Slow-release cilostazol tablet having an improved elution rate and minimal side effects
一种西洛他唑片剂、西洛他唑的技术,应用在非有效成分的医用配制品、含有效成分的医用配制品、丸剂输送等方向,达到长溶出时间、头痛低的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
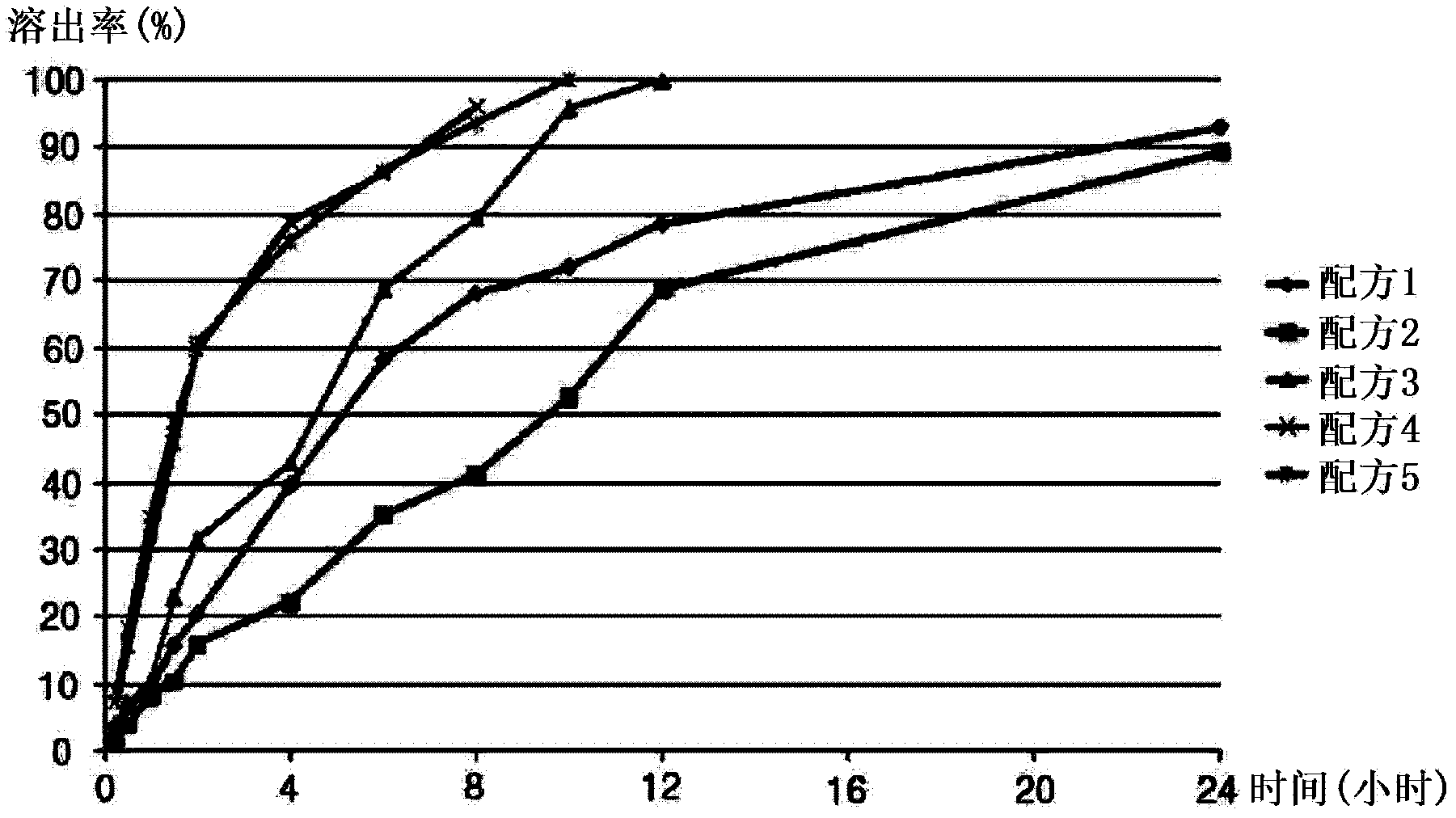
[0042] Sustained release tablets were prepared using the following method using the formulation listed in Table 1 below. First, Povidone K-30 and Carbomer (50%) were dispersed and dissolved in ethanol to prepare a binder solution. Next, cilostazol, microcrystalline cellulose, carbomer (50%), and hydroxypropyl methylcellulose were mixed thoroughly using a high-speed mixer, and then granulated in a cylindrical granulator using a binder solution. Made into wet granules. The prepared granules were dried in a drying oven (40° C.) for 12 hours, and then passed through a 40-mesh sieve. Afterwards, light anhydrous silicic acid and magnesium stearate were further mixed with the sieved semi-finished product, and formed into tablets, whereby one tablet could reach the weights listed in Table 1 below. The sustained-release cilostazol tablets prepared as in the following examples with a constant content were subjected to a dissolution test according to the method described in Korean Herb...
