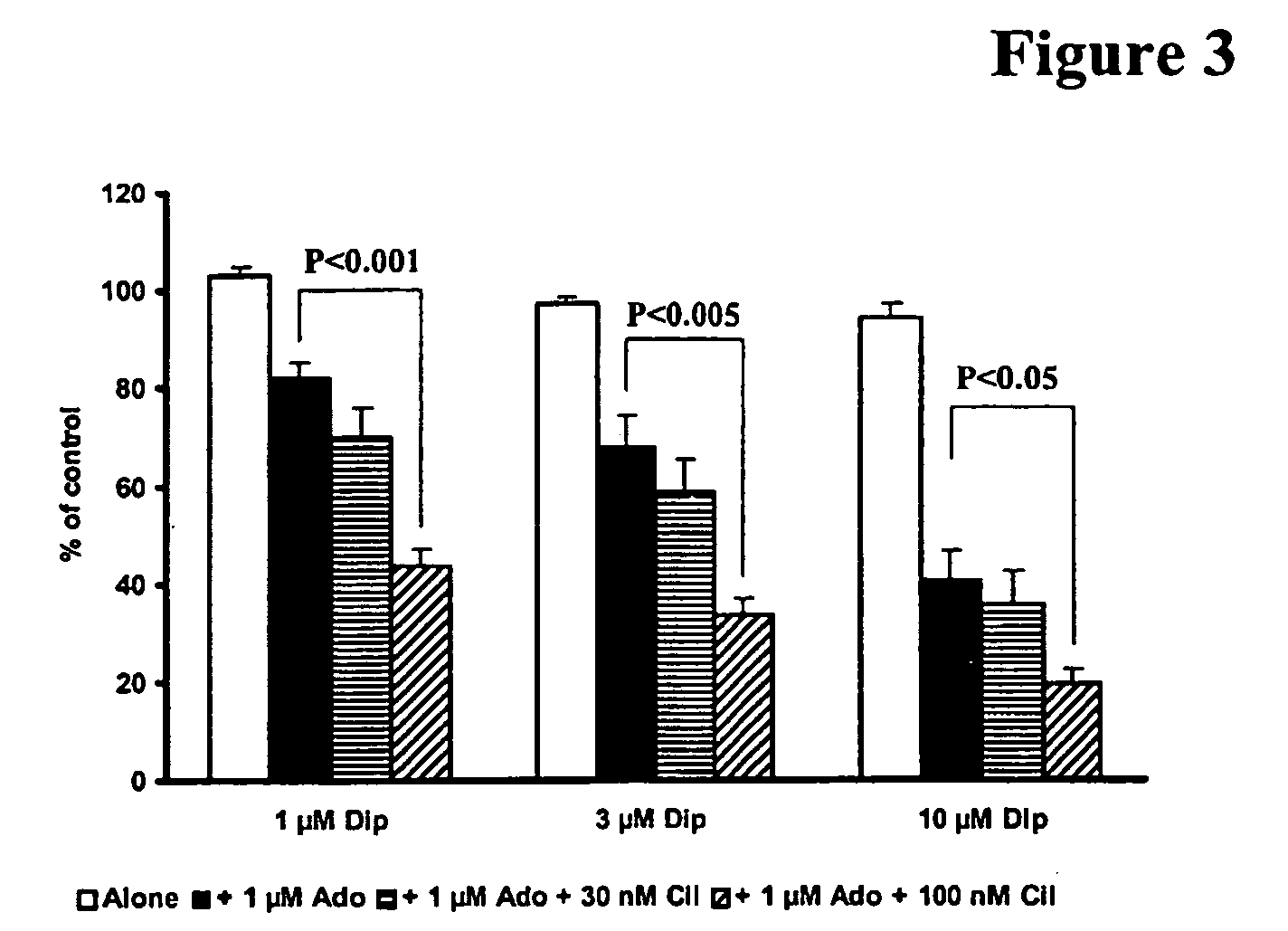
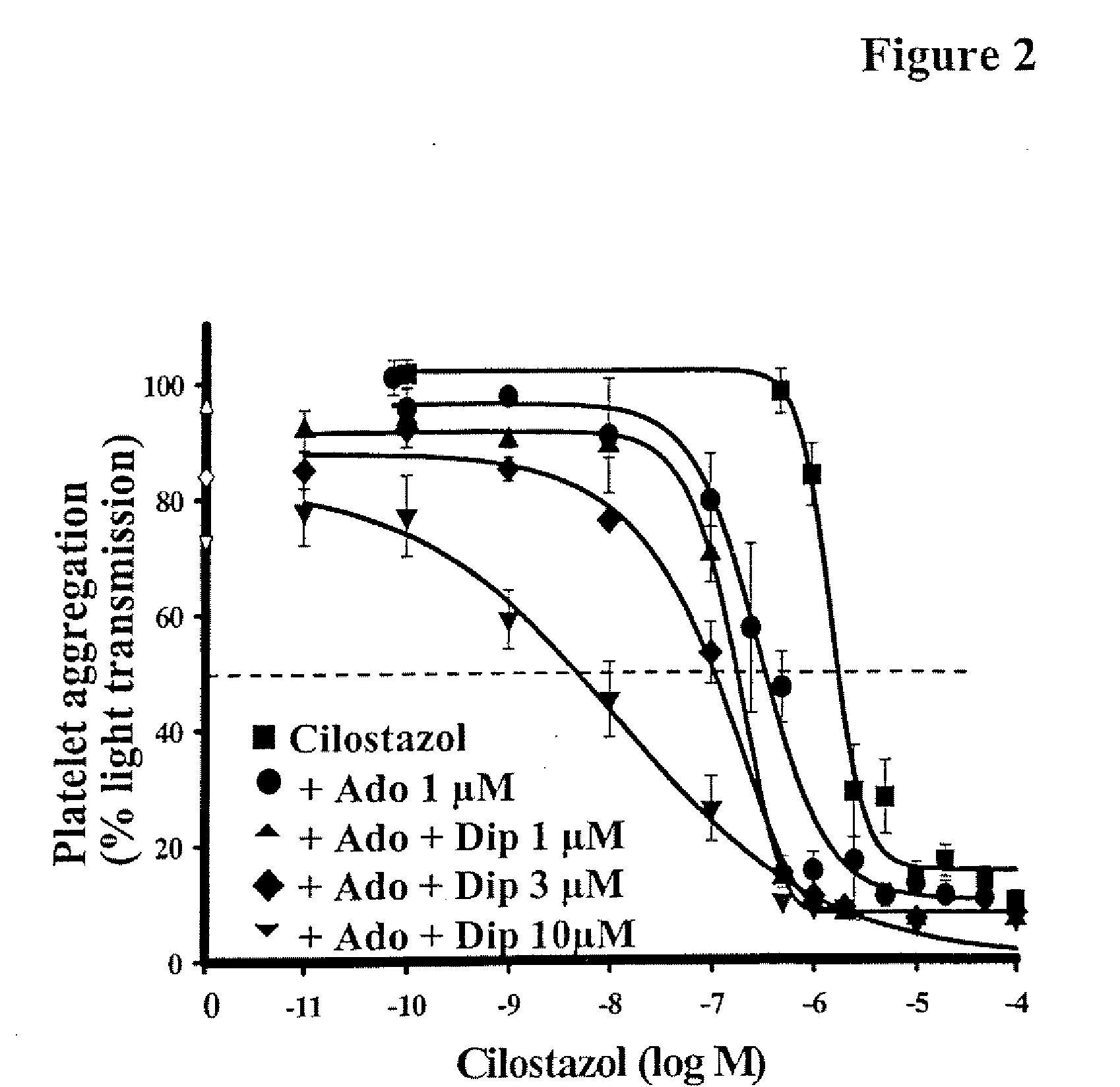
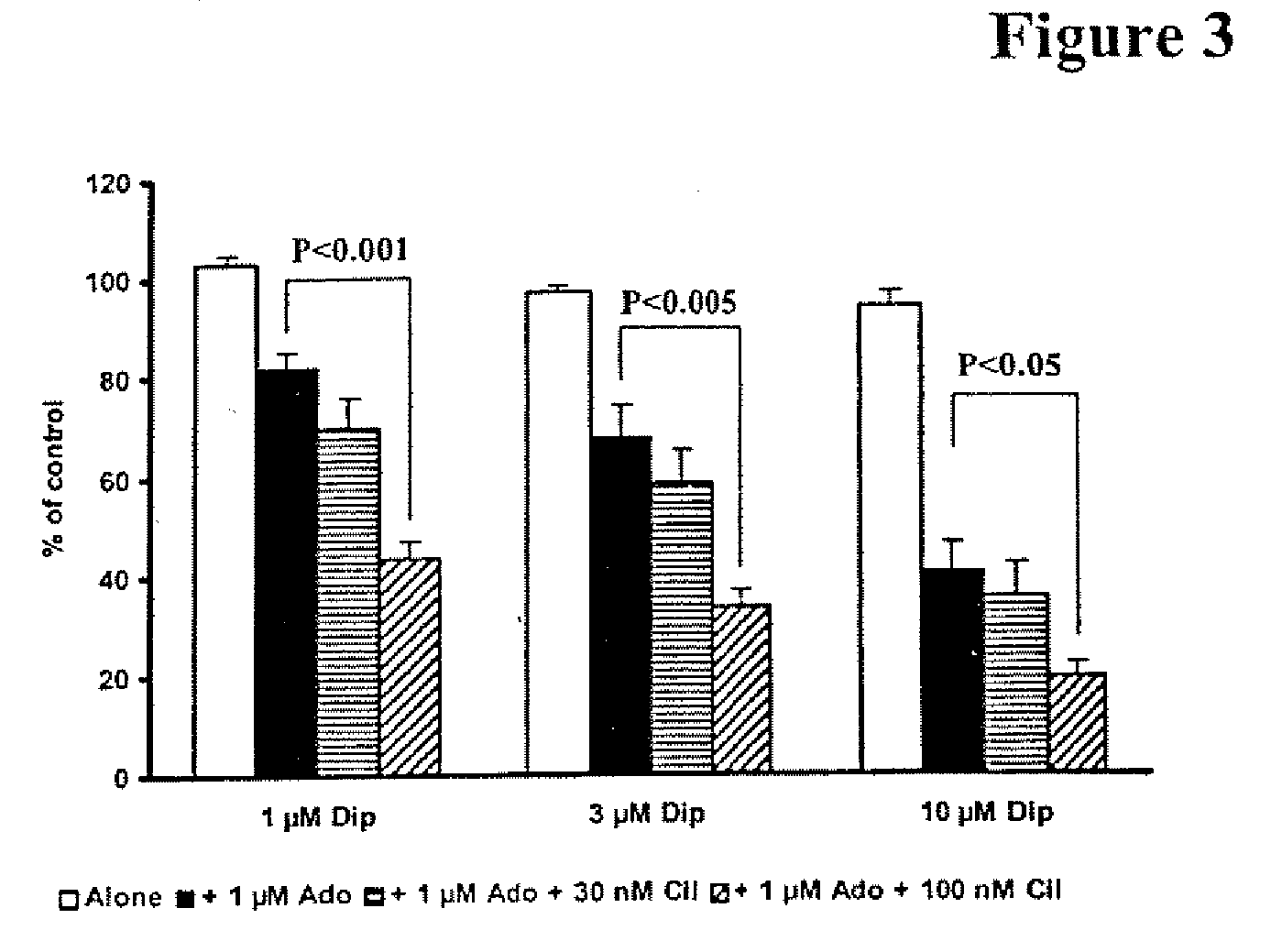
Pharmaceutical compositions comprising a multifunctional phosphodiesterase inhibitor and an adenosine uptake inhibitor
InactiveUS20050165030A1Increase antiplatelet effectIncrease vasodilationBiocideMuscular disorderDipyridamoleAdenosine
The present invention relates to pharmaceutical compositions comprising at least one multifunctional phosphodiesterase inhibitor (MPDEI) and at least one adenosine uptake inhibitor. The present invention also relates to compositions comprising cilostazol and dipyridamole and their use.
Owner:OTSUKA PHARM CO LTD
Cilostazol preparation
InactiveUS7144585B1Promote absorptionEasy to operatePowder deliveryOrganic active ingredientsSolubilityThrombolytic drug
Provided is a cilostazol preparation which comprises incorporating a fine powder of cilostazol into a dispersing and / or solubilizing agent thereby to enhance the dispersibility and / or solubility. Further, provided is a process for improving absorbability of a slightly soluble drug such as cilostazol even at the lower portion of the digestive tract, wherein said drug is hard to be absorbed at the lower portion of the digestive tract when a conventional method is used. According to the present invention, cilostazol is absorbed enough even at the lower portion of the digestive tract to have an effect as thrombolytic drug, cerebral circulation improving drug or the like.
Owner:OTSUKA PHARM CO LTD
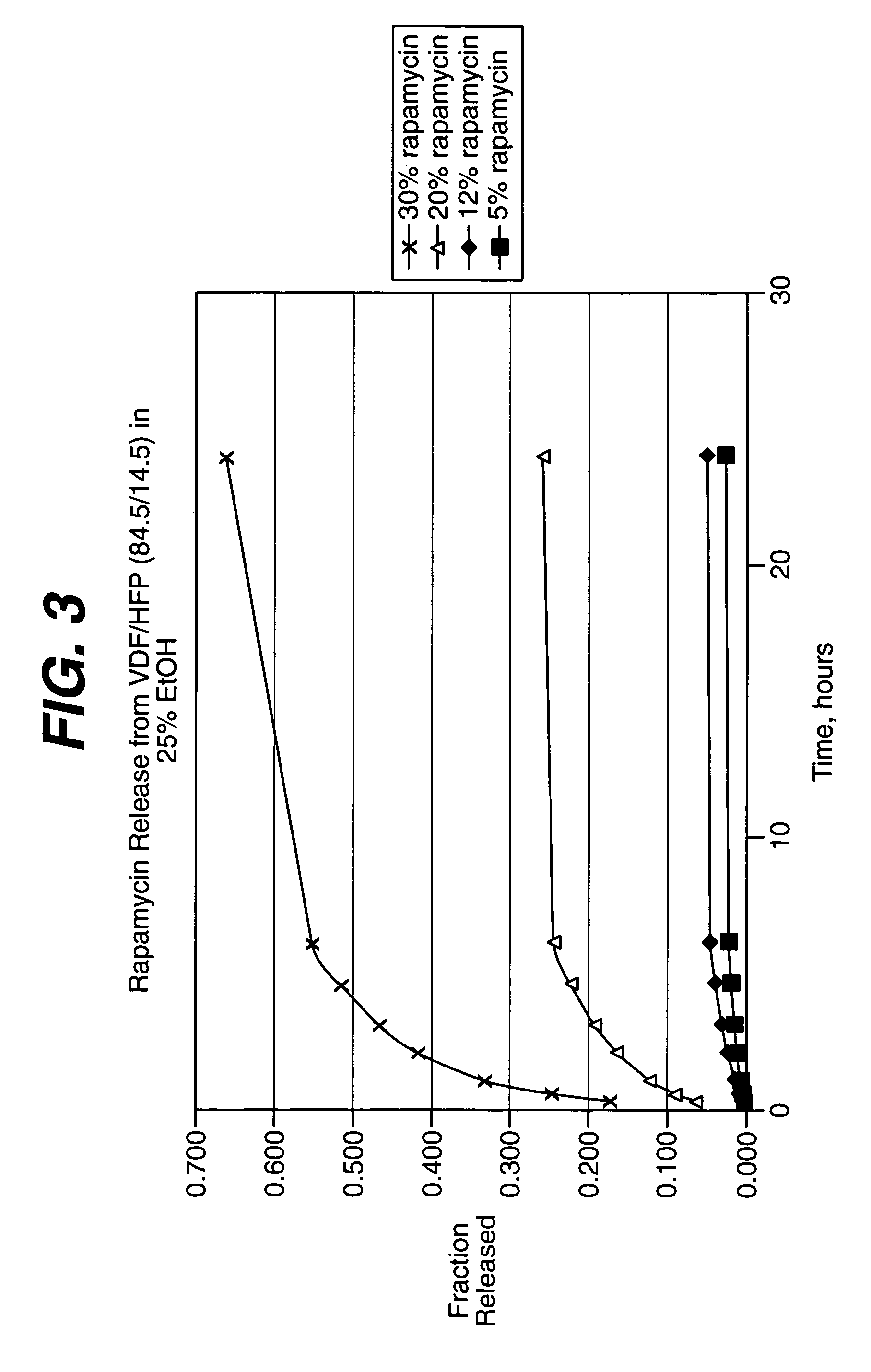
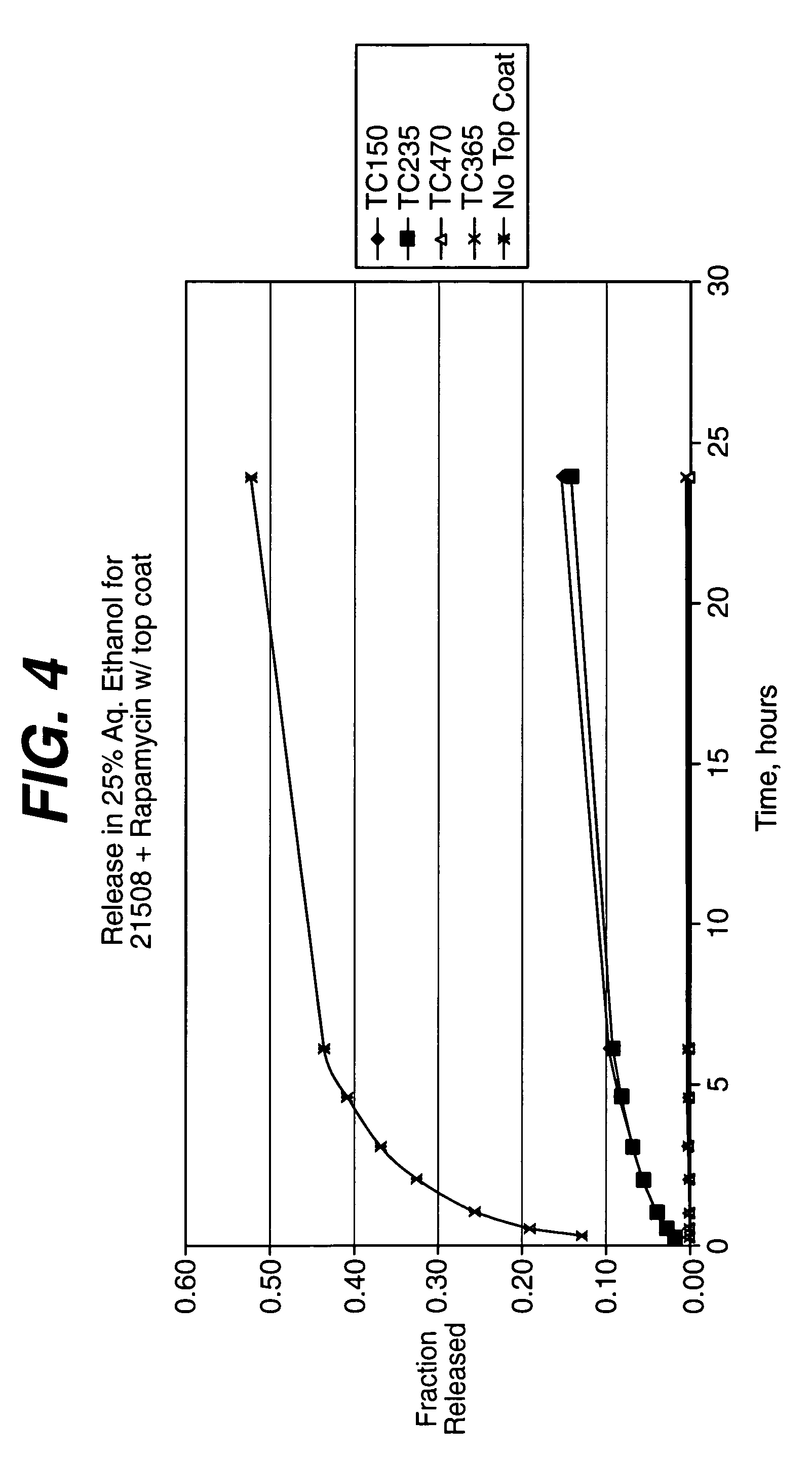
Local administration of a combination of rapamycin and cilostazol for the treatment of vascular disease
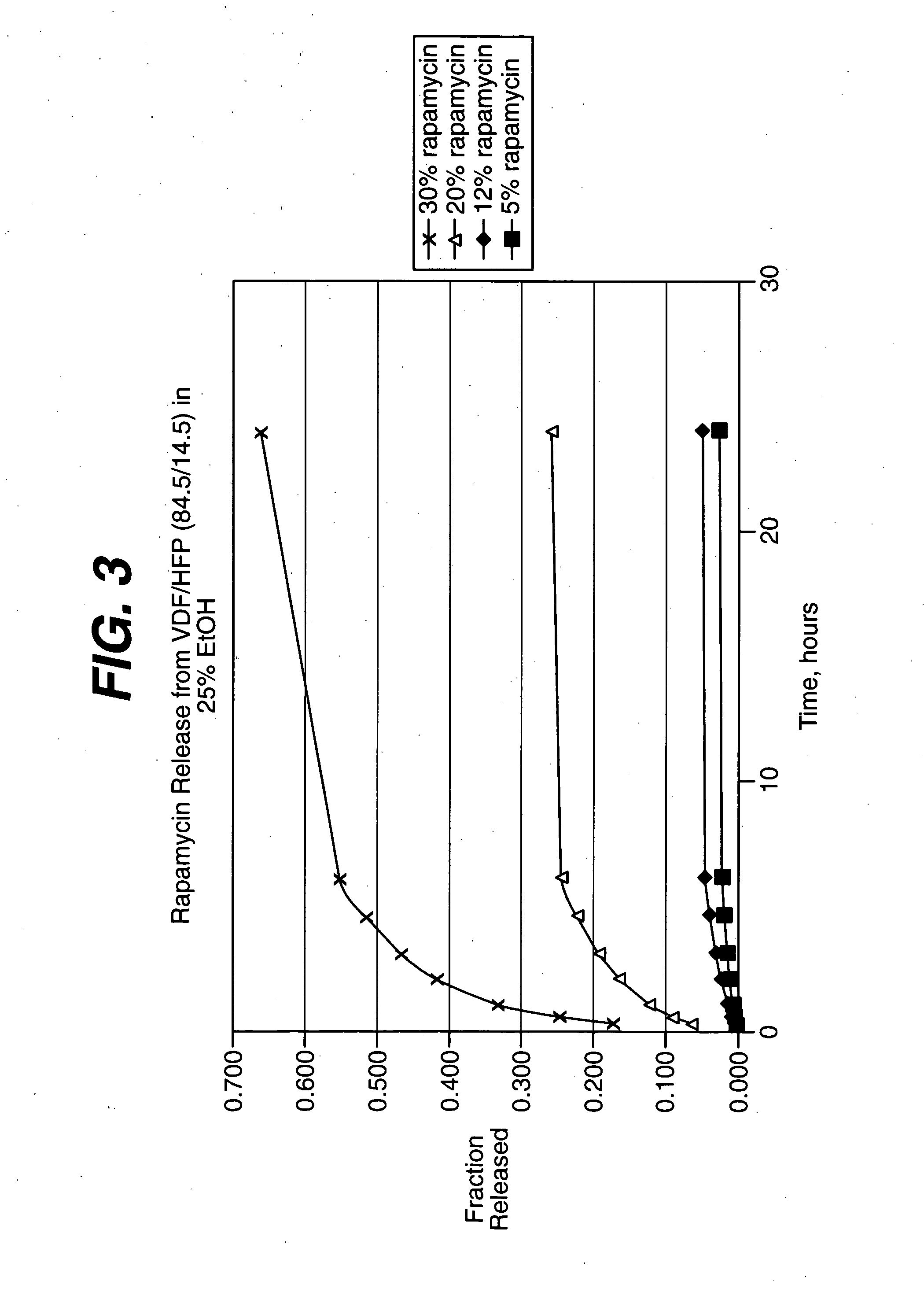
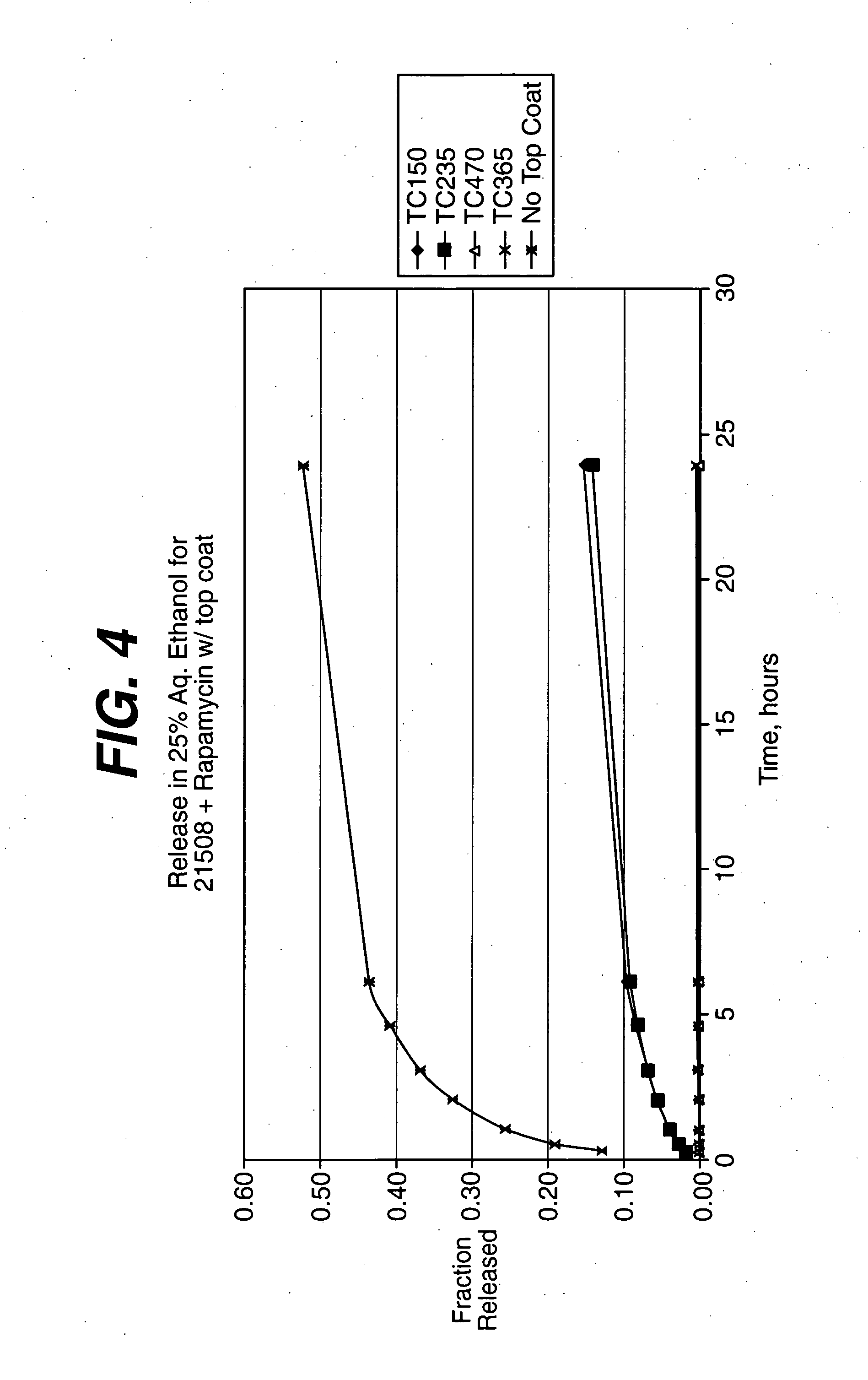
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots. The drugs, agents, and / or compounds may also be utilized to treat specific diseases, including vulnerable plaque. Therapeutic agents may also be delivered to the region of a disease site. In regional delivery, liquid formulations may be desirable to increase the efficacy and deliverability of the particular drug. Also, the devices may be modified to promote endothelialization. Various materials and coating methodologies may be utilized to maintain the drugs, agents or compounds on the medical device until delivered and positioned. In addition, the devices utilized to deliver the implantable medical devices may be modified to reduce the potential for damaging the implantable medical device during deployment. Medical devices include stents, grafts, anastomotic devices, perivascular wraps, sutures and staples. In addition, various polymer combinations may be utilized to control the elution rates of the therapeutic drugs, agents and / or compounds from the implantable medical devices.
