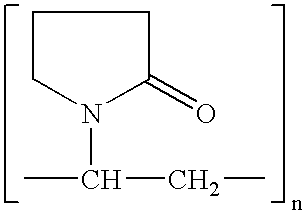
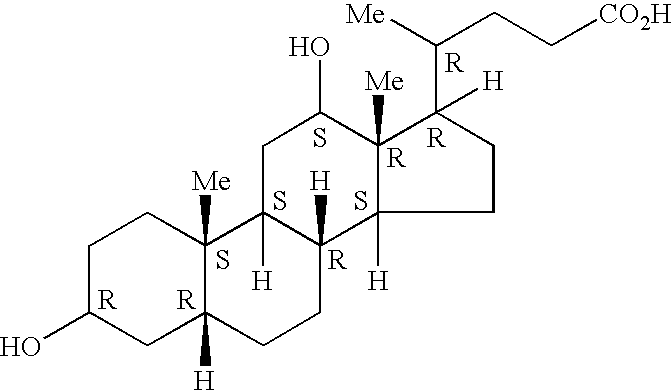
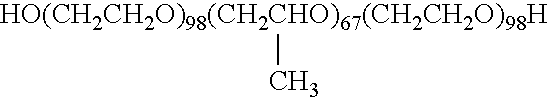Patents