Embodiment 2
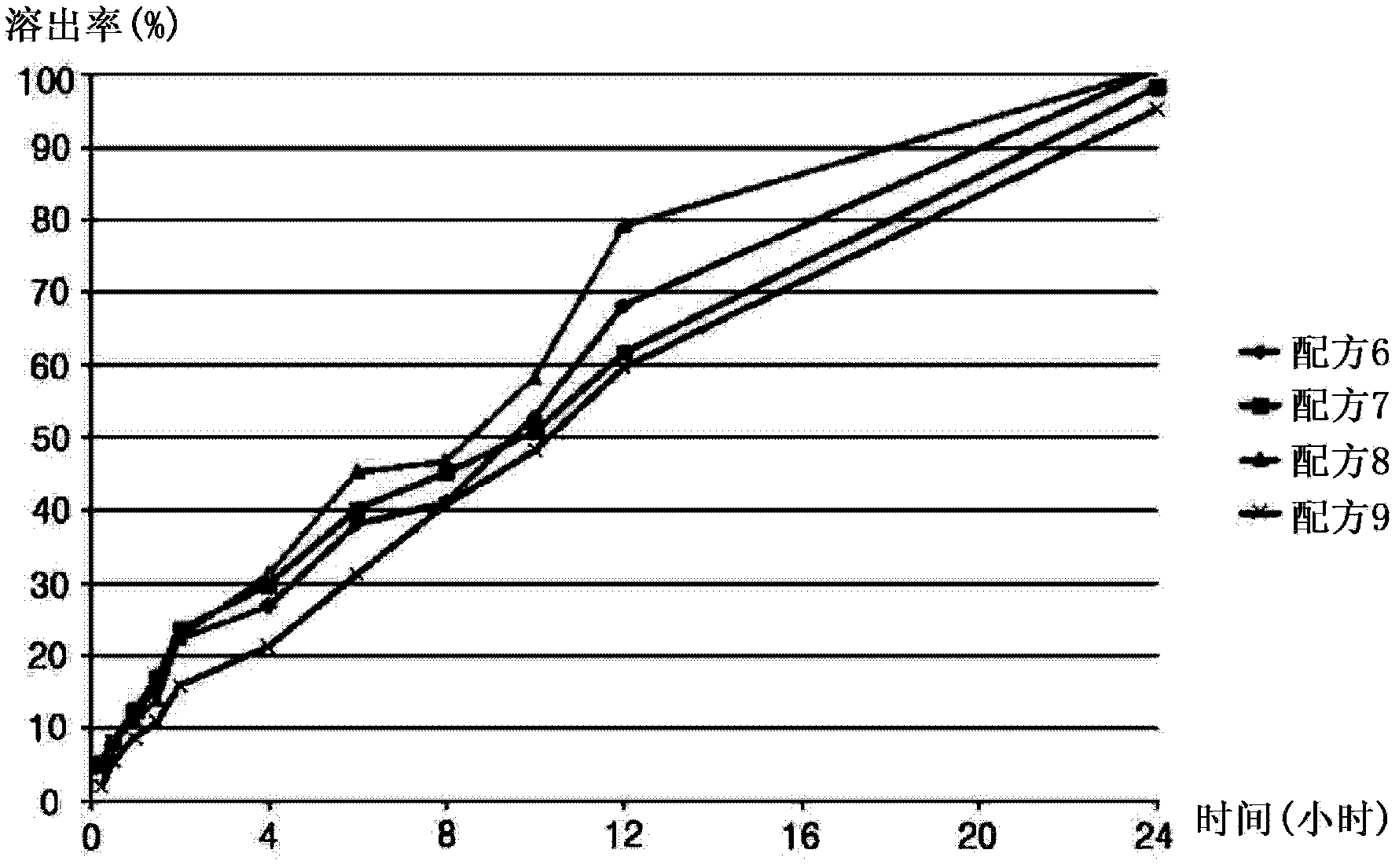
[0054] The formulations listed in Table 5 below were prepared in the same manner as in Example 1 except that the ingredients listed in Table 5 below were used.