Owner:WYETH LLC
Controlled release hydrogel formulation
InactiveUS20110165236A1Controlled release rateBiocideOrganic chemistryControlled releasePharmaceutical drug
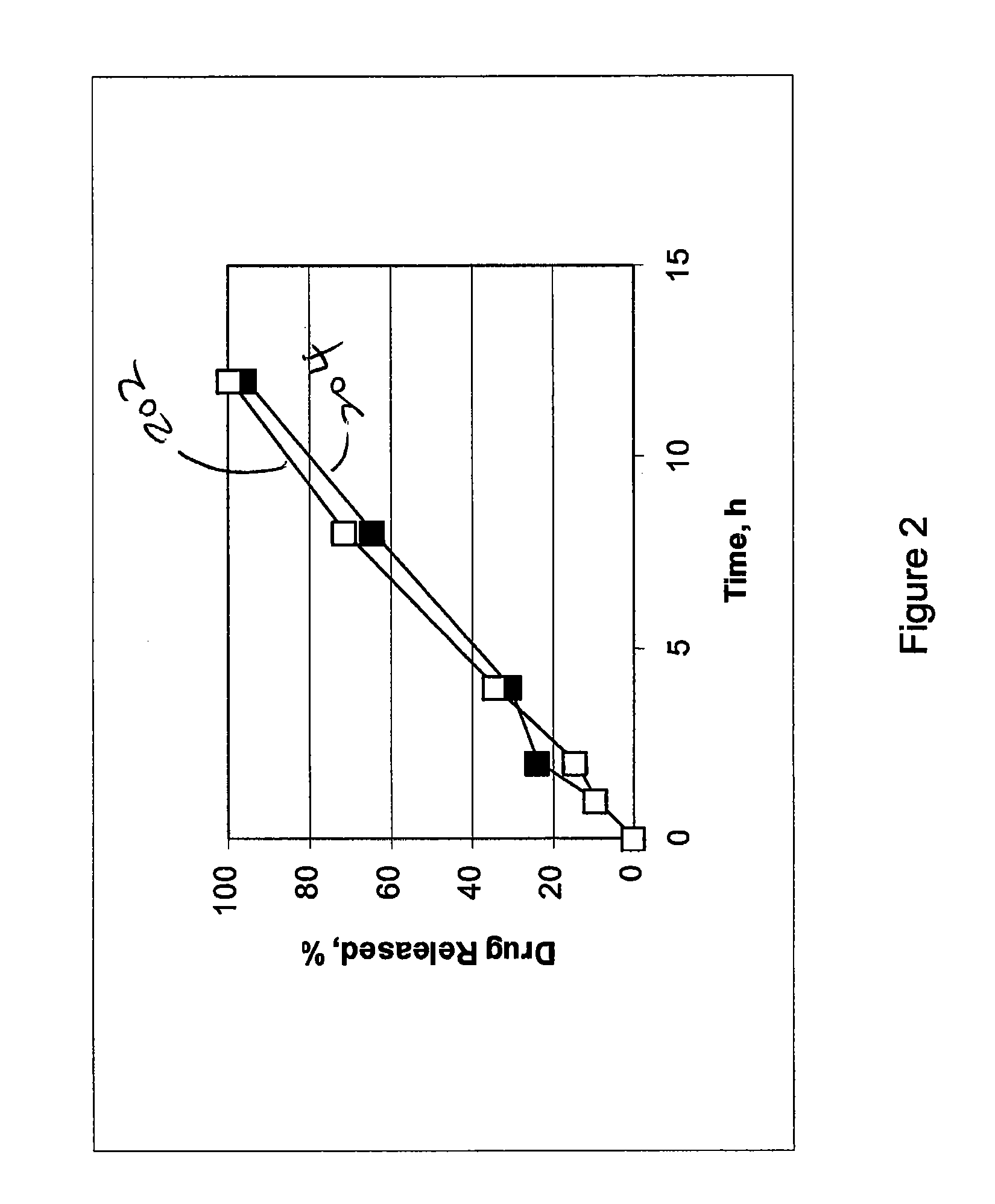
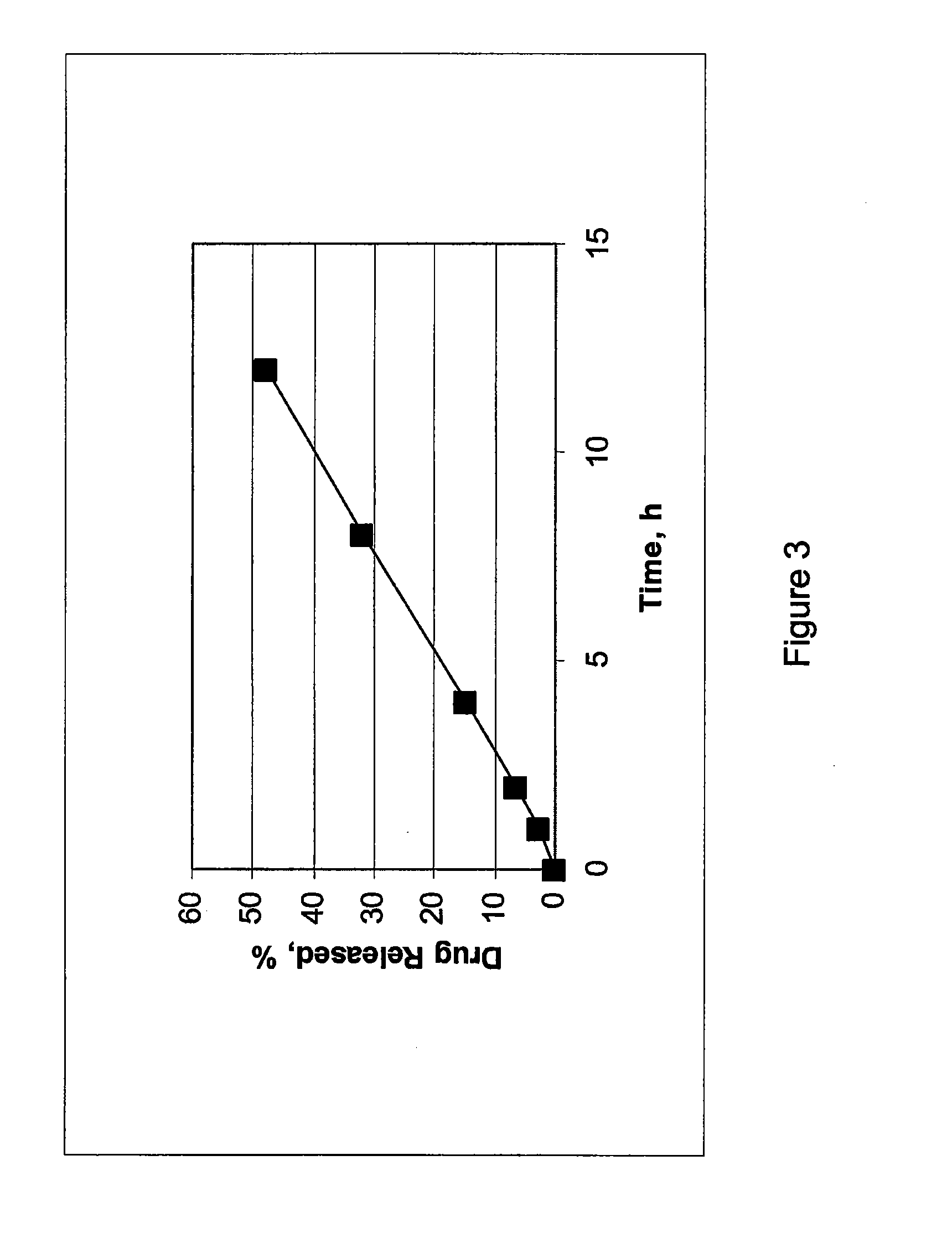
Embodiments of the invention generally provide pharmaceutical drug compositions, methods of preparing oral drug compositions, such as controlled release dosage compositions for hydrophobic active ingredients. In one aspect, the invention provides a pharmaceutical formulation comprising a therapeutically effective amount of a hydrophobic drug, an adjustable ratio of a non-cross linked hydrogel polymer and a non-gelling insoluble polymer. One example is a controlled release pharmaceutical composition which includes 1% to 80% of a therapeutically amount of cilostazol, 4% to 80% of a water-swelling hydrogel polymer, and 4% to 80% of a non-gelling insoluble polymer. In another aspect, a constant release profile of the pharmaceutical formulation is obtained. In another aspect, a zero degree release profile of the pharmaceutical formulation is obtained. Further, a method for treating intermittent claudication using the pharmaceutical formulation is provided.
Owner:BIOKEY
Modified release cilostazol compositions
Pharmaceutical composition comprising a therapeutically effective amount of micronized particles of cilostazol or pharmaceutically acceptable salts or esters thereof, wherein at least about 50% of the micronized cilostazol particles have an effective average particle size of less than about 10 microns is provided.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Nanoparticulate and Controlled Release Compositions Comprising a Platelet Aggregation Inhibitor
InactiveUS20090297596A1Reduces and eliminates developmentQuick releasePowder deliveryBiocideControlled releaseNanoparticle
The present invention provides a composition comprising a platelet aggregation inhibitor, for example, cilostazol, or a salt or derivative thereof, useful in the treatment and prevention of ischemic symptoms. The invention provides a composition which comprises nanoparticulate particles comprising the platelet aggregation inhibitor and at least one surface stabilizer. The nanoparticulate particles have an effective average particle size of less than about 2000 nm. The invention provides also a composition that delivers a platelet aggregation inhibitor, or nanoparticles comprising the same, in a pulsatile or continuous manner.
