Method of producing drug-containing wax matrix particles, extruder to be used in the method and sustained-release preparation containing cilostazol
A manufacturing method and technology of sustained-release preparations, which are applied in the field of wax-based particle manufacturing, can solve the problems of drug or additive stability damage, wax-based particle preparation technology, solidification, etc., and achieve high biological availability , effective pharmacological effects, and the effect of ensuring sustained release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
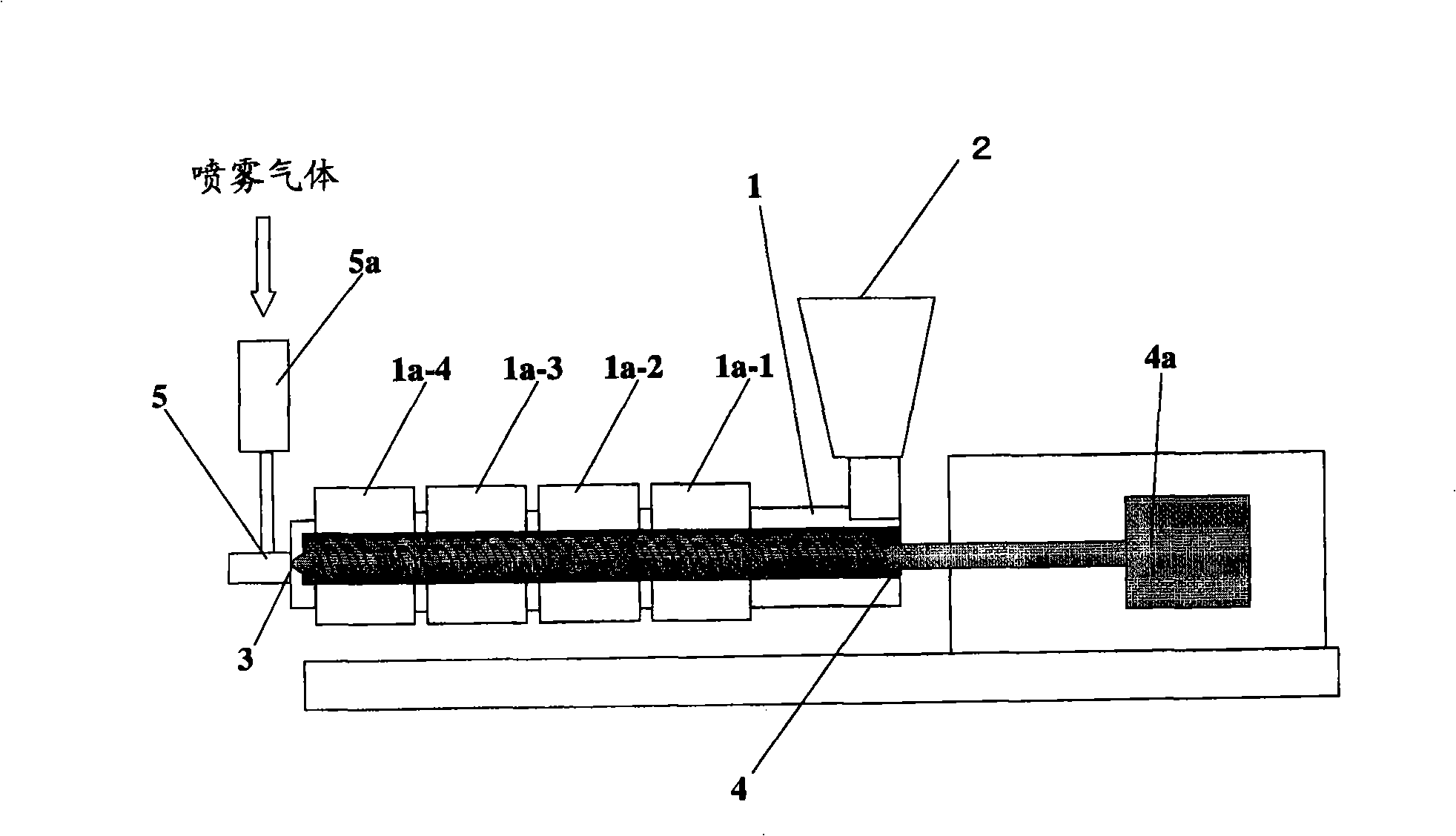
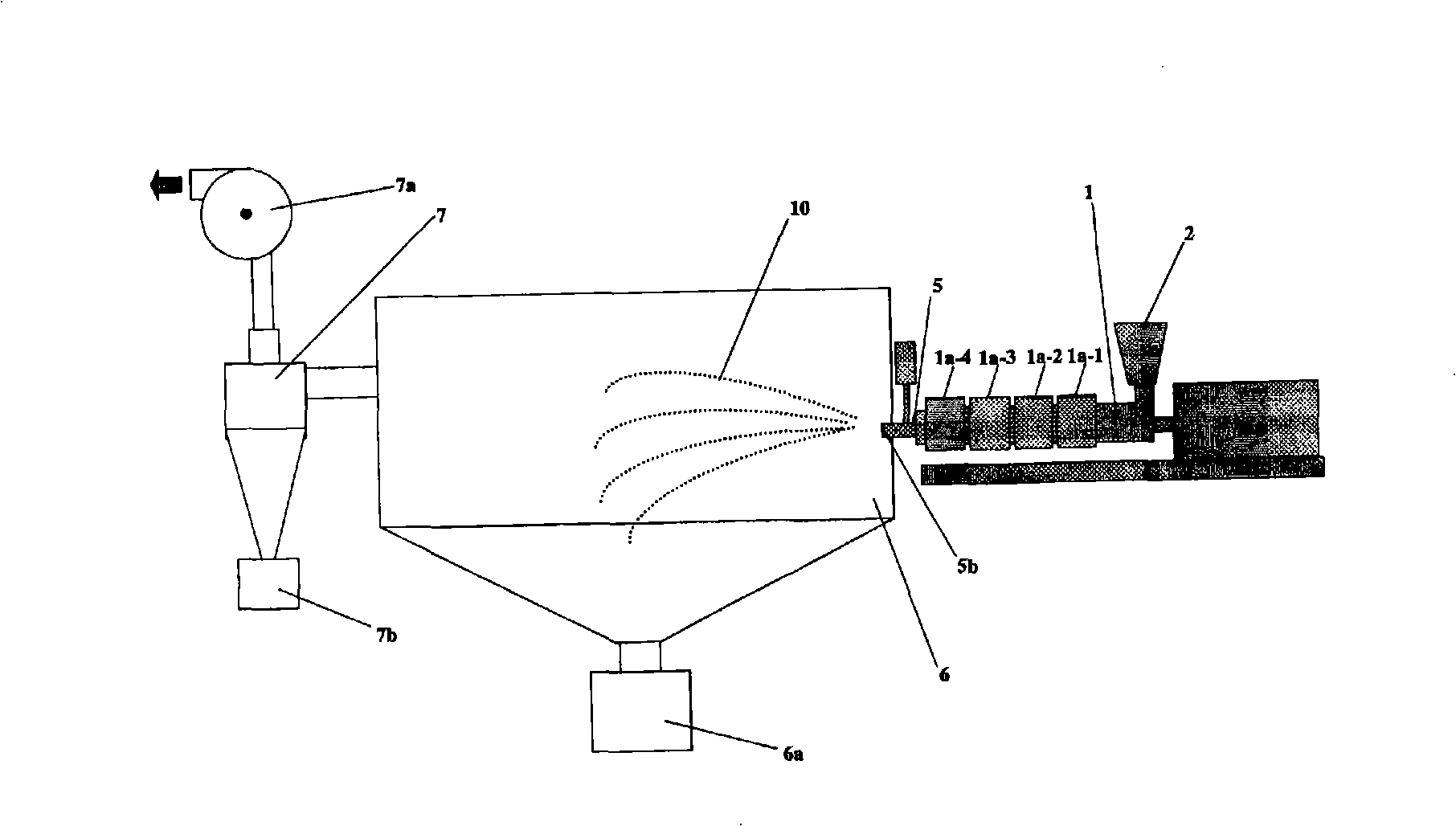
[0152] use figure 2 The extruder of the configuration shown, produces wax matrix particles. As an extruder, the structure and its operating conditions are shown below.
[0153] Extruder type: Twin-screw extruder (KEX-25, manufactured by Kurimoto Iron Works)
[0154] Screw shape: From the downstream side to the upstream side, the shape in which the conveying, kneading, and mixing sections are connected in sequence
[0155] Nozzle: Dual Fluid Nozzle
[0156] Screw part length: Approximately 50cm
[0157] Rotation speed of the screw: 125rpm
[0158] The shape and diameter of the discharge hole of the nozzle: circular, 0.5mm
[0159] The residence time of raw materials in the drum: about 2 minutes
[0160] Drum temperature setting temperature: drum sleeve 1a-1 is 140°C, drum sleeve 1a-2 is 150°C, drum sleeve 1a-3 and 1a-4 are 160°C
[0161] Temperature and introduction speed of spray gas: about 200°C, 25L / min
[0162] Discharge speed per discharge hole of molten kneaded...
Embodiment 2
[0168] As raw materials, 300 g of theophylline, 10 g of ethyl cellulose, and 690 g of fatty acid glyceride (glycerol monobehenate; melting point of about 75° C.) were mixed, and wax was produced under the same conditions as in Example 1 above using the obtained mixed raw material. matrix particles.