[0055] table 5
[0056]
[0057] Table 6
[0058]
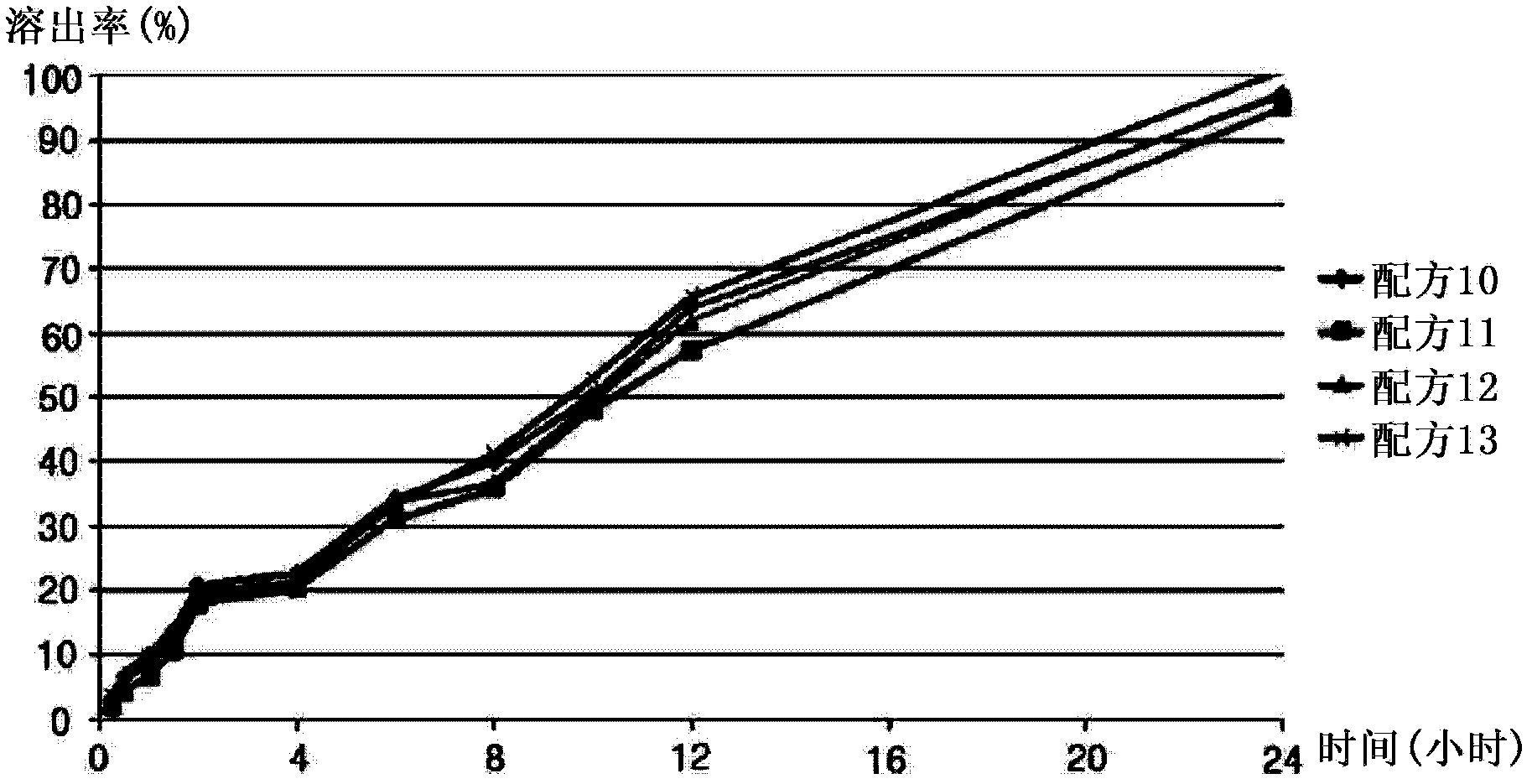
Embodiment 3
[0060] The formulations listed in Table 7 below were prepared in the same manner as in Example 1, except that the ingredients listed in Table 7 below were used.
[0061] Table 7
[0062]
[0063] Table 8
[0064]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com