Owner:ELAN PHRMA INT LTD
Process for production cilostazol
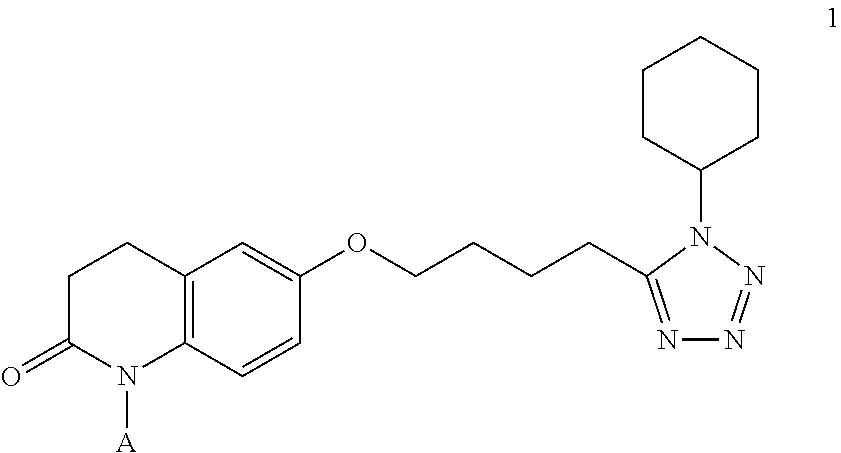
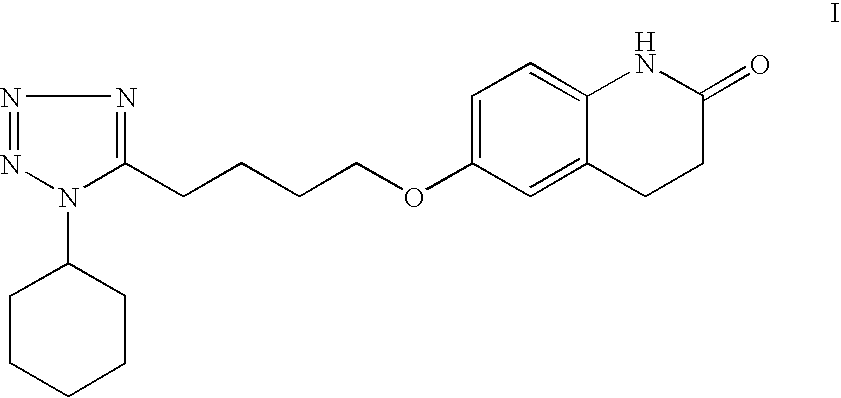
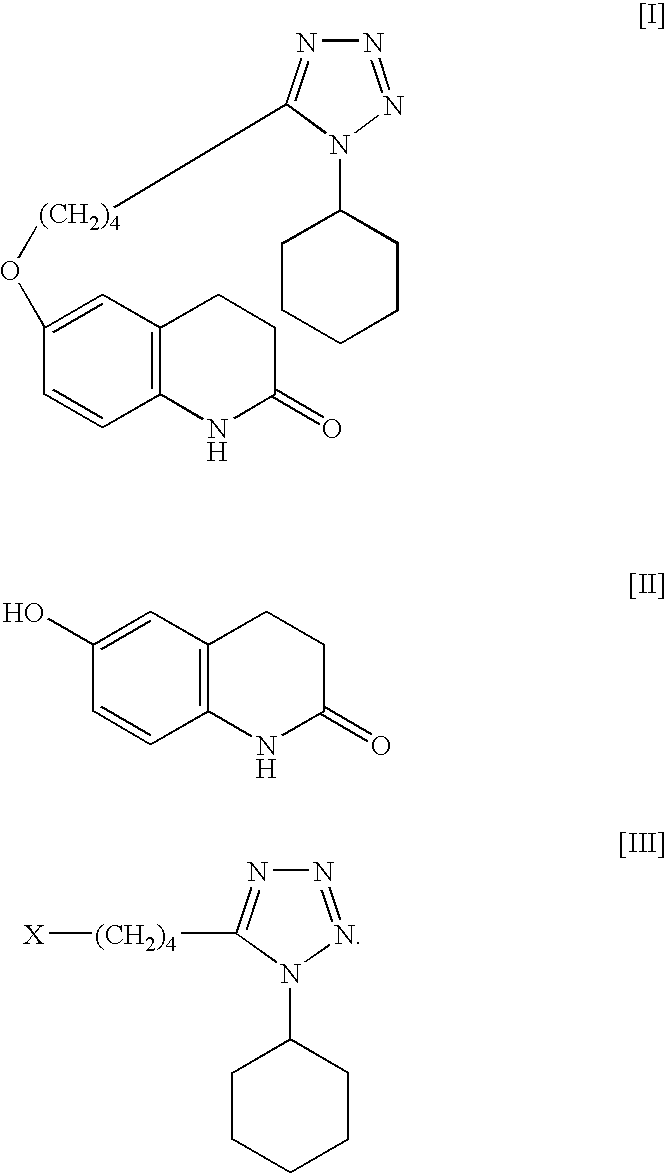
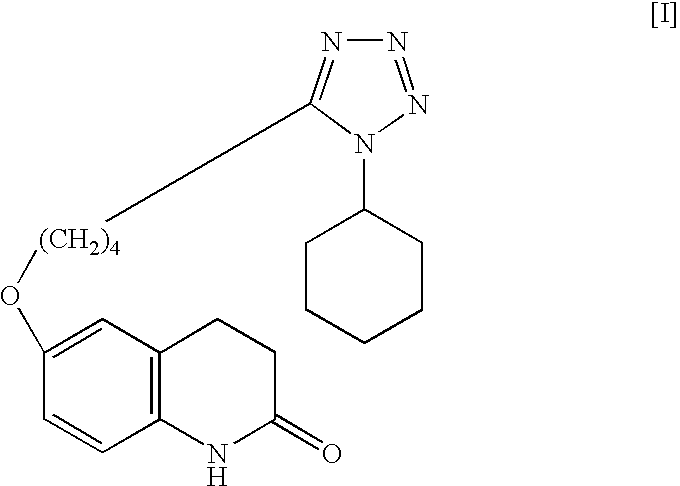
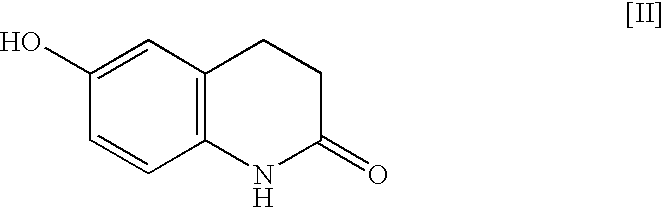
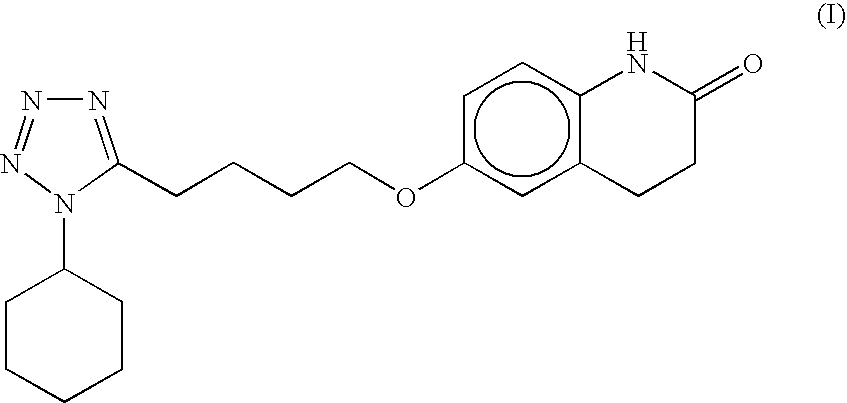
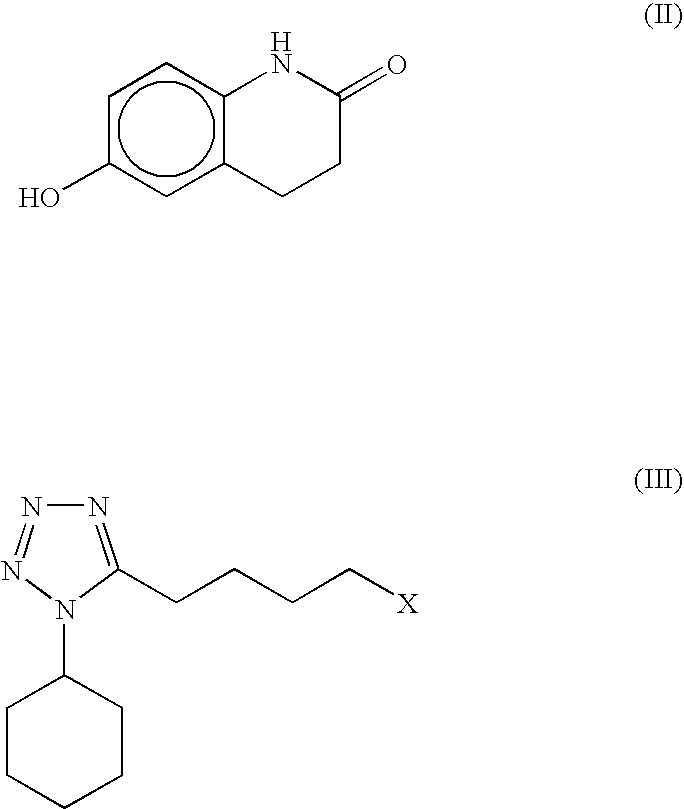
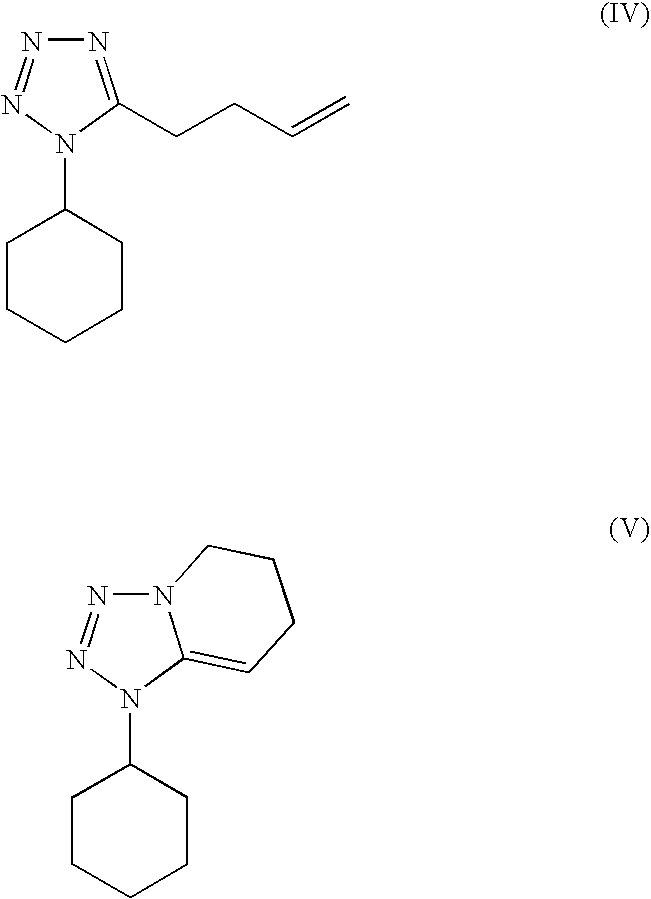
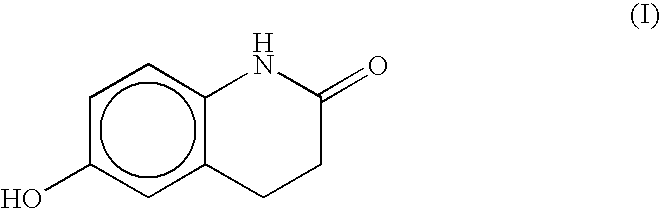
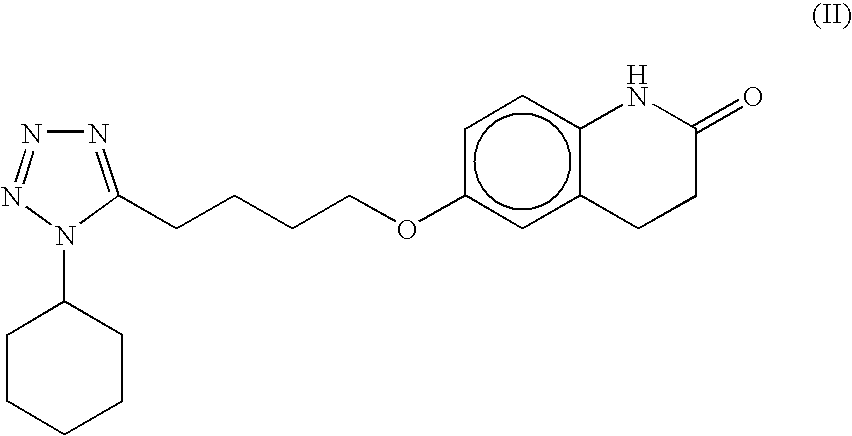
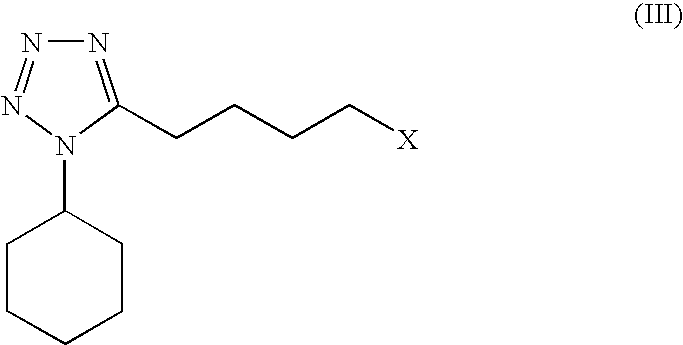
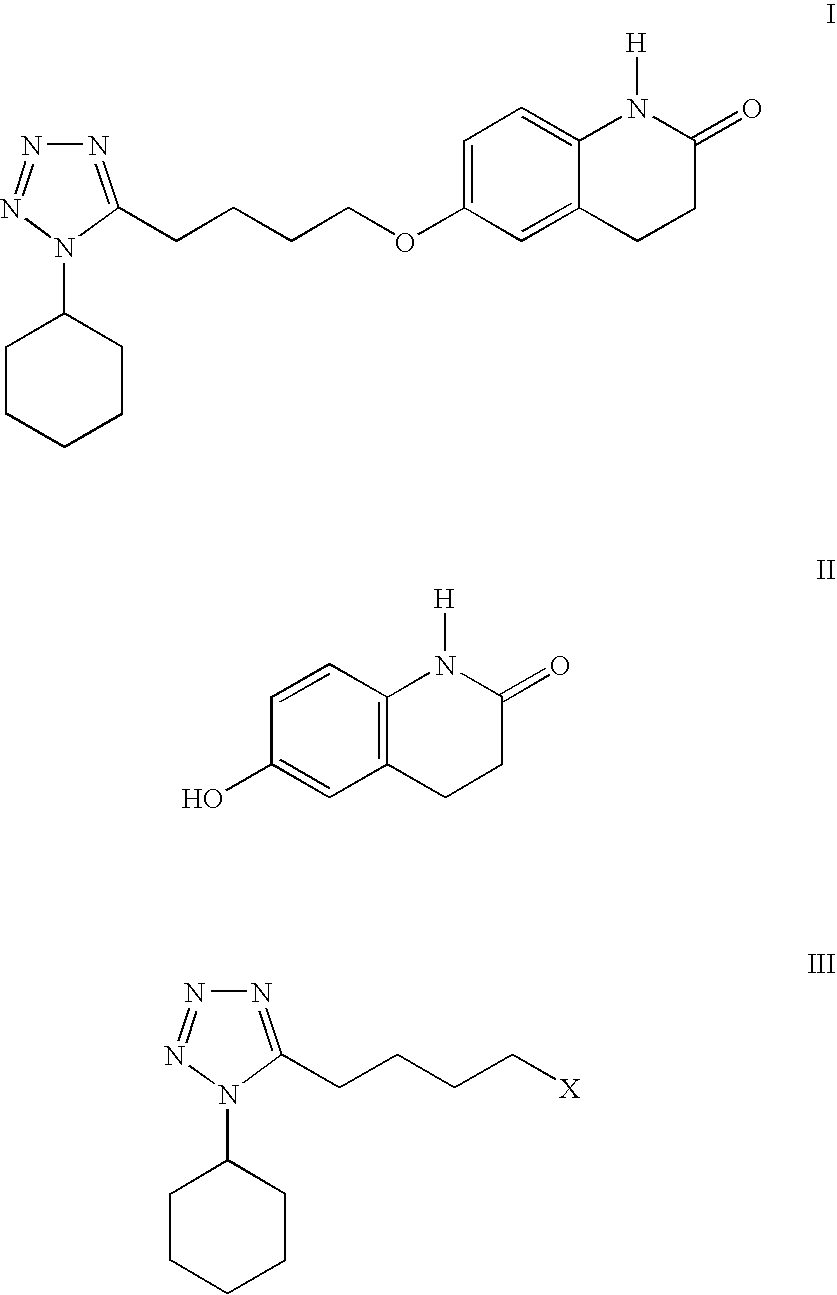
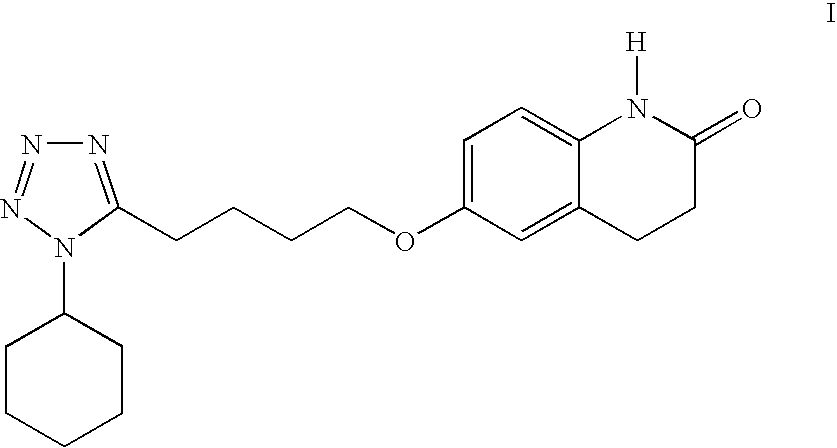
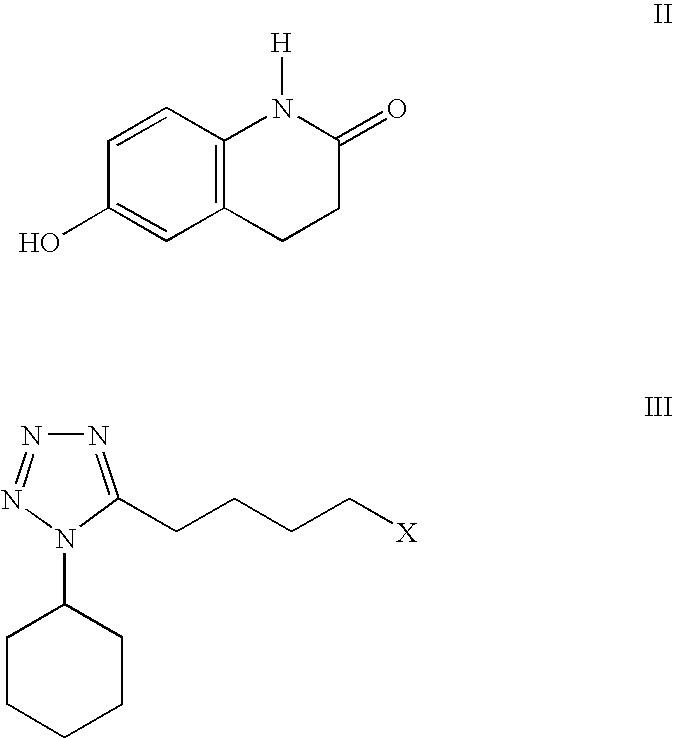
The present invention provides a process for producing cilostazol [I] in a high yield and a high purity, by reacting a carbostyril derivative [II] with a tetrazole derivative [III] in the presence of an inorganic basic compound in a solvent of water, wherein water is used in an amount of 3 to 7-fold weight to that of the carbostyril derivative [II] and the inorganic basic compound is used in an amount of 1 to 6 mol per mol of the carbostyril derivative [II]. The process of the present invention is the improved and environment-friendly process for producing cilostazol being useful for pharmaceuticals
Owner:OTSUKA PHARM CO LTD
Substantially pure cilostazol and processing for making same
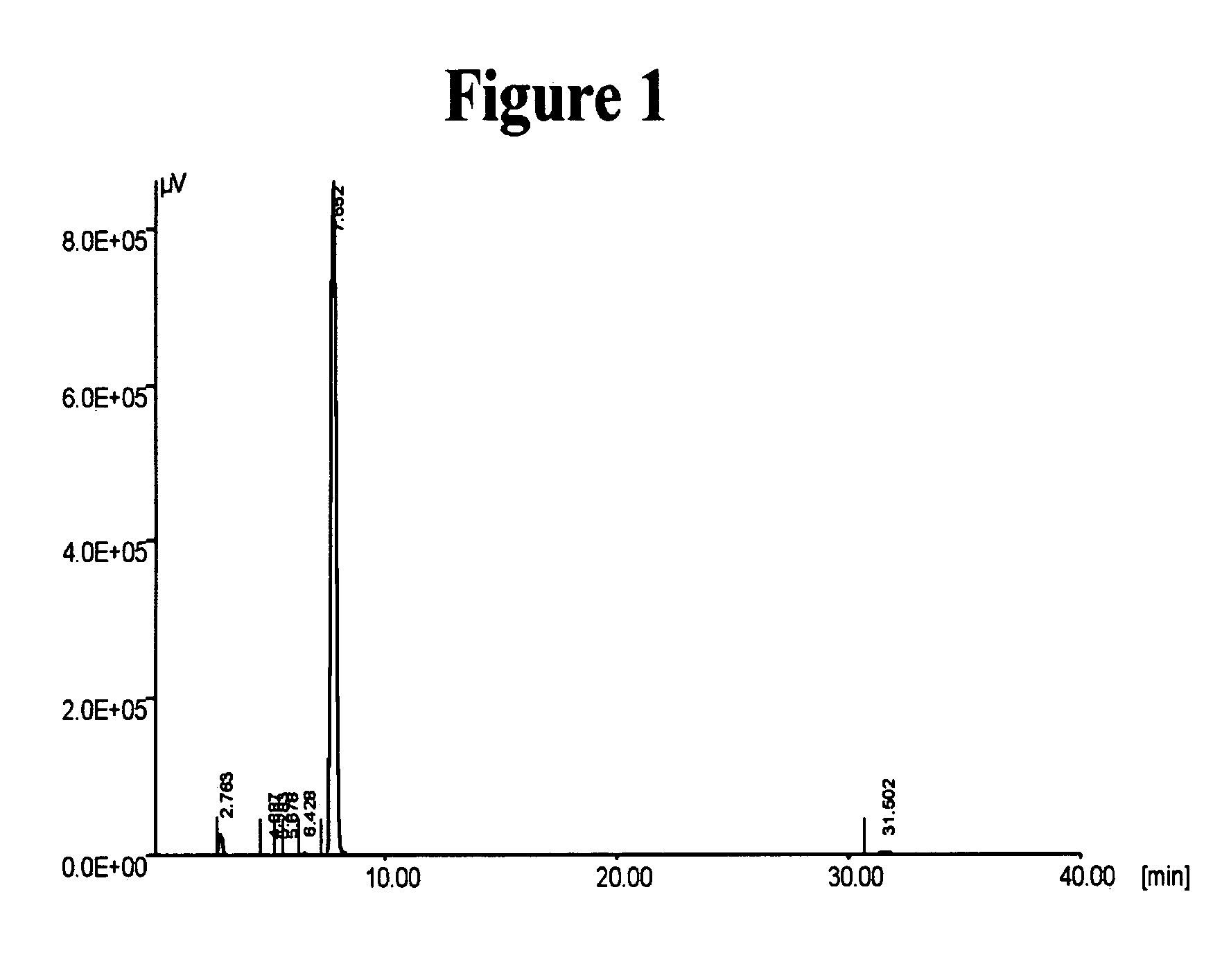
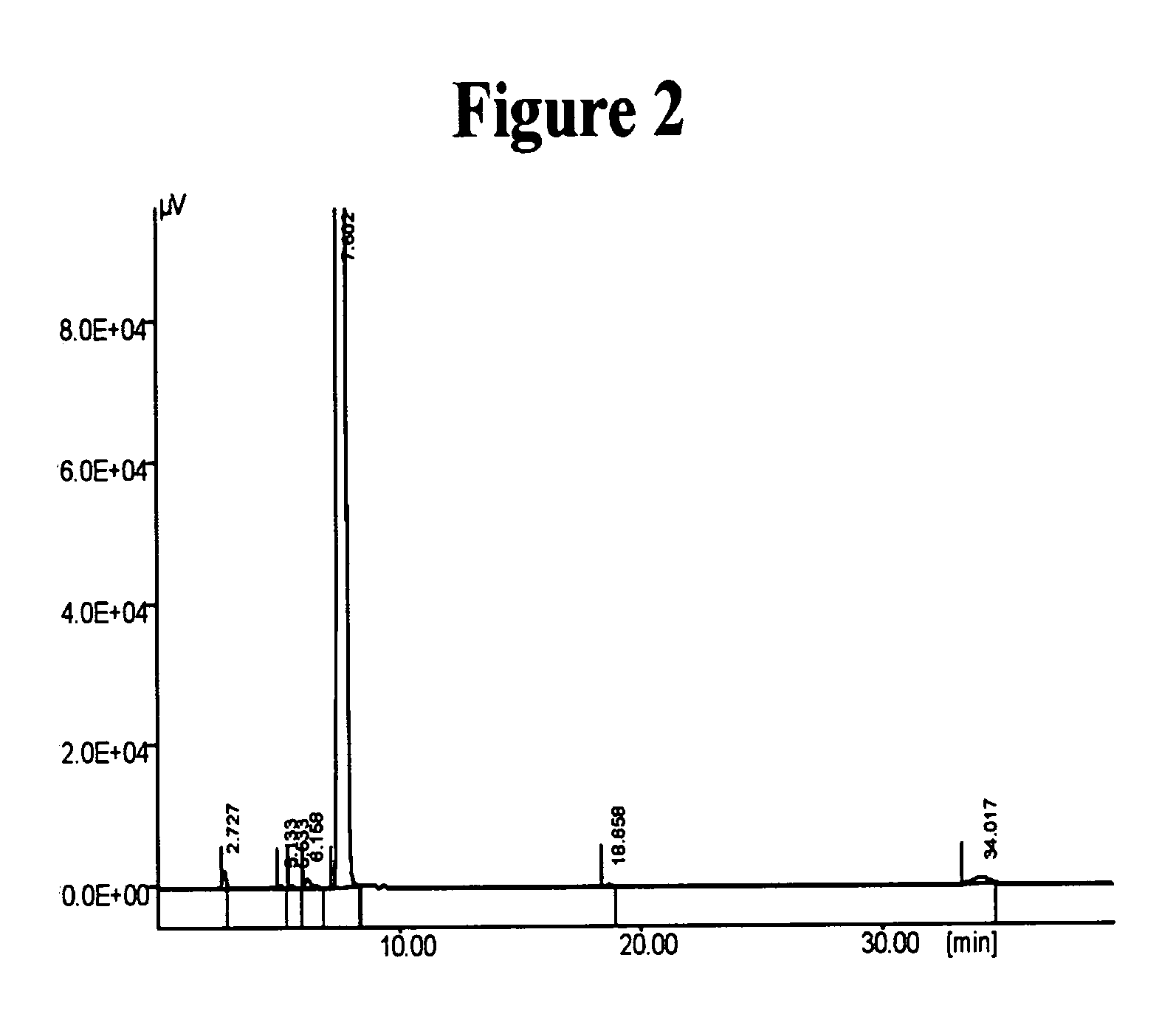
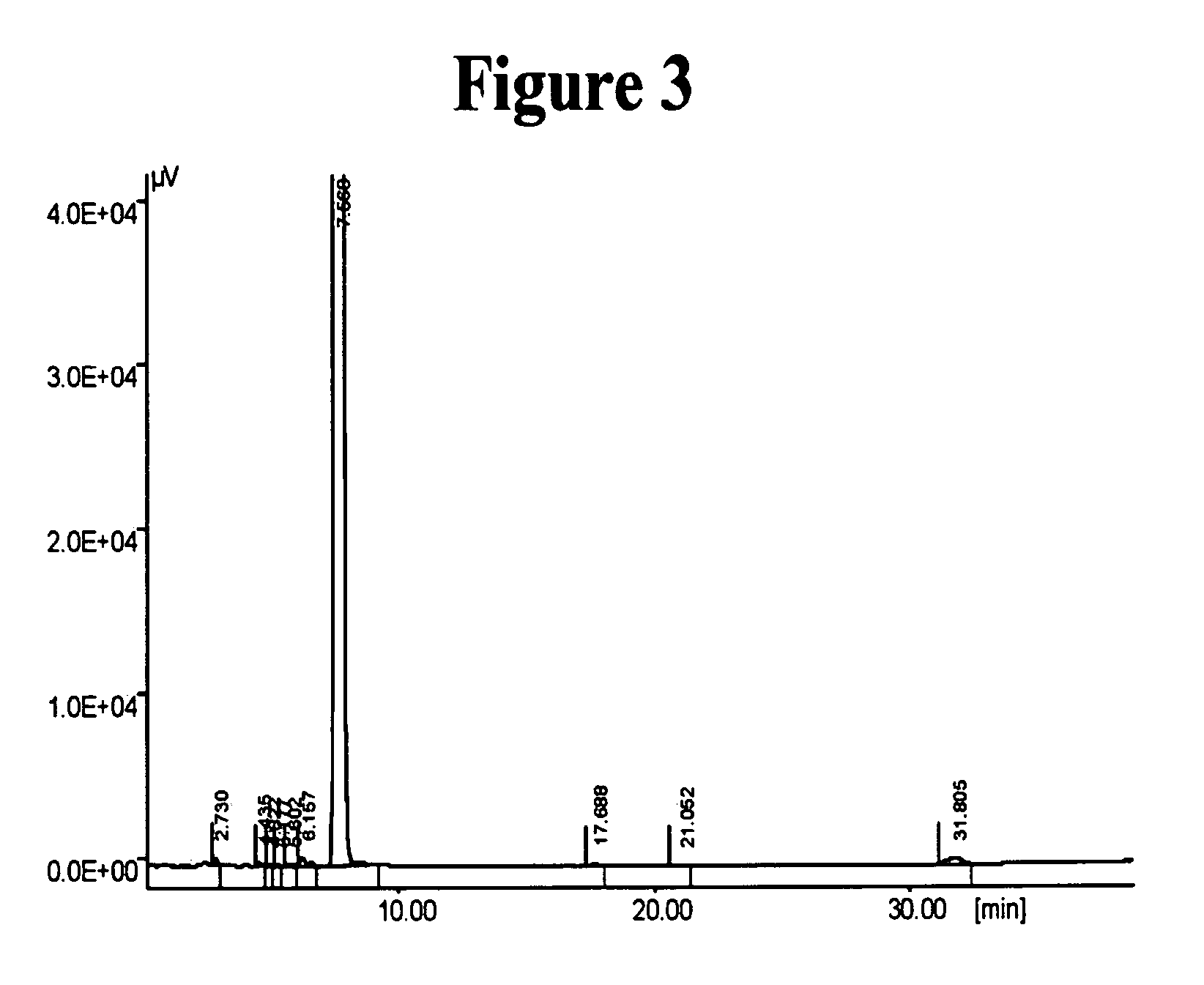
InactiveUS20050065343A1Minimizes decompositionIncrease productionBiocideOrganic compound preparationAnalytical chemistryCilostazol