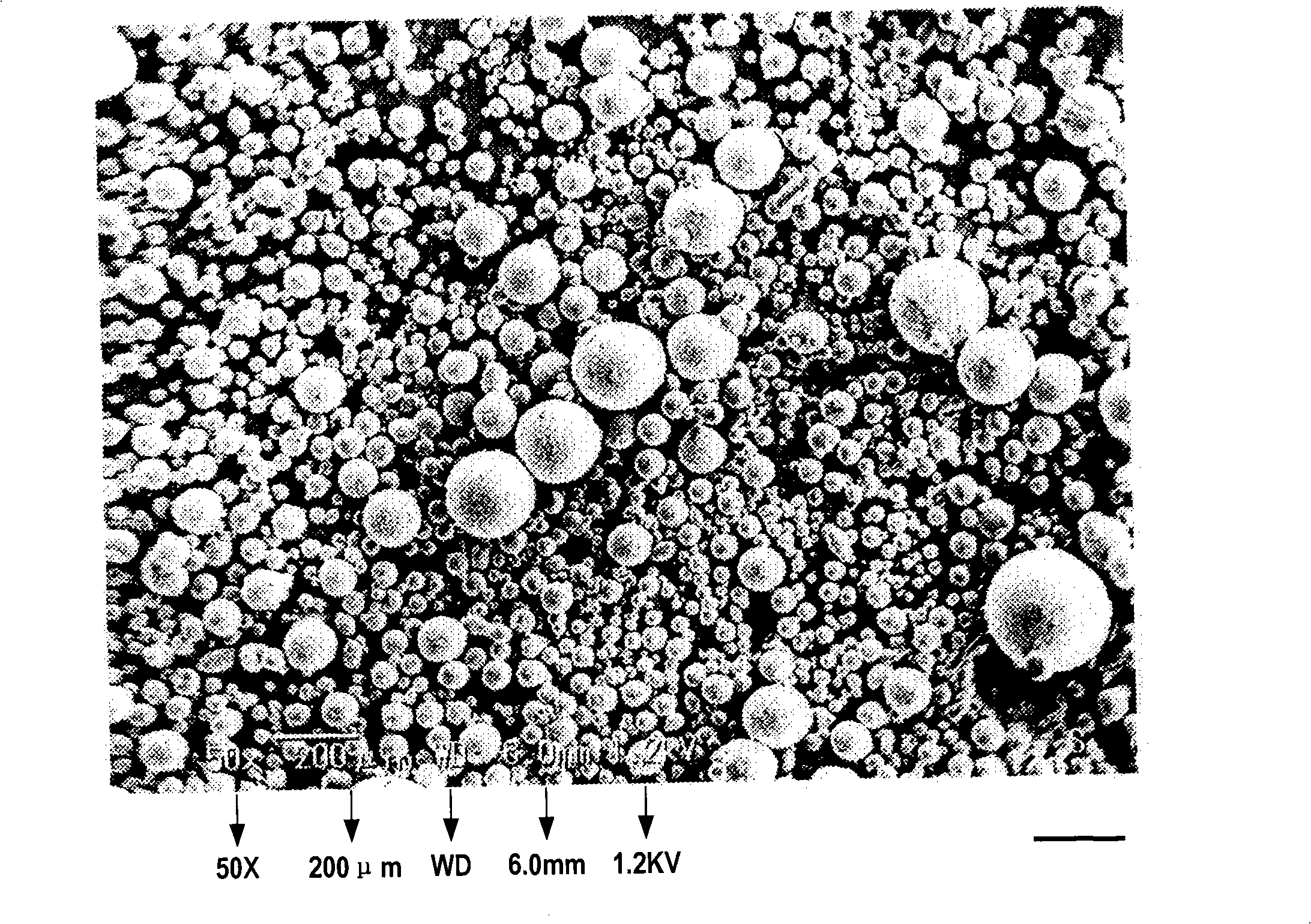
[0169] The obtained wax matrix particles were spherical, and when the particle size distribution was measured with a laser diffraction particle size distribution meter (Tohnichi Computer Application), the 10% cumulative diameter was 43 μm, the 50% cumulative diameter (average particle diameter) was 88 μm, and the 90% cumulative diameter was 160 μm, 99% cumulative diameter is 204 μm.
[0170] In addition, it was confirmed that no troubles such as theophylline precipitation in the extruder and liquid clogging occurred during the production process. In addition, the content of theophylline in the obtained wax-based particles was about 100% relative to the theoretical value.
Embodiment 3
[0172] As raw materials, 300 g of theophylline and 700 g of hardened oil (melting point: about 86° C.) were mixed, and using the obtained mixed raw material, wax matrix particles were produced under the same conditions as in Example 1 above.
[0173] The obtained wax matrix particles were spherical, and when the particle size distribution was measured with a laser diffraction particle size distribution meter (Tohnichi Computer Application), the 10% cumulative diameter was 48 μm, the 50% cumulative diameter (average particle diameter) was 96 μm, and the 90% cumulative diameter was 169 μm, 99% cumulative diameter is 221 μm.
[0174] In addition, it was confirmed that no troubles such as theophylline precipitation in the extruder and liquid clogging occurred during the production process. In addition, the content of cilostazol in the obtained wax-based particles was about 100% relative to the theoretical value.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com