Controlled-release preparation containing cilostazol and process for the preparation thereof
A technology of cilostazol and controlled-release preparations, which is applied in the field of controlled-release preparations containing cilostazol and its preparation, which can solve the problems of complex preparation methods, irregular absorption rates, and difficulty in taking drugs for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 and comparative example 1 to 3
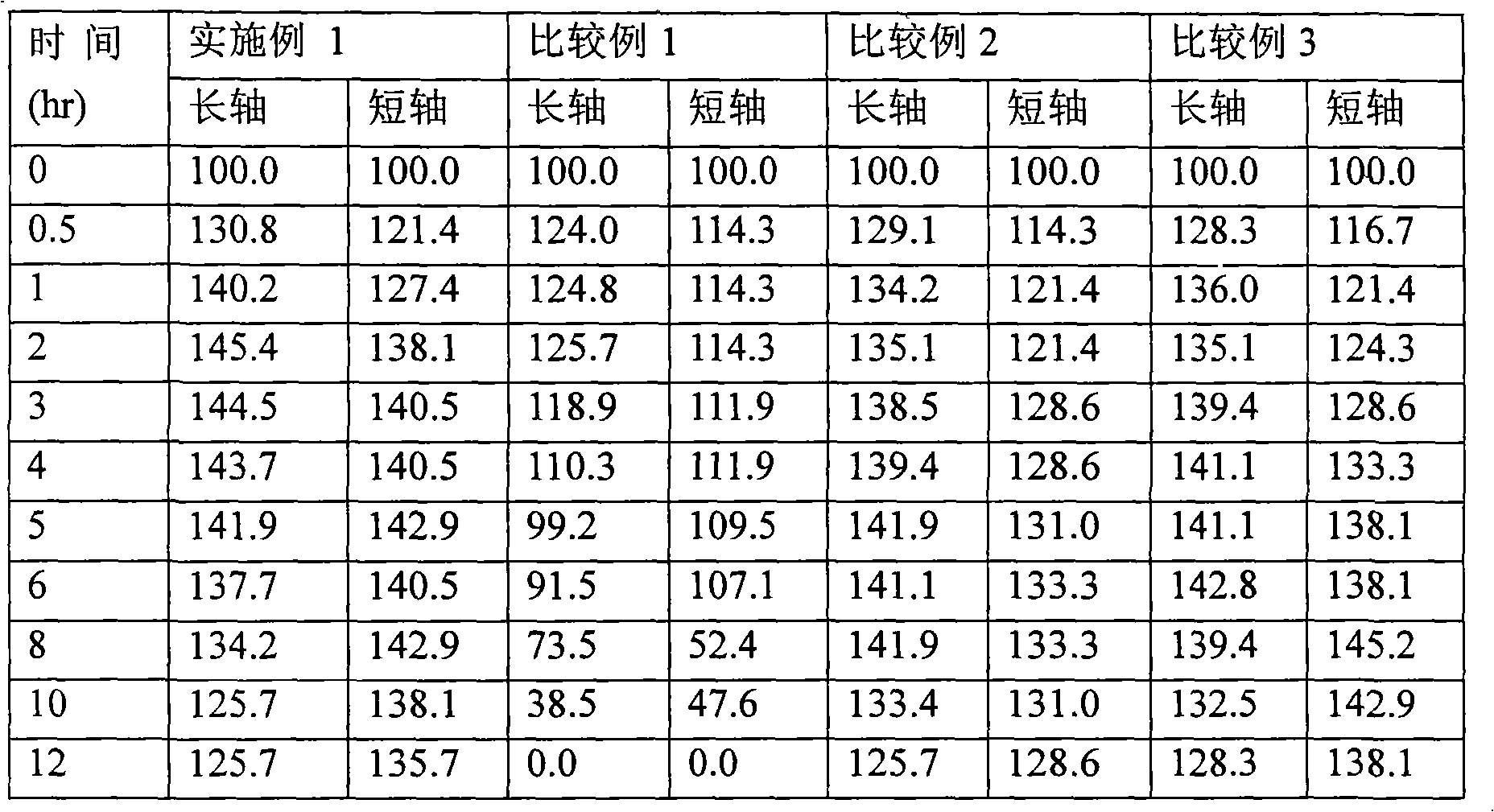
[0056] Example 1 and Comparative Examples 1 to 3: Preparation of Matrix Tablets Containing Cilostazol
[0057] Using the ingredients shown in Table 1, the cilostazol matrix tablets of Example 1 and Comparative Examples 1 to 3 were prepared as follows.