The present invention provides substantially pure cilostazol. The present invention also provides cilostazol particles that have reduced particle size.
Owner:TEVA PHARMA IND LTD
Nanoparticulate and controlled release compositions comprising a platelet aggregation inhibitor
InactiveCN101287451AImprove conveniencePrevention and treatment of ischemic symptomsBiocideNanomedicineControlled releaseNanoparticle
The present invention provides a composition comprising a platelet aggregation inhibitor, for example, cilostazol, or a salt or derivative thereof, useful in the treatment and prevention of ischemic symptoms. The invention provides a composition which comprises nanoparticulate particles comprising the platelet aggregation inhibitor and at least one surface stabilizer. The nanoparticulate particles have an effective average particle size of less than about 2000 nm. The invention provides also a composition that delivers a platelet aggregation inhibitor, or nanoparticles comprising the same, in a pulsatile or continuous manner.
Owner:ELAN PHRMA INT LTD
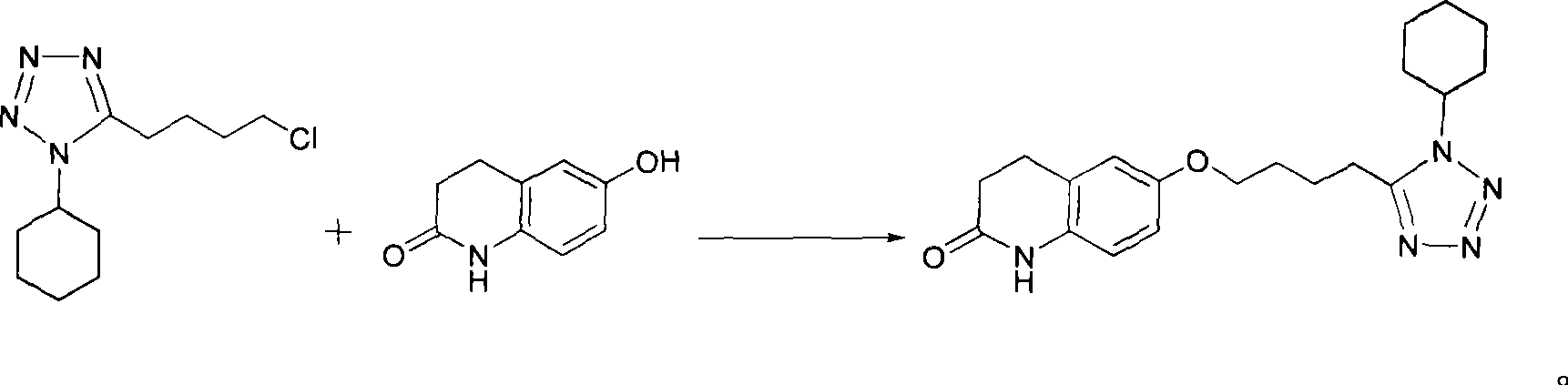
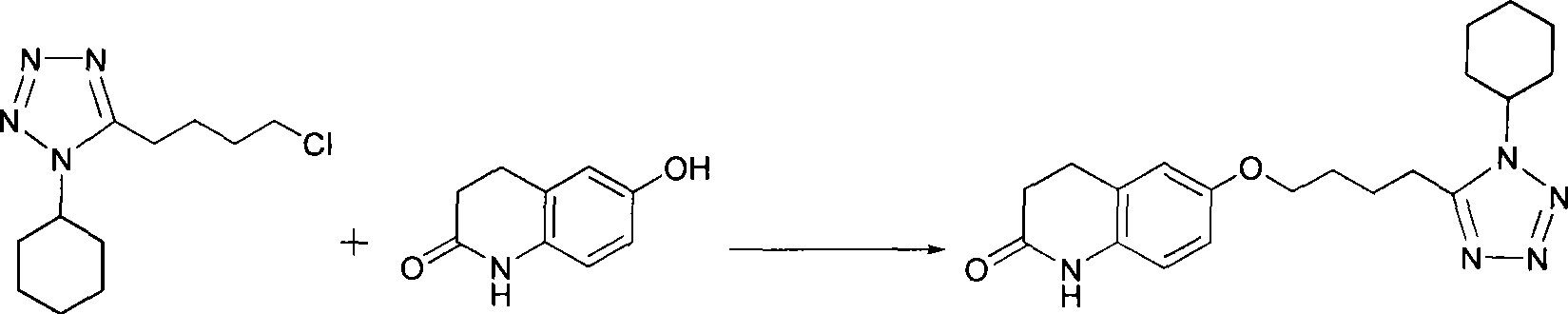
Preparation of cilostazol
The invention discloses a preparation method of cilostazol. 1-cyclohexyl-5-(4-chlorobutyl)-1H-tetrazolium and 6-hydroxy-3, 4-dihydroquinolone is subjected to substitution reaction with the existence of an inorganic base catalyst and an alcohols solvent to prepare the cilostazol; the preparation method has products with high yield and good quality, avoids the application of toxical reagents and expensive reagents, has little environmental pollution and low production cost, and is suitable for industrialized production.
Owner:CHONGQING KANGLE PHARMA
Methods for enhancing the release and absorption of water insoluble active agents
InactiveUS8524280B2Improved profilePromote absorptionBiocidePowder deliveryHydrophilic polymersActive agent
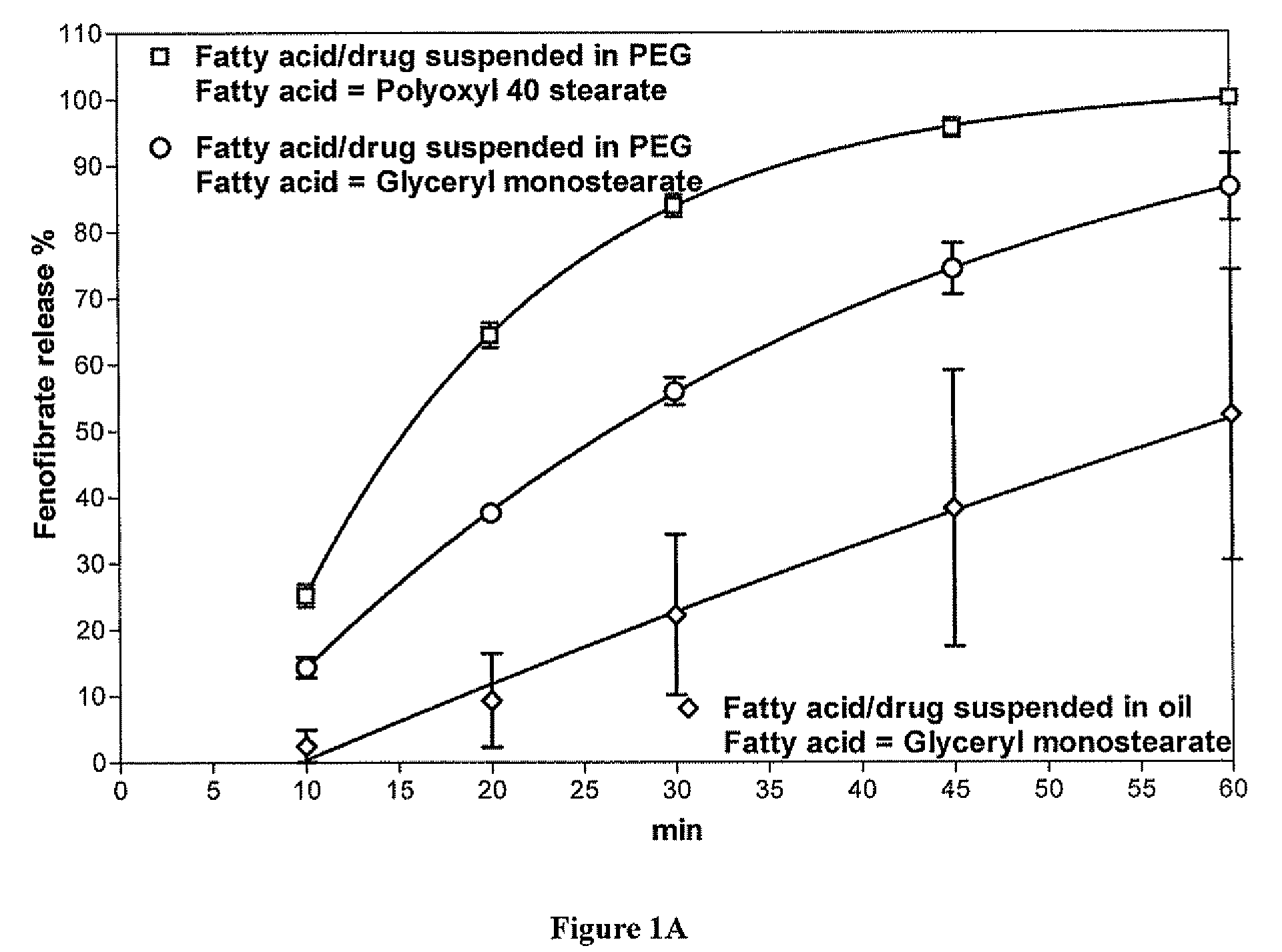
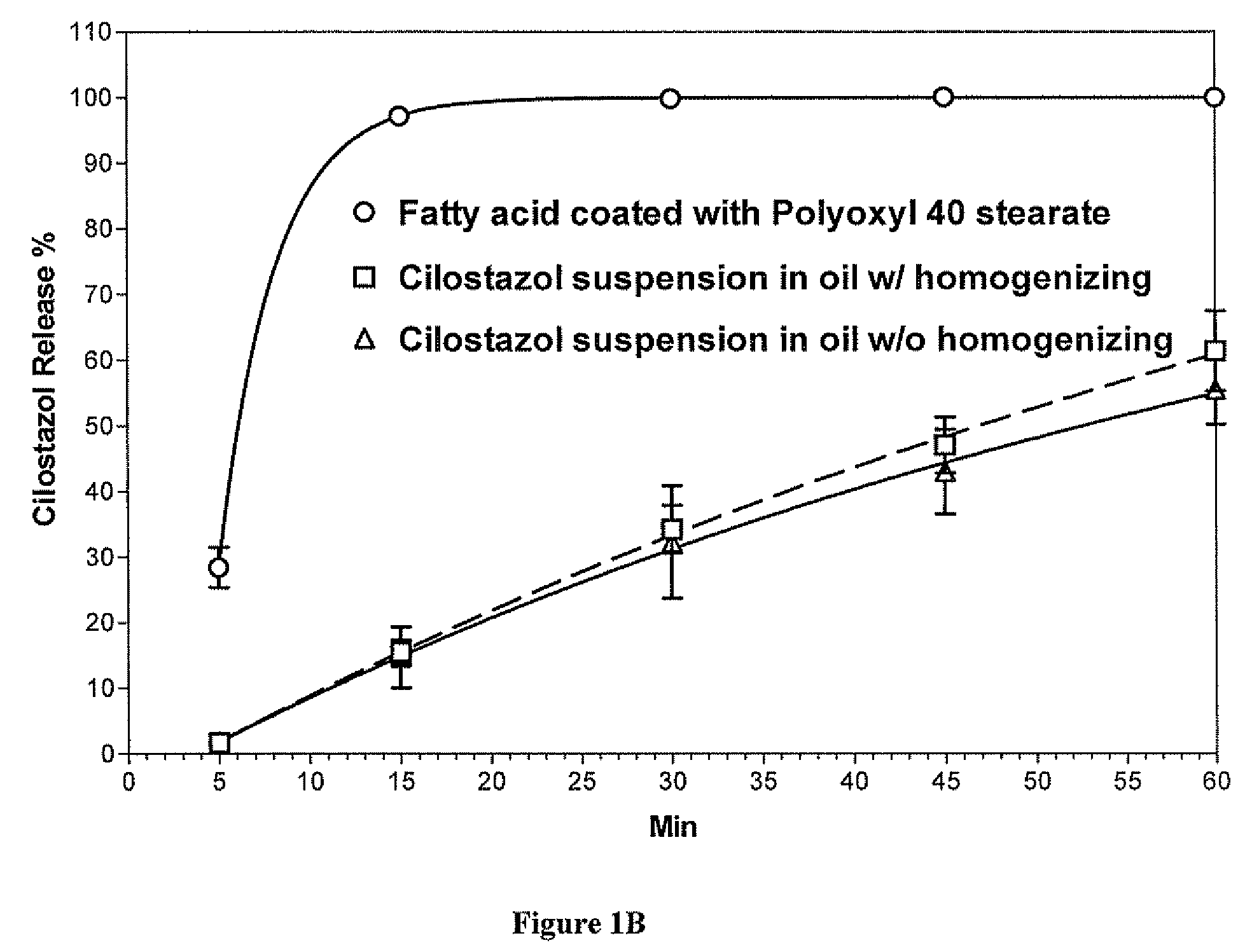
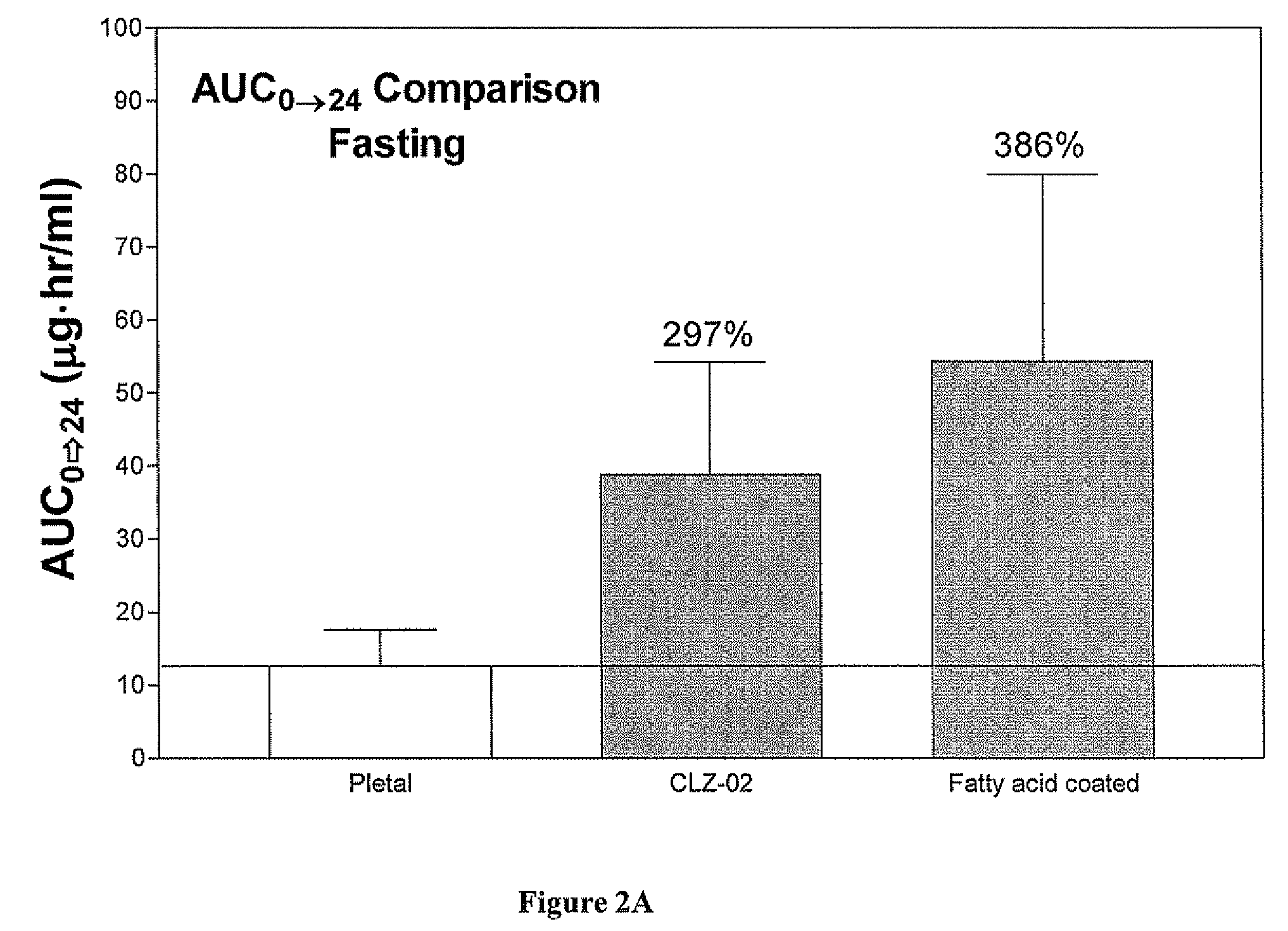
Methods for enhancing the release and / or absorption of poorly water soluble active agents are described herein. The method involves dissolving, melting, or suspending a poorly water soluble active agent in one or more molten fatty acids, conjugated fatty acids, (semi-) solid surfactants of high HLB value, and / or hydrophilic polymers. The molten active agent mixture is then suspended and homogenized in a hydrophilic or lipophilic carrier to form microparticles suspended in the hydrophilic or lipophilic carrier. The particles suspended in the hydrophilic or lipophilic carrier can be encapsulated in a hard or soft gelatin or non-gelatin capsule. It is believed that the microparticles produced by the method described above will exhibit enhanced dissolution profiles. In vitro release studies of formulations containing cilostazol and fenofibrate showed 100% dissolution of cilostazol in 15 minutes and over 90% dissolution of fenofibrate in 35 minutes.
Owner:PATHEON SOFTGELS INC
Method of producing drug-containing wax matrix particles, extruder to be used in the method and sustained-release preparation containing cilostazol
InactiveCN101340882AEasy to manufactureNon-blockingPowder deliveryOrganic non-active ingredientsSpray nozzleFailure causes
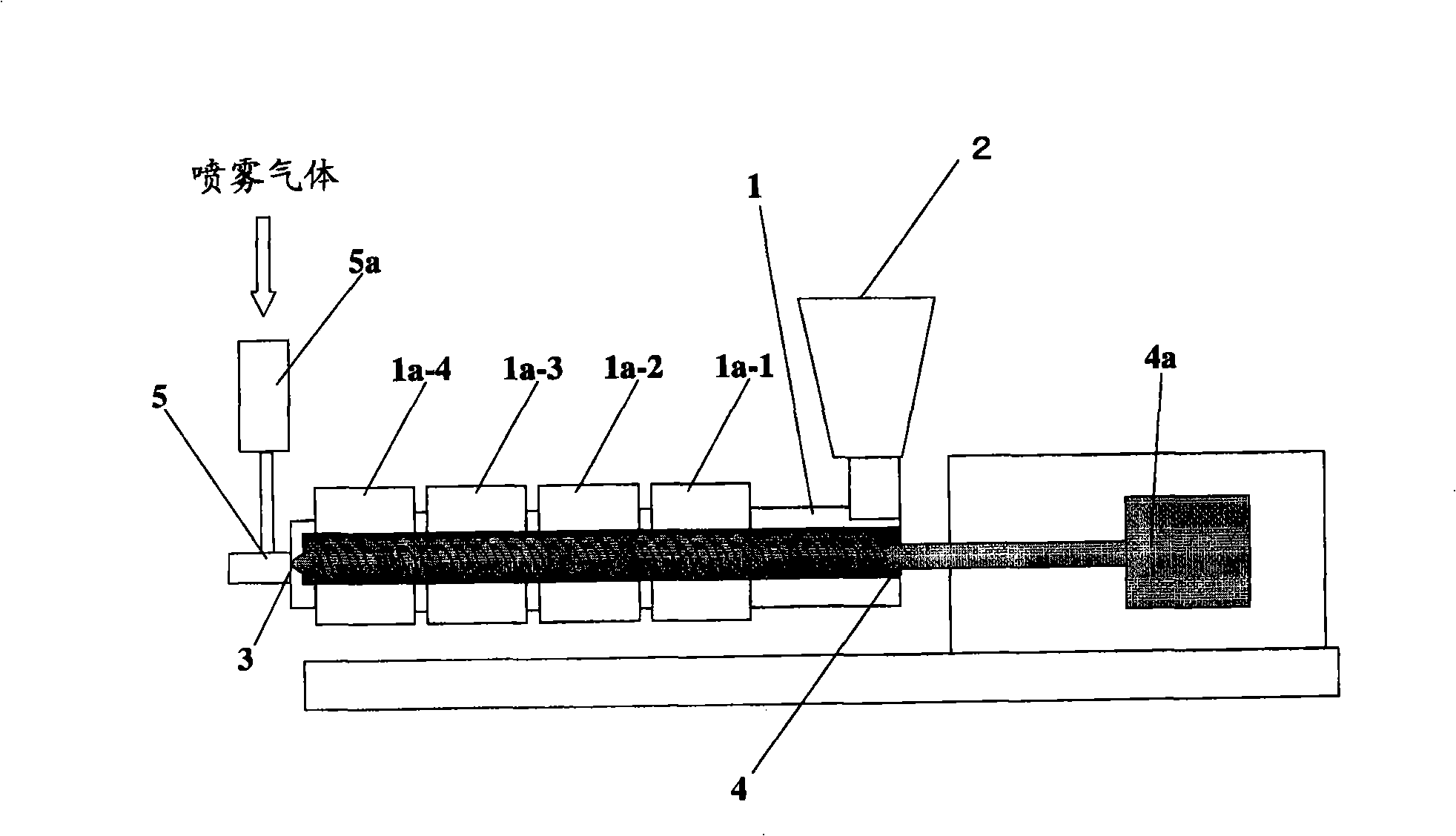
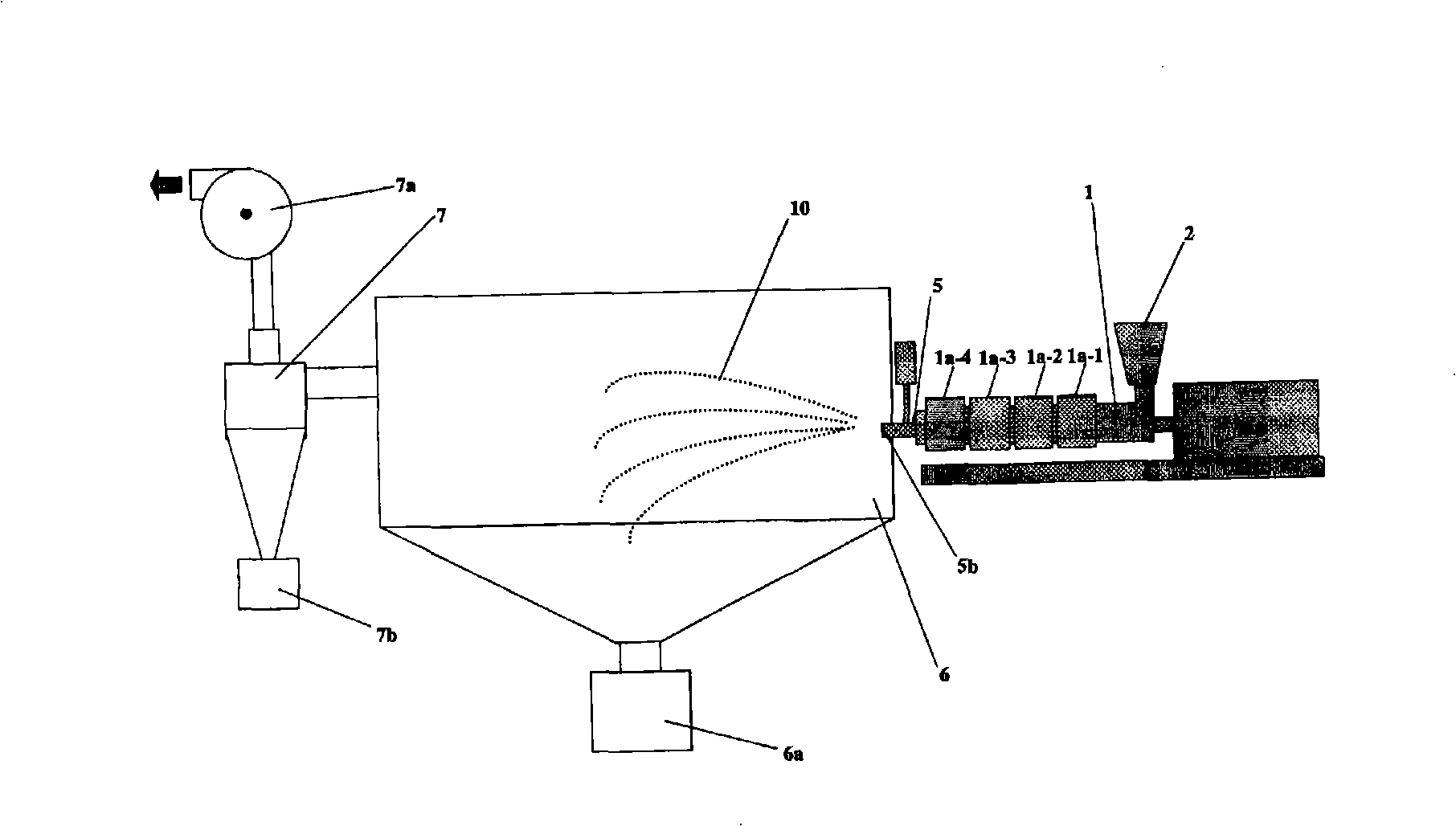
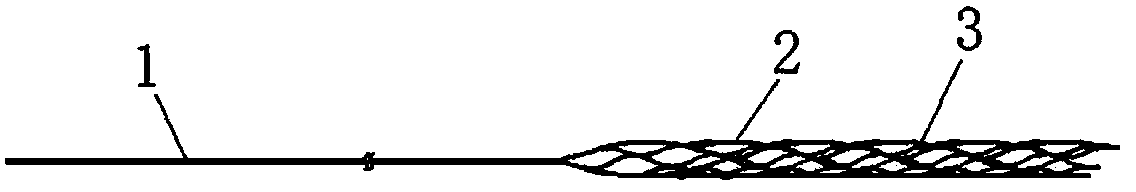
It is intended to provide a method of conveniently producing drug-containing wax matrix particles (in particular, drug-containing wax matrix particles having an average particle diameter of 1 mm or less) through melting and spraying steps without suffering from a jamming failure caused by the recrystallization of the once molten drug. Namely, drug-containing wax matrix particles containing a drug and a wax are produced via the following steps (i) and (ii): (i) the step of feeding the drug and wax as described above into an extruder wherein the temperatures of a barrel (1) and a die have been controlled to a level higher than the melting point of the wax; and (ii) the step of melt-kneading the drug with the wax in the extruder as described above and simultaneously spraying out the melt-kneaded mixture comprising the drug and the wax from a spray nozzle (5), which is attached directly to a die (3) provided at the tip of the barrel (1) in the extruder, into an atmosphere at a temperature lower than the melting point of the wax to thereby form particles.
Owner:OTSUKA PHARM CO LTD
Bi-functional antiplatelet aggregation medicine and application thereof
The invention, belonging to the technical field of medicine, relates to a bi-functional anti-platelet aggregation medicine and an application of the medicine in preventing and treating arterial tlirombotic diseases. The medicine of the invention has both a P2Y12 receptor antagonist property and a phosphodiesterase inhibitory activity. The results of in vivo and in vitro anti-platelet aggregation activity experiments of the medicine provide the anti-platelet mechanism of the medicine, the structural formula and anti-platelet mechanism of the medicine are different from those of known anti-platelet medicines, aspirin, Clopidogrel, Prasugrel, Cilostazol, and platelet and fibrinogen receptor antagonist. The medicine of the invention has good inhibition effect to various platelet aggregation induced by agonists. The results of mice in vivo experiment models of arterial thrombosis show that the medicine of the invention has significantly similar antithrombotic activity with Clopidogrel and non-obvious side effect of hemorrhage. The medicine of the invention can be used as antithrombotic medications for treating coronary heart disease, apoplexy and other arterial tlirombotic diseases.
Owner:FUDAN UNIV
Pharmaceutical Compositions Comprising A Multifunctional Phosphodiesterase Inhibitor and An Adenosine Uptake Inhibitor
InactiveUS20080200484A1Limiting positive inotropic effectGood effectBiocideMuscular disorderAdenosine Deaminase InhibitorDipyridamole
Owner:OTSUKA PHARM CO LTD
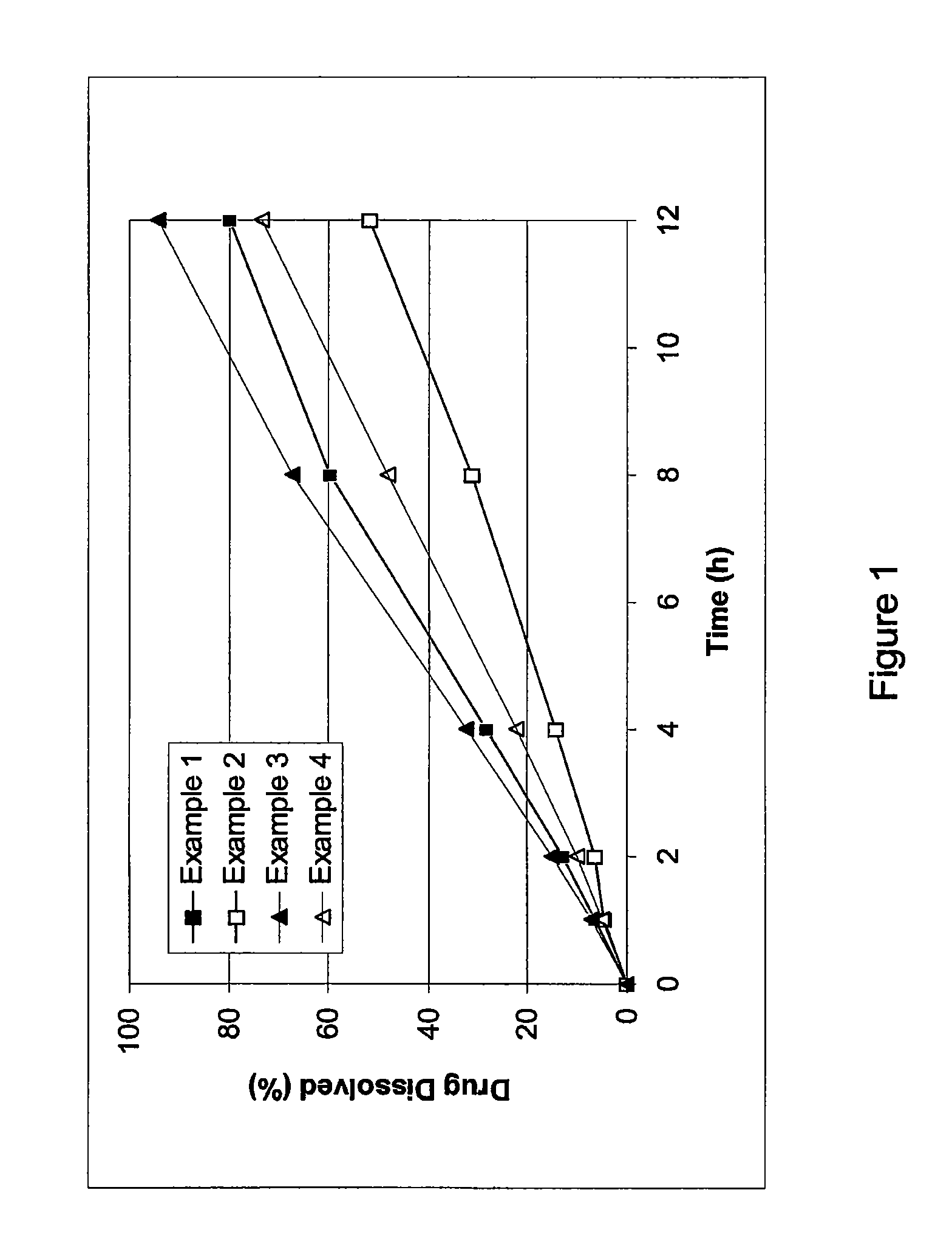
Slow release preparation of cilostazol
InactiveCN101006990AImprove securityImprove effectivenessOrganic active ingredientsInorganic non-active ingredientsBlood concentrationCurative effect
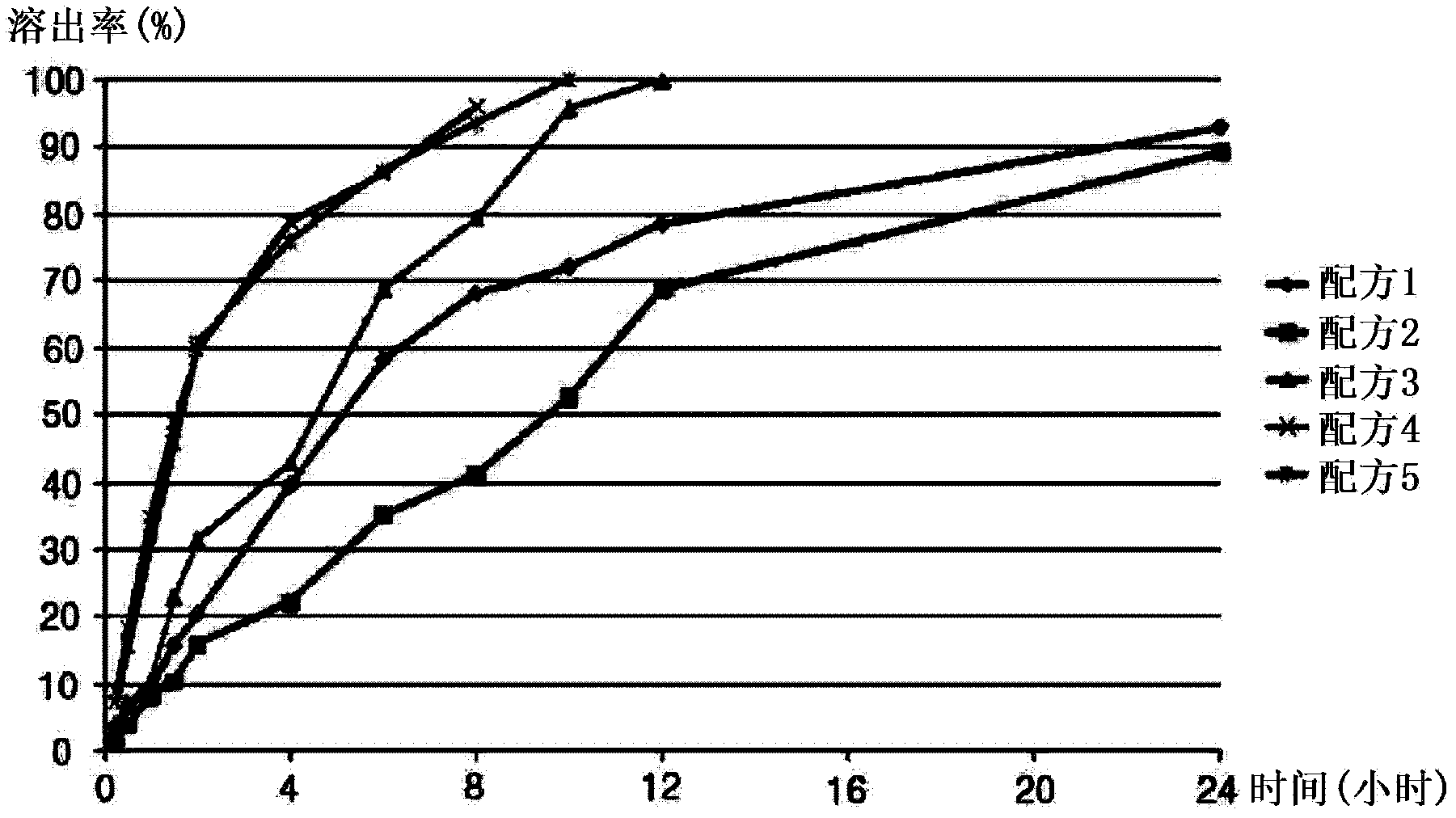
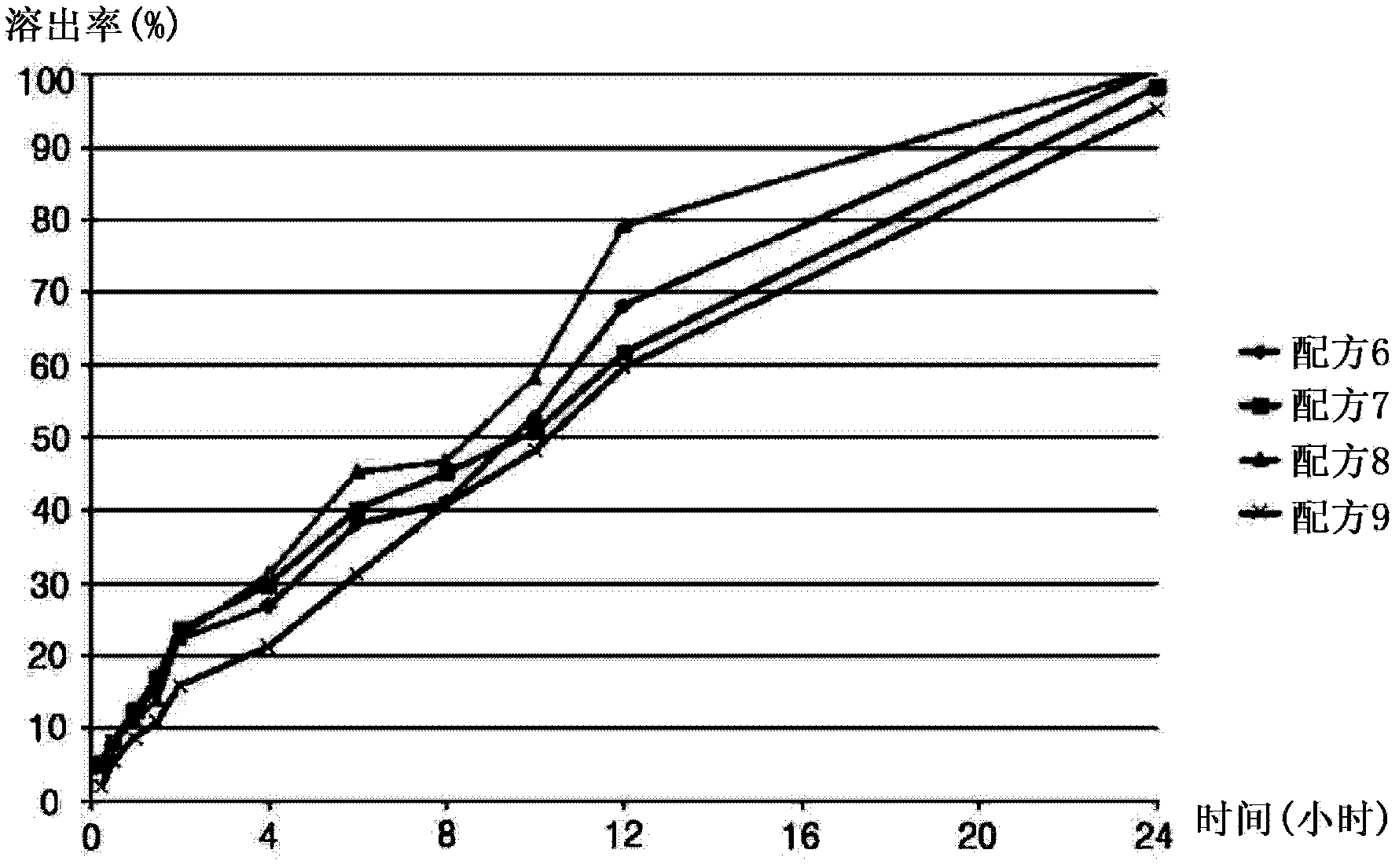
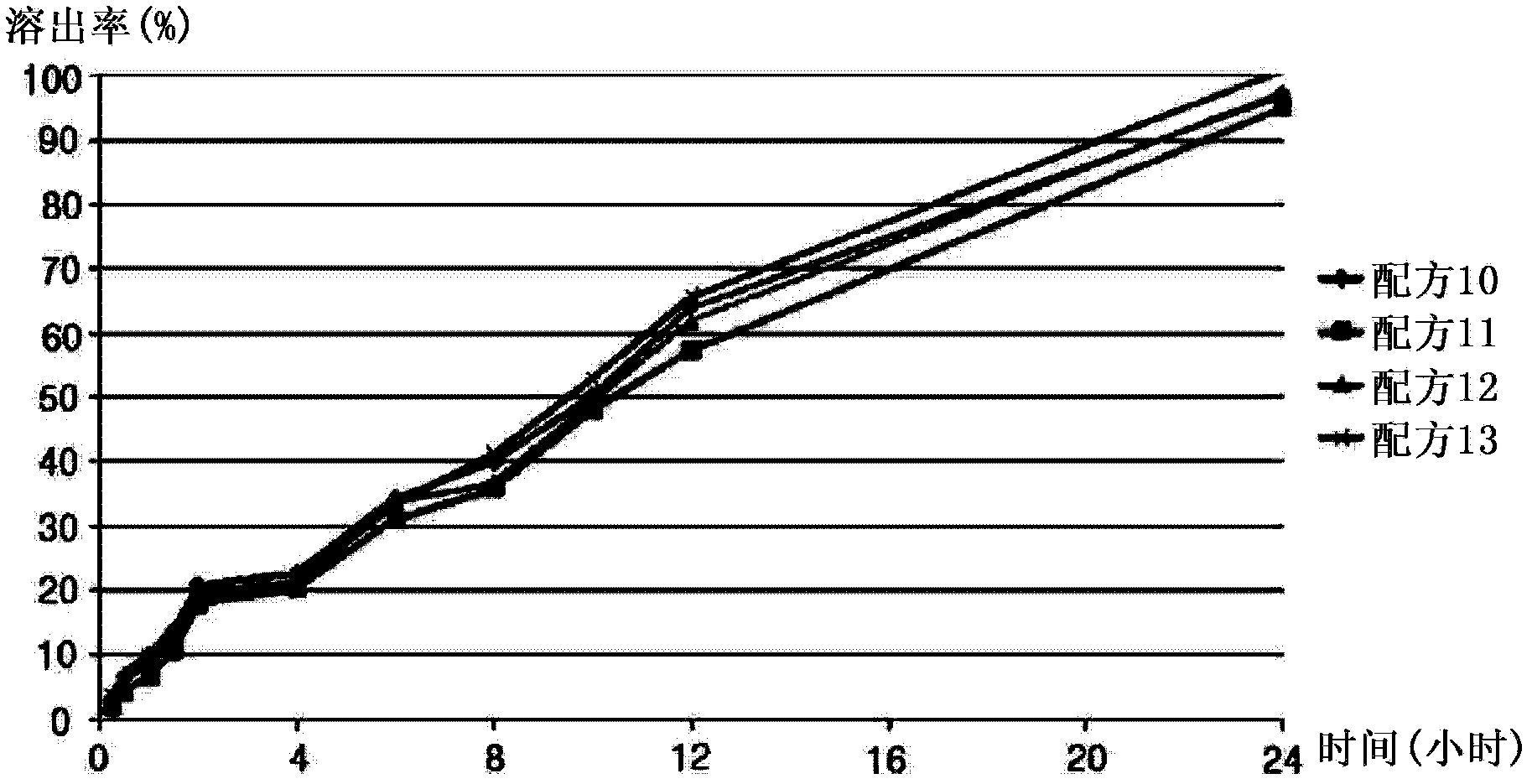
The invention discloses a slow release preparation of cilostazol and its preparing process, wherein the raw materials of the preparation include cilostazol of a predetermined proportion, slow release matrix material and medicinal material, the preparation can be prepared into solid dispersing agent, wherein the medicament can be released slowly and continuously after being administrated, the effective concentration in blood can be maintained, and long action can be achieved. The advantages of the invention include decreased frequency of medicinal administration, improved patient's adaptability, lowered blood concentration peak-valley, increased medicinal effect and safety, and reduced total medicinal dose, thereby optimum curative effect can be achieved through minimum dose, thus the preparation is more suitable for patients.