Literature
31 results about "Enhanced dissolution" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
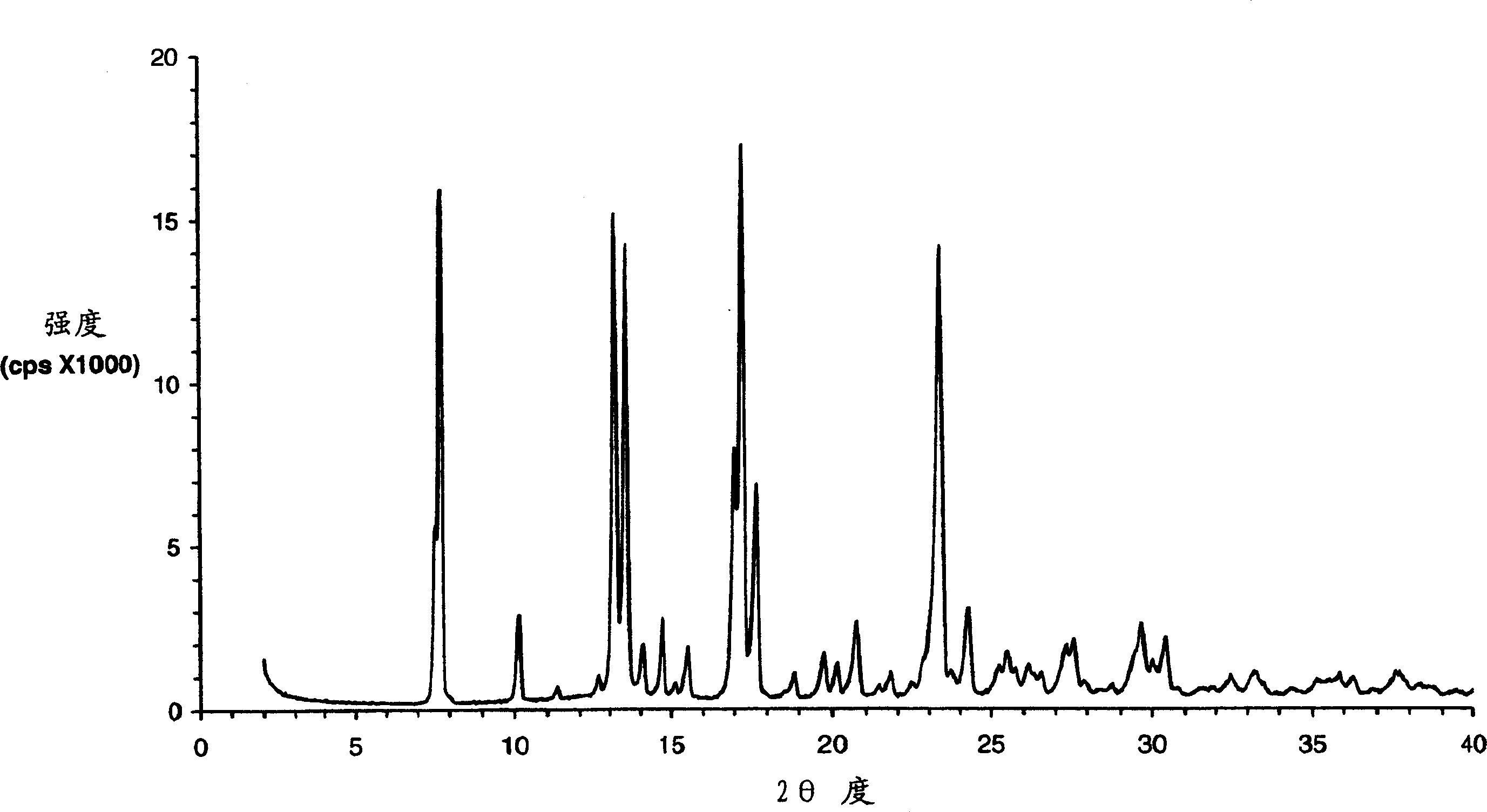
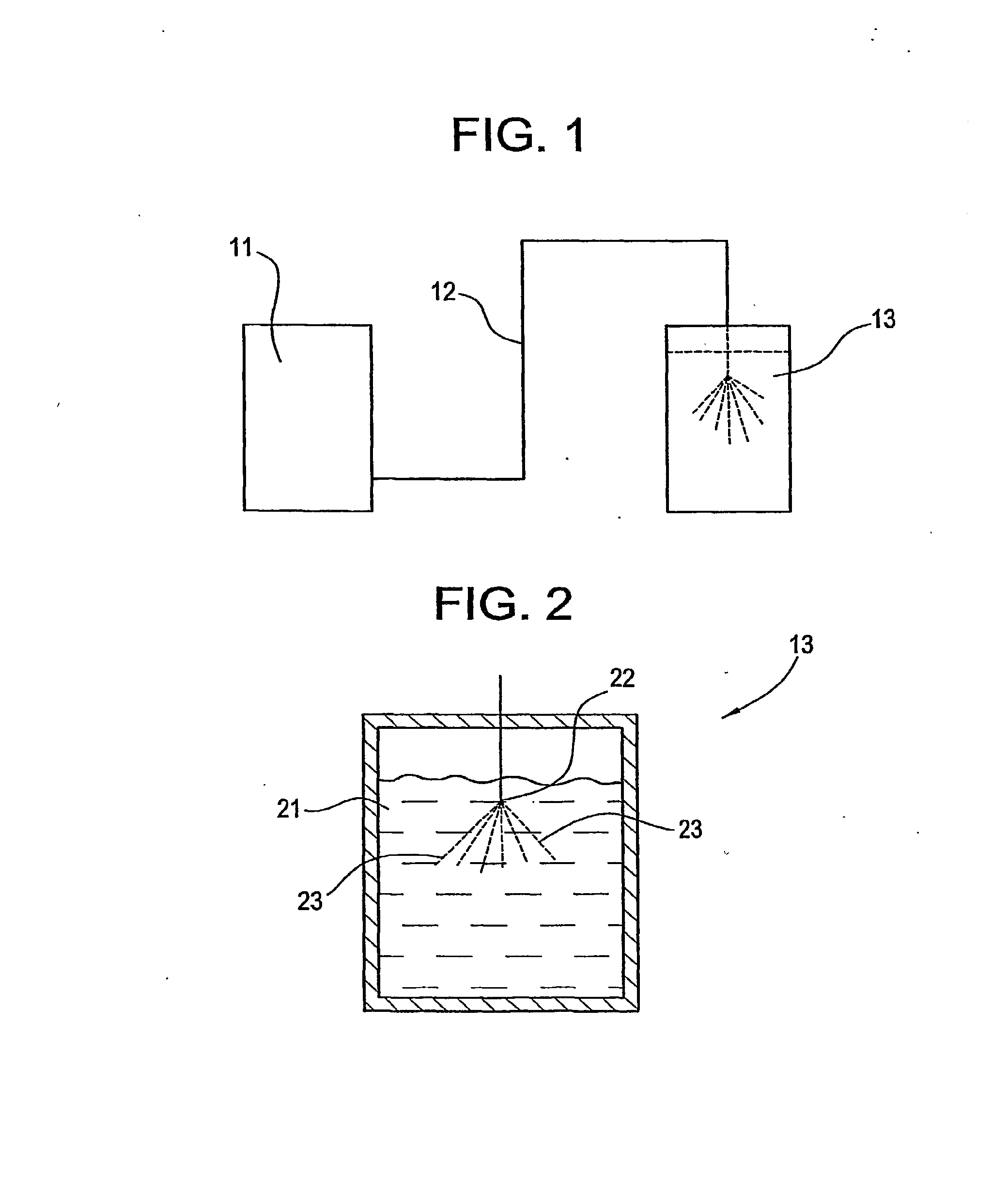
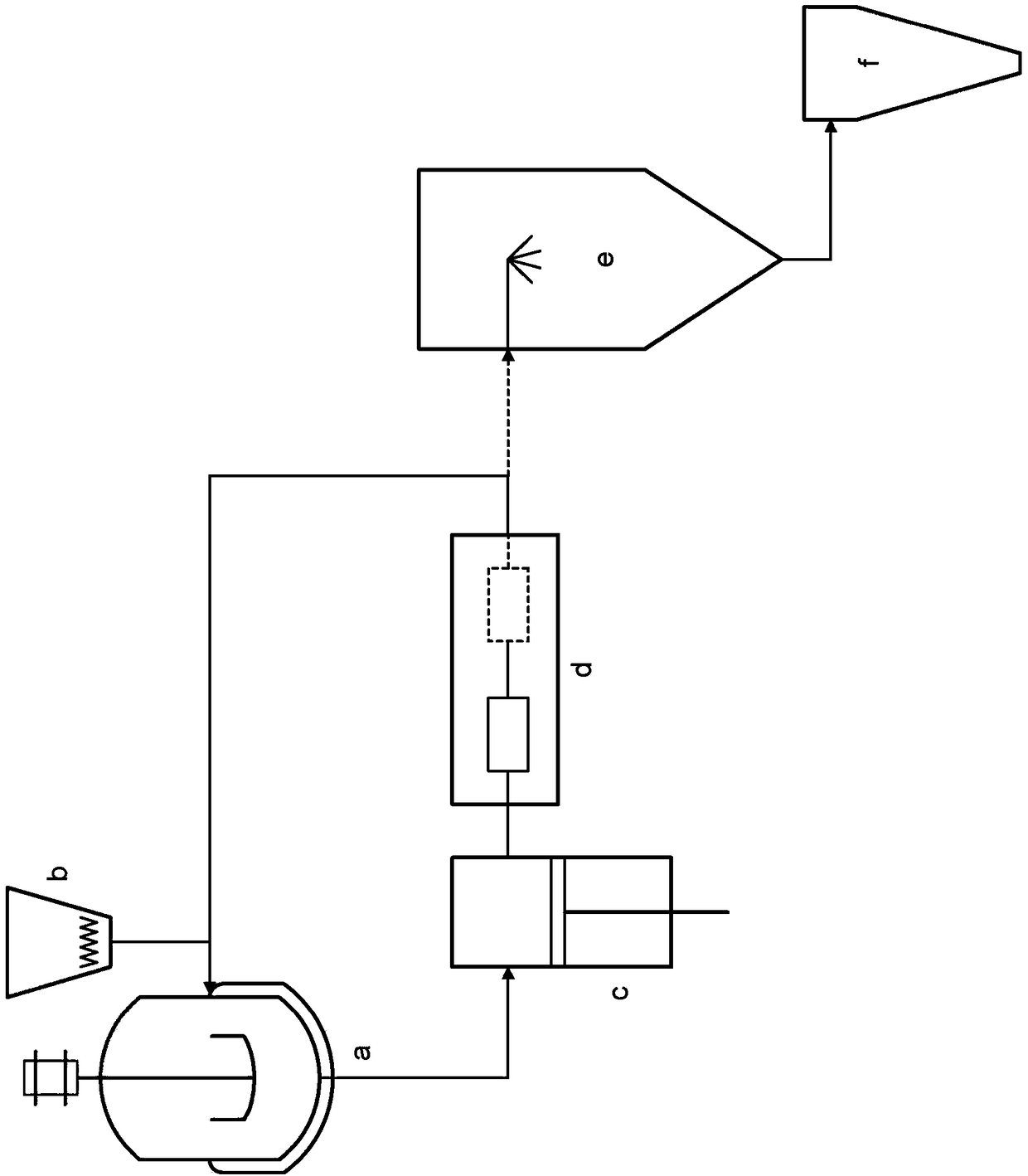