[0058] Table 1
[0059] Element
[0060] A mixture of cilostazol, sodium lauryl sulfate, croscarmellose sodium or crospovidone, and lactose was granulated, and an ethanol solution of hydroxypropylcellulose was added thereto and mixed. The resulting granules were classified using a 14-mesh sieve, dried, and the granules passed through were further classified using a 18-mesh sieve, and the granules passed through the sieve were mixed with: polyethylene oxide (molecular weight: 5,000,000) and light anhydrous silicon acid (Comparative Examples 1 to 3); or polyethylene oxide (molecular weight: 5,000,000), light anhydrous silicic acid, citric acid, and sodium bicarbonate (Example 1). After magnesium stearate was added th...
Embodiment 2 to 11
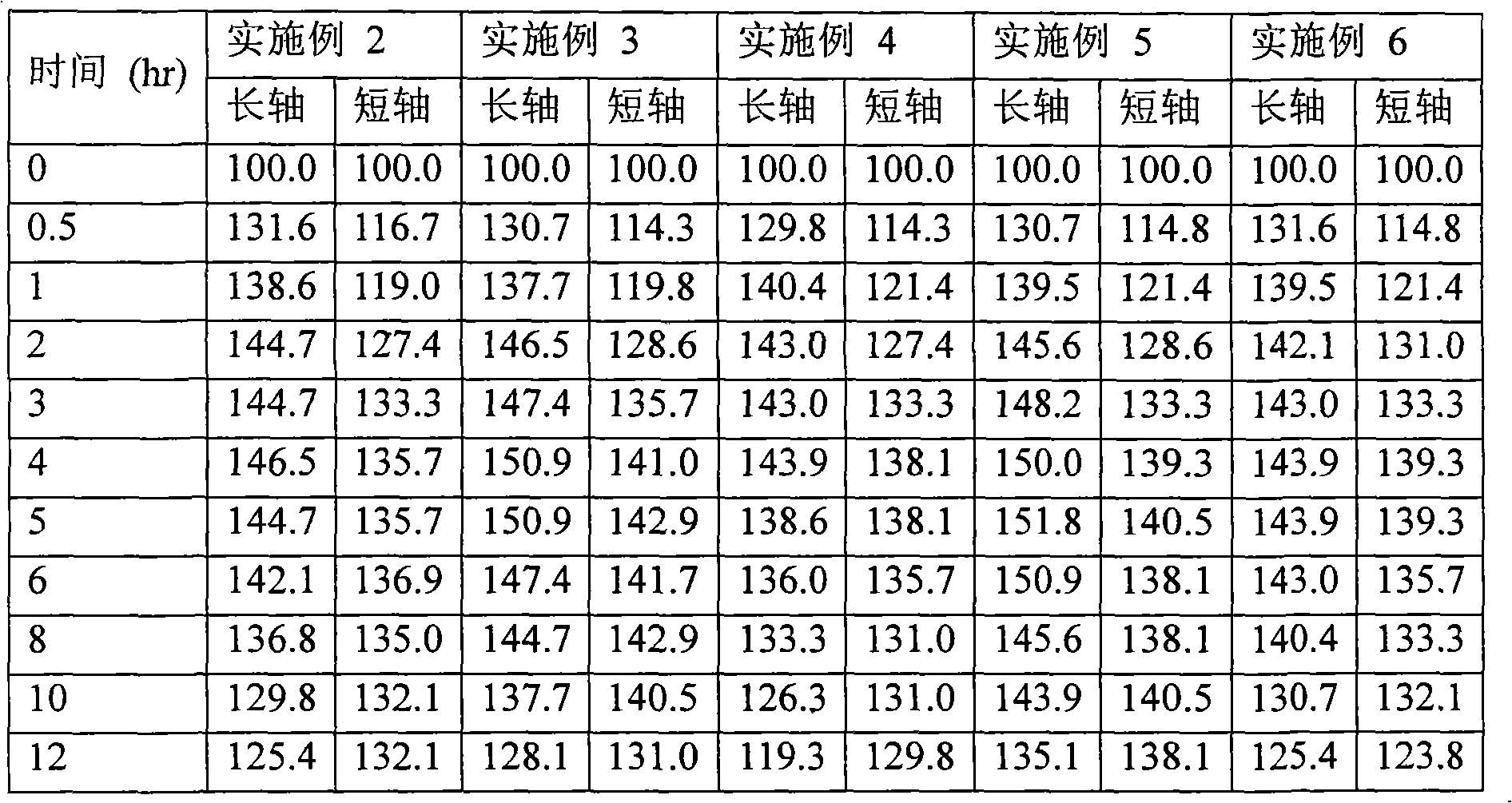
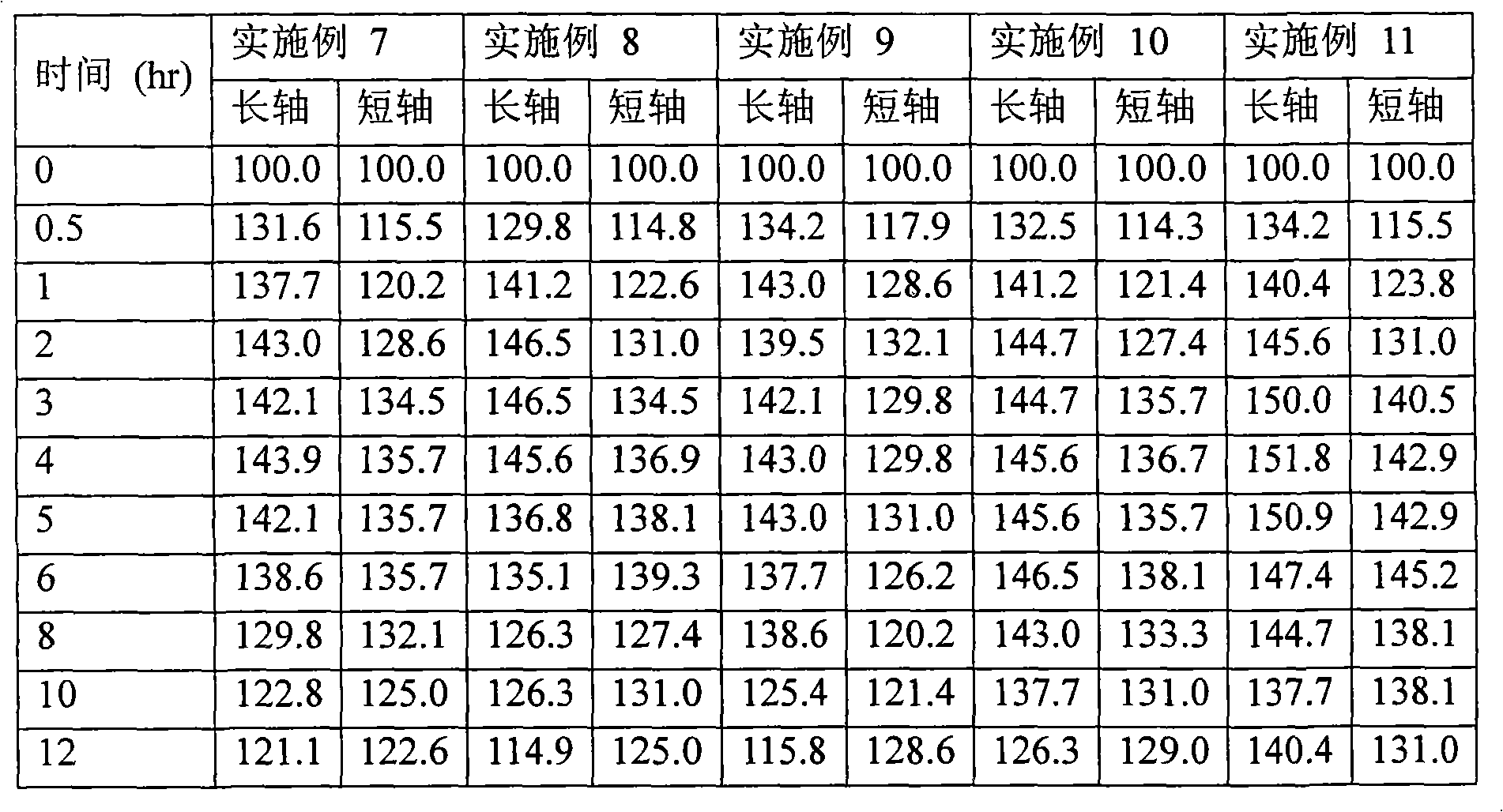
[0073] Examples 2 to 11: Preparation of matrix tablets comprising cilostazol
[0074] Cilostazol matrix tablets of Examples 2 to 11 were prepared as follows using the ingredients shown in Tables 4a and 4b.
[0075] Table 4a
[0076] Element
[0077] citric acid
[0078] Table 4b
[0079]
[0080] A mixture of cilostazol, sodium lauryl sulfate, croscarmellose sodium or crospovidone, and lactose was granulated, an ethanol solution of hydroxypropylcellulose-L was added thereto, and mix. The obtained granules were classified using a 14-mesh sieve, dried and further classified using a 18-mesh sieve, and the granules passed through the sieve were mixed with the following: polyethylene oxide (molecular weight: 5,000,000), light anhydrous silicic acid, lemon acids and sodium bicarbonate. After magnesium stearate was added thereto, the resulting mixture was lubricated and formulated into tablets using a compounder to obtain a controlled release dosage...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com