Owner:刘凤鸣
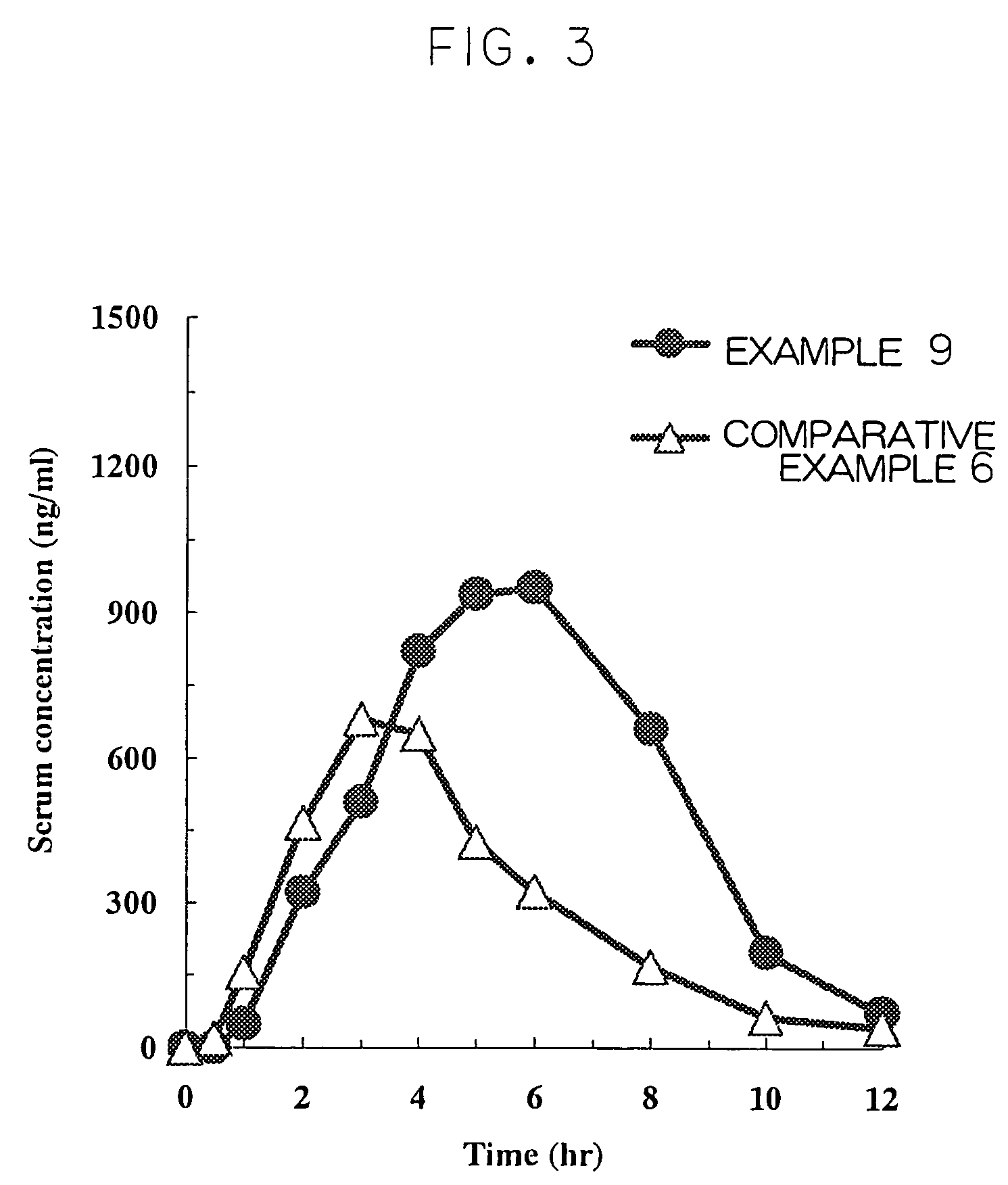
Controlled-release preparation containing cilostazol and process for the preparation thereof
InactiveCN101636152APromote absorptionImprove complianceAntipyreticAnalgesicsSmall intestineControlled-Release Formulations
The present invention relates to a controlled-release formulation comprising cilostazol and a method for preparing said formultion. The inventive controlled-release formulation comprising cilostazol or a pharmaceutically acceptable salt thereof, a solubilizing agent, a swelling agent, a swell-controlling agent and a gas generating material has advantages in that it maintains a constant cilostazol level in the blood through a slow release while it resides in the stomach and intestines over a long period of time, thereby increasing the absorption of cilostazol in the small intestine, the major absorption site of cilostazol, as well as minimizing adverse effects caused by rapid release and making it easy for a patient to take the drug.
Owner:AMOREPACIFIC CORP +1
Local administration of a combination of rapamycin and cilostazol for the treatment of vascular disease
ActiveUS8784860B2Improve storage and shelf lifeIncrease concentrationBiocideStentsBuccal administrationMedical treatment
Medical devices, and in particular implantable medical devices, may be coated to minimize or substantially eliminate a biological organism's reaction to the introduction of the medical device to the organism. The medical devices may be coated with any number of biocompatible materials. Therapeutic drugs, agents or compounds may be mixed with the biocompatible materials and affixed to at least a portion of the medical device. These therapeutic drugs, agents or compounds may also further reduce a biological organism's reaction to the introduction of the medical device to the organism. In addition, these therapeutic drugs, agents and / or compounds may be utilized to promote healing, including the formation of blood clots.
Owner:WYETH LLC
Highly pure cilostazol and an improved process for obtaining same
InactiveUS20050222202A1Easy to operateImprove efficiencyBiocideOrganic chemistry1H-tetrazoleCilostazol
A novel process for preparing highly pure cilostazol, effected by reacting 6-hydroxy-3,4-dihydroquinolinone and 5-(4-chlorobutyl)-1-cyclohexyl-1H-tetrazole in the presence of a hydrated inorganic base, is disclosed. Further disclosed is highly pure cilostazol, and particularly highly pure cilostazol that is substantially free of 6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)-butoxy]-1-[4-(1-cyclohexyl-1H-tetrazol-5-yl)-butyl]-3,4-dihydro-1H-quinolin-2-one.
Owner:CHEMAGIS
Sustained release preparation containing cilostazol and its preparation method
The invention belongs to the field of pharmaceutical preparation, and relates to a slow release preparation of Cilostazol, which comprises Cilostazol 35-60%, slow release material 10-60% and balancing medicinal adjuvant.
Owner:SHENZHEN XINGYIN PHARML
Processes for preparing 6-hydroxy-3,4-dihydroquinolinone, cilostazol and N-(4-methoxyphenyl)-3-chloropropionamide
InactiveUS6967209B2Quick responseHigh yieldBiocideOrganic compound preparationAlkyl transferBoiling point
A process for preparing 6-hydroxy-3,4-dihydroquinolinone by intramolecular Friedel-Crafts alkylation of N-(4-methoxyphenyl)-3-chloropropionamide in which an equivalent of N-(4-methoxyphenyl)-3-chloropropionamide is contacted with a Lewis acid in DMSO or a high boiling amide or amine at an elevated temperature of from about 150° C. to about 220° C. is provided. The process produces 6-HQ in high yield and a high state of purity such that it may be used in subsequent reactions toward the preparation of cilostazol without intermediate purification. A process for preparing cilostazol from 6-hydroxy-3,4-dihydroquinolinone prepared by the process and improved processes for preparing N-(4-methoxyphenyl)-3-chloropropionamide are also provided.
Owner:TEVA PHARMA IND LTD
Controlled-release preparation containing cilostazol and process for the preparation thereof
InactiveUS20100098759A1Promote absorptionConstant drug level of cilostazolBiocideAntipyreticIntestinal structureSmall intestine
The present invention relates to a controlled-release formulation comprising cilostazol and a method for preparing said formulation. The inventive controlled-release formulation comprising cilostazol or a pharmaceutically acceptable salt thereof, a solubilizing agent, a swelling agent, a swell-controlling agent and a gas generating material has advantages in that it maintains a constant cilostazol level in the blood through a slow release while it resides in the stomach and intestines over a long period of time, thereby increasing the absorption of cilostazol in the small intestine, the major absorption site of cilostazol, as well as minimizing adverse effects caused by rapid release and making it easy for a patient to take the drug.
Owner:AMOREPACIFIC CORP +1
Process for the production of cilostazol
InactiveUS20070105898A1Mass productionExcellent starting materialBiocideOrganic chemistryOrganic solventHalogen
A process for the preparation of cilostazol of formula I from 6-hydroxy-3,4-dihydroquinolinone of formula II and 1-cyclohexyl-5-(4-halobutyl)-tetrazole of formula III, wherein X is a halogen atom such as Cl, Br, and I, that includes combining compounds II, III, a water-miscible organic solvent, a water-soluble base and water. The cilostazol can then be separated from the reaction mixture and dissolved in a solvent A. The resulting cilostazol solution is mixed with a solvent B to precipitate cilostazol particles of defined particle size range, milling the precipitate if desired, and filtering and drying the product.
Owner:APOTEX PHARMACHEN INC
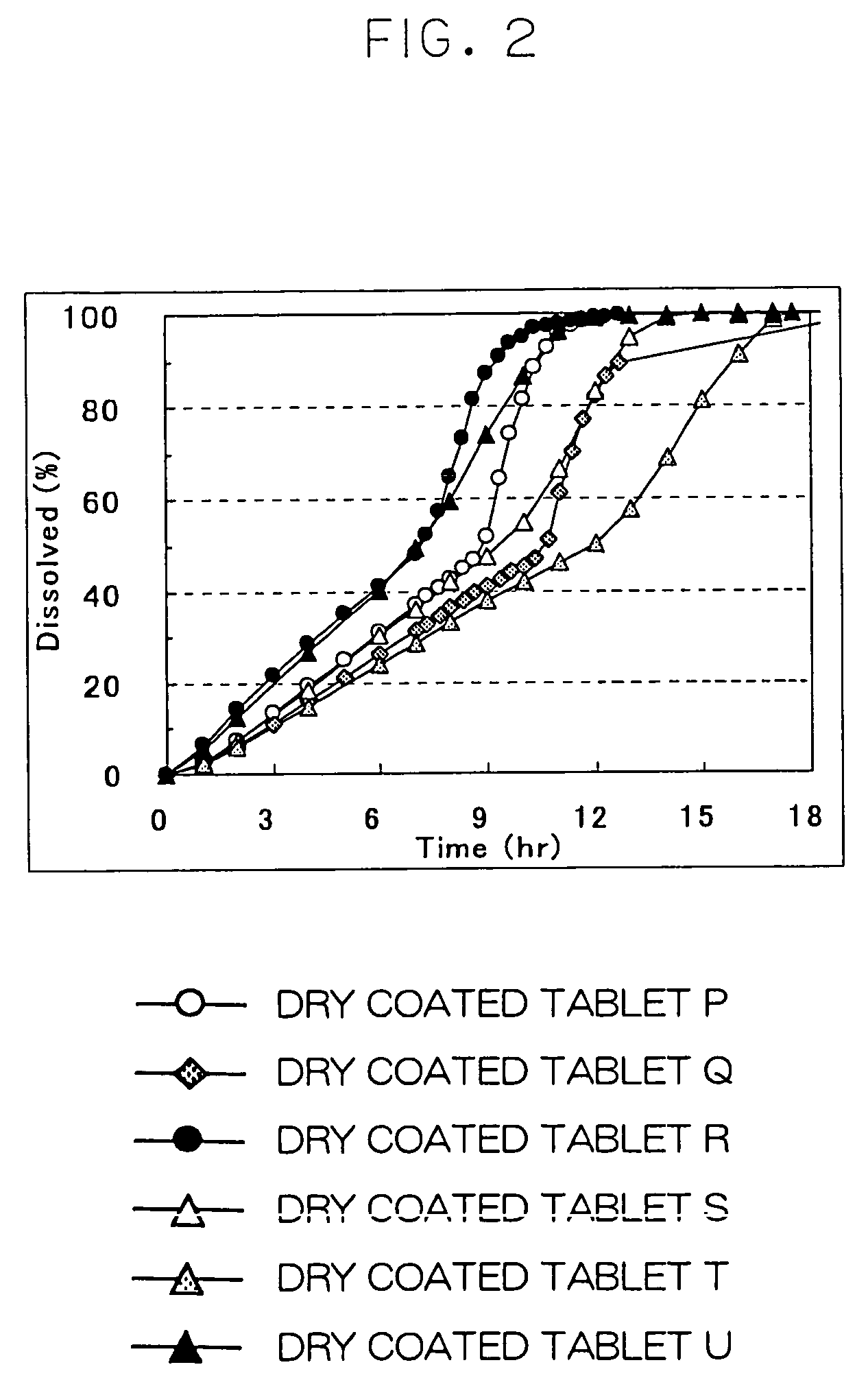
Slow-release cilostazol tablet having an improved elution rate and minimal side effects
ActiveCN102548543ALong dissolution timeConstant dissolution ratePill deliveryPharmaceutical non-active ingredientsPhosphodiesteraseSide effect
The present invention relates to a slow-release tablet of cilostazol which is a pharmacologically active component efficacious in the suppression of blood platelet aggregation and the promotion of vascular relaxation by inhibiting phosphodiesterase types; and, more specifically, provided is a slow-release cilostazol tablet of which the elution time is extended such that it can be taken once a day for expediency of drug-taking, and which minimises the occurrence of headache which is a side effect during the taking of cilostazol preparations of the prior art and therefore improves the convenience of drug-taking for women, the elderly and children. Also provided is a slow-release cilostazol tablet which exhibits a stable elution pattern without any variation in the percentage eluted depending on the pH in the stomach in addition to an effect whereby release of the drug is delayed, by using a release-controlling polymer consisting of a mixture of hydroxypropyl methyl cellulose and a carbomer.
Owner:KOREA UNITED PHARMA
Controlled release preparation containing cilostazoland process for the preparation thereof
A controlled release preparation which comprises particles containing cilostazol or its pharmaceutically acceptable salt dispersed in a solubilizing agent and an erodible material encasing said particles which is capable of forming a hydrogel, can maintain a constant level of cilostazol in the blood through its slow release during its prolonged residence time in the stomach and intestines, thereby minimizing adverse effects caused by rapid release of the drug or solubilizing agent.
Owner:AMOREPACIFIC CORP +1
Pharmaceutical composition for treating or preventing alcoholic liver diseases, containing cilostazol as active ingredient
InactiveUS20130267558A1Suppressed expression levelGood effectBiocideOrganic chemistryPentoxifyllineFatty Acid Synthetases
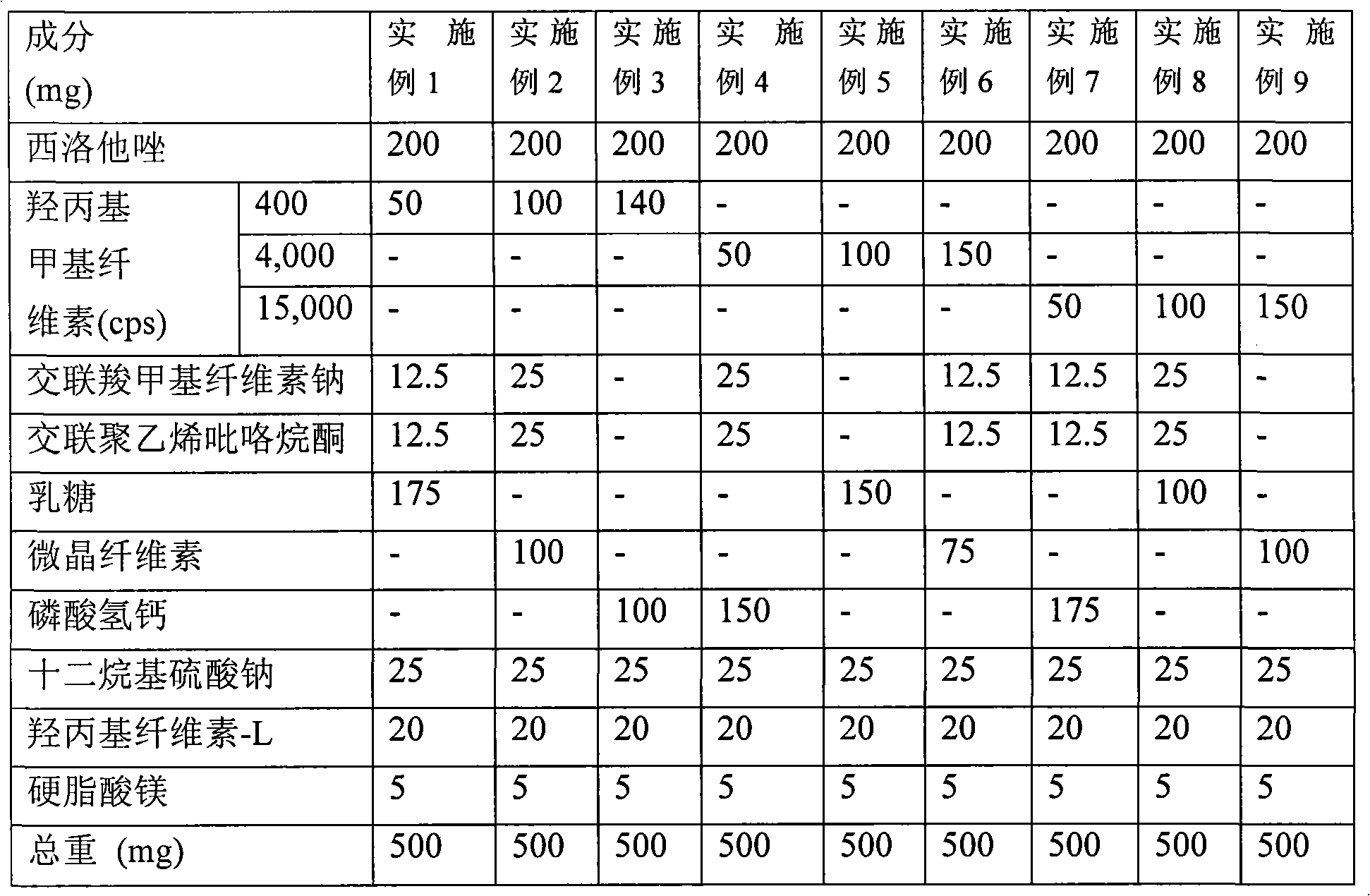
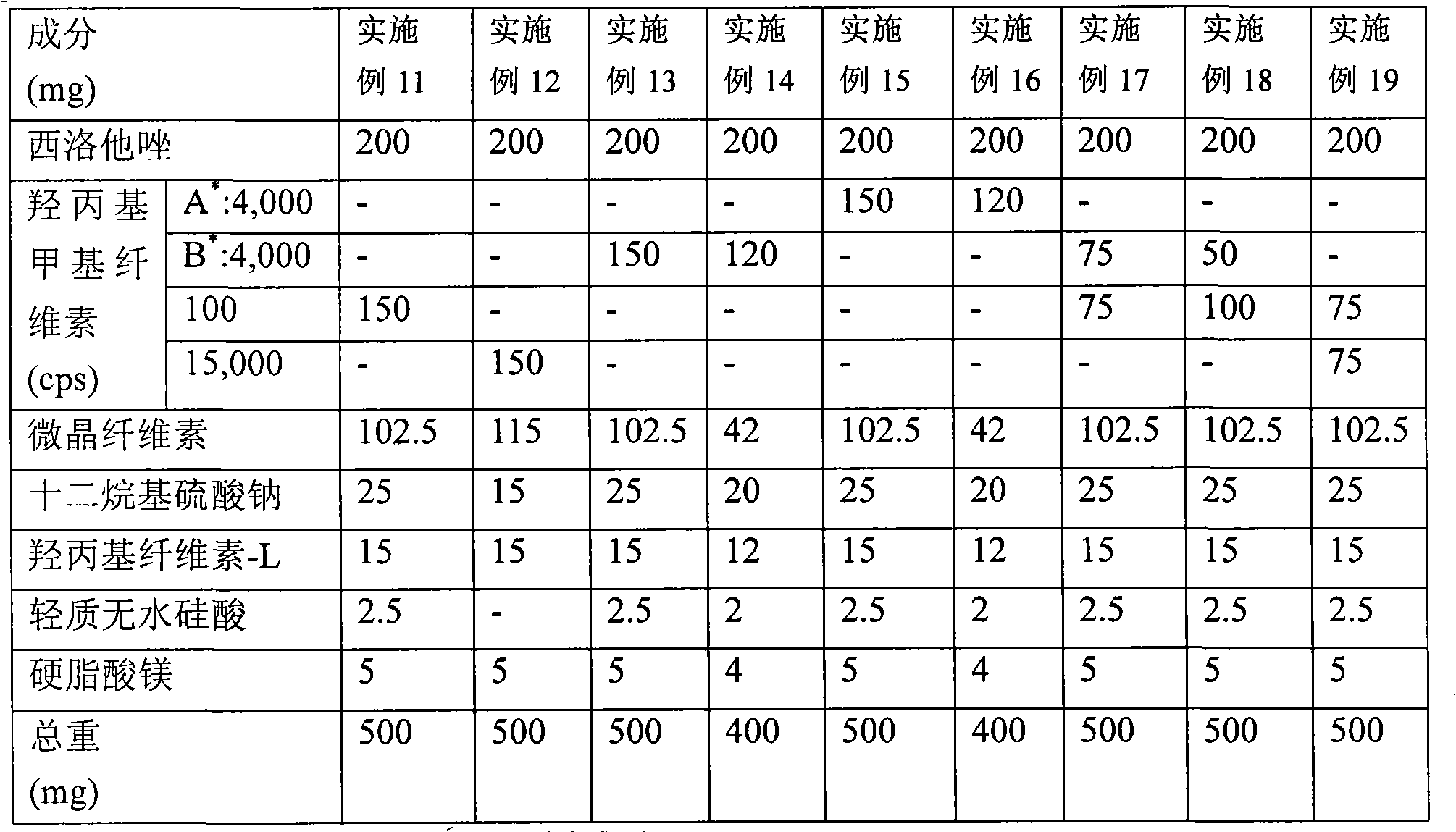
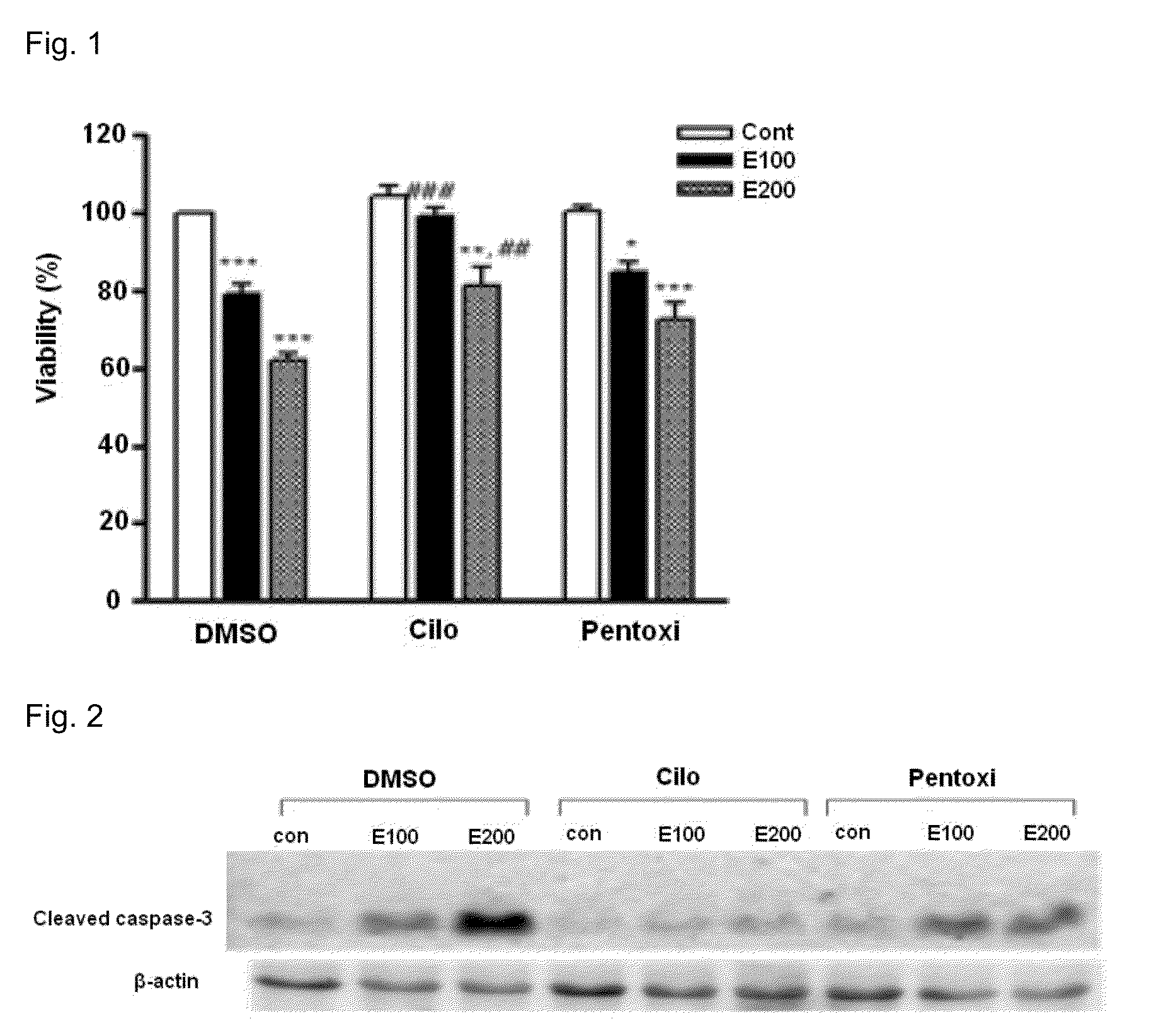
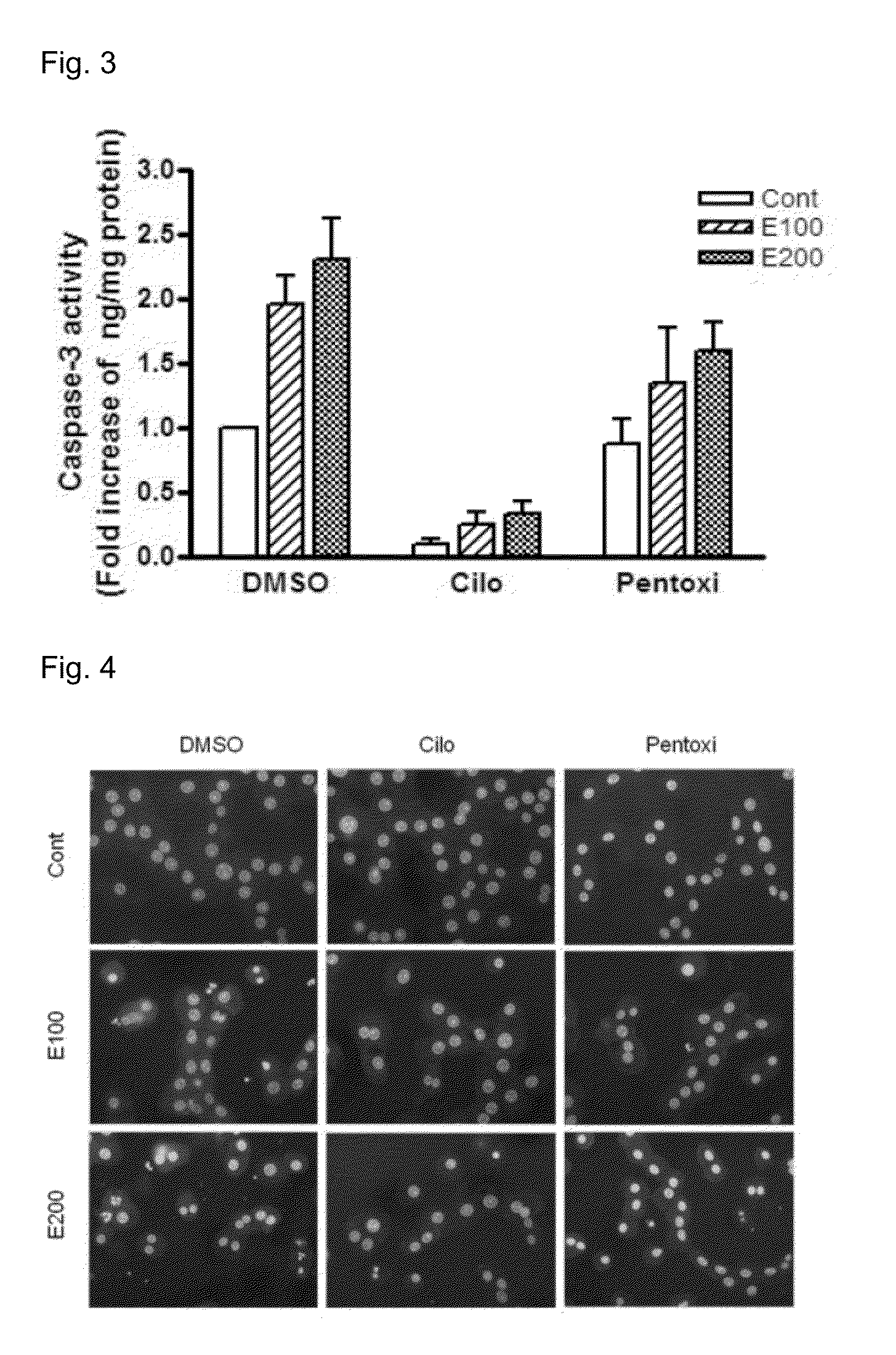
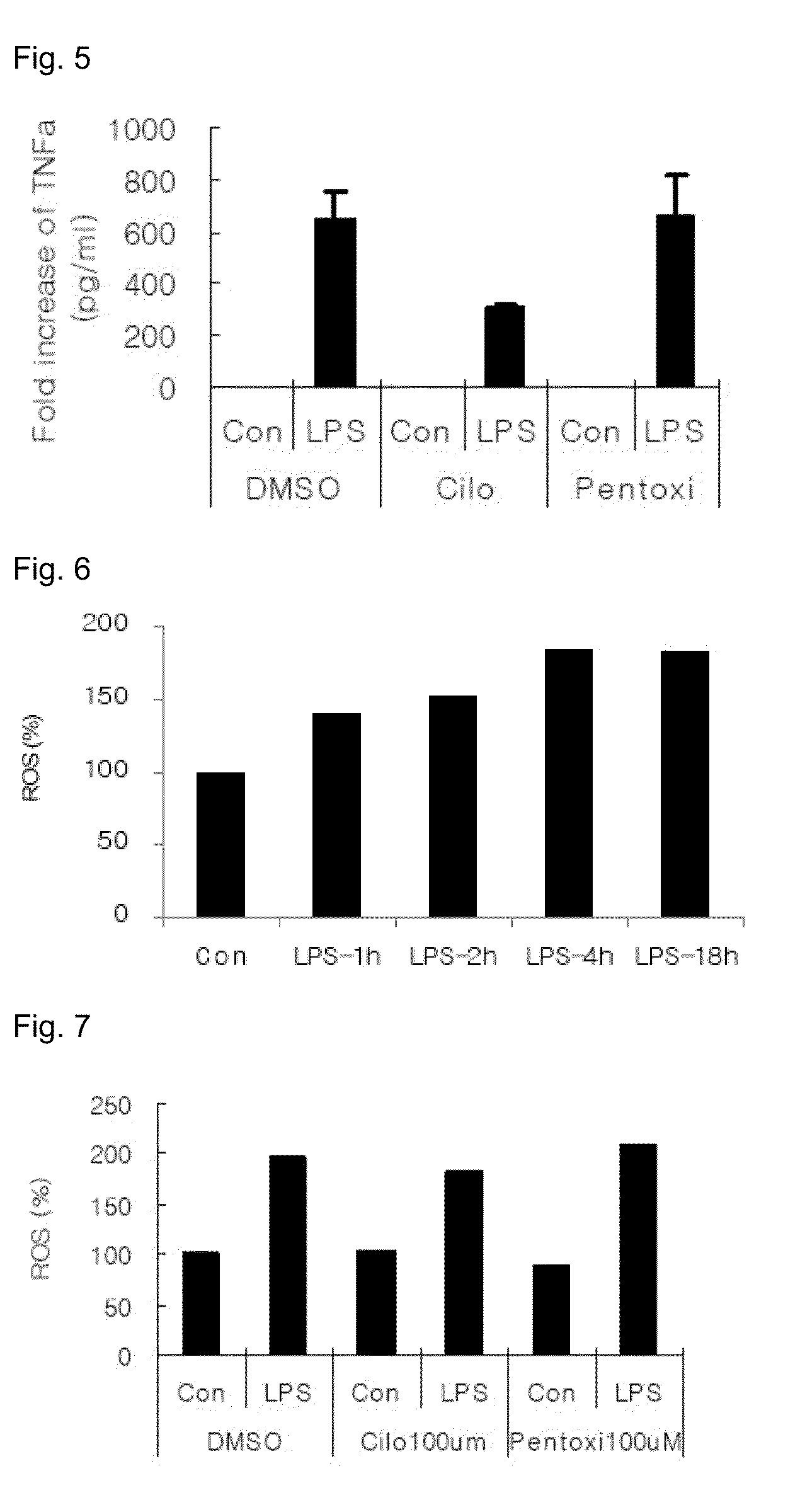
Provided is a pharmaceutical composition for the treatment and prevention of alcoholic liver diseases, including cilostazol as an active ingredient. Cilostazol inhibits expression levels of TNF-α and FAS (fatty acid synthase) gene in a concentration-dependent manner, and also significantly inhibits the activity of caspase-3. Accordingly, cilostazol shows superior effects for the treatment or prevention of alcoholic liver diseases, in particular, alcoholic hepatitis compared to pentoxifylline which is conventionally used as a therapeutic agent for the treatment for alcoholic hepatitis. Thus, cilostazol is suitable for use as a drug for the treatment or prevention of alcoholic hepatitis.
Owner:RES COOPERATION FOUND OF YEUNGNAM UNIV
Production method of cilostazol
InactiveCN104744436AEasy to produceReduce manufacturing costOrganic chemistryValeramidePotassium hydroxide
The invention relates to a production method of cilostazol. The method comprises the specific steps of: slowly adding 1.9g of phosphorus pentachloride into 1.75g of a raw material 5-chloro-N-cyclohexyl valeramide in 15ml of benzene solution, adding 1.4 mol / L H3N in 11 ml of benzene solution to obtain 1.7g of 5-(4-chlorobutyl)-1-cyclohexyl tetrazolium, dissolving 3.2g of 6-hydroxy-3,4-two-dihydro-2(1H)-quinolinone and 1.4g of potassium hydroxide in 20ml of isopropanol, extracting with, followed by 1 mol / L sodium hydroxide solution, dilute hydrochloric acid and water washing, conducting silica gel chromatography on residual liquid, eluting with chloroform methanol (30:1), recrystallizing with methanol-water to obtain 6.0g of colorless acicular crystal cilostazol. The production method of cilostazol has low manufacture cost and convenient production, and can realize batch production.
Owner:张云
Cilostazol-Containing Pharmaceutical Composition Based On Particles Of Less Than 50 Micrometers
The present invention relates to cilostazol compositions, process for their preparation, and methods for their administration to treat a condition. In the cilostazol compositions, 90% of the cilostazol particles have a particle size less than about 50 μm.
Owner:RANBAXY LAB LTD
Encephalic drug eluting stent
InactiveCN103948458AInhibit the inflammatory responsePrevent restenosisStentsSurgeryDiseaseAntiplatelet drug
The invention discloses an encephalic drug eluting stent in the field of medicines, and particularly relates to the drug eluting stent used for curing the encephalic atherosclerotic stenosis disease. Diseased blood vessels are expanded by the stent to improve the condition of stenosis, so as to improve the blood flow state, meanwhile, drug carried by the stent can be used for preventing a tunica elastica interna from hyperplasia, and the probability of in-stent restenosis can be lowered. The encephalic drug eluting stent comprises a conveying system, a stent body and a stent drug carrying layer, and is characterized in that one or more drug carrying layers are attached to the surface of the encephalic drug eluting stent; each drug carrying layer is composed of a polymer and an active ingredient, the active ingredient is an antiplatelet drug cilostazol, and each drug carrying layer comprises the following components by weight percent: 5 to 50 percent of the polymer and the balance of active ingredients; the active ingredients in all medicine carrying layers are the same or different. The encephalic drug eluting stent provided by the invention has the advantages that the functions of protecting blood vessels, restraining inflammation reactions, expanding blood vessels and the like can be achieved, and the purpose of preventing encephalic vascular in-stent restenosis can be achieved.
Owner:ACHIEVA MEDICAL SHANGHAI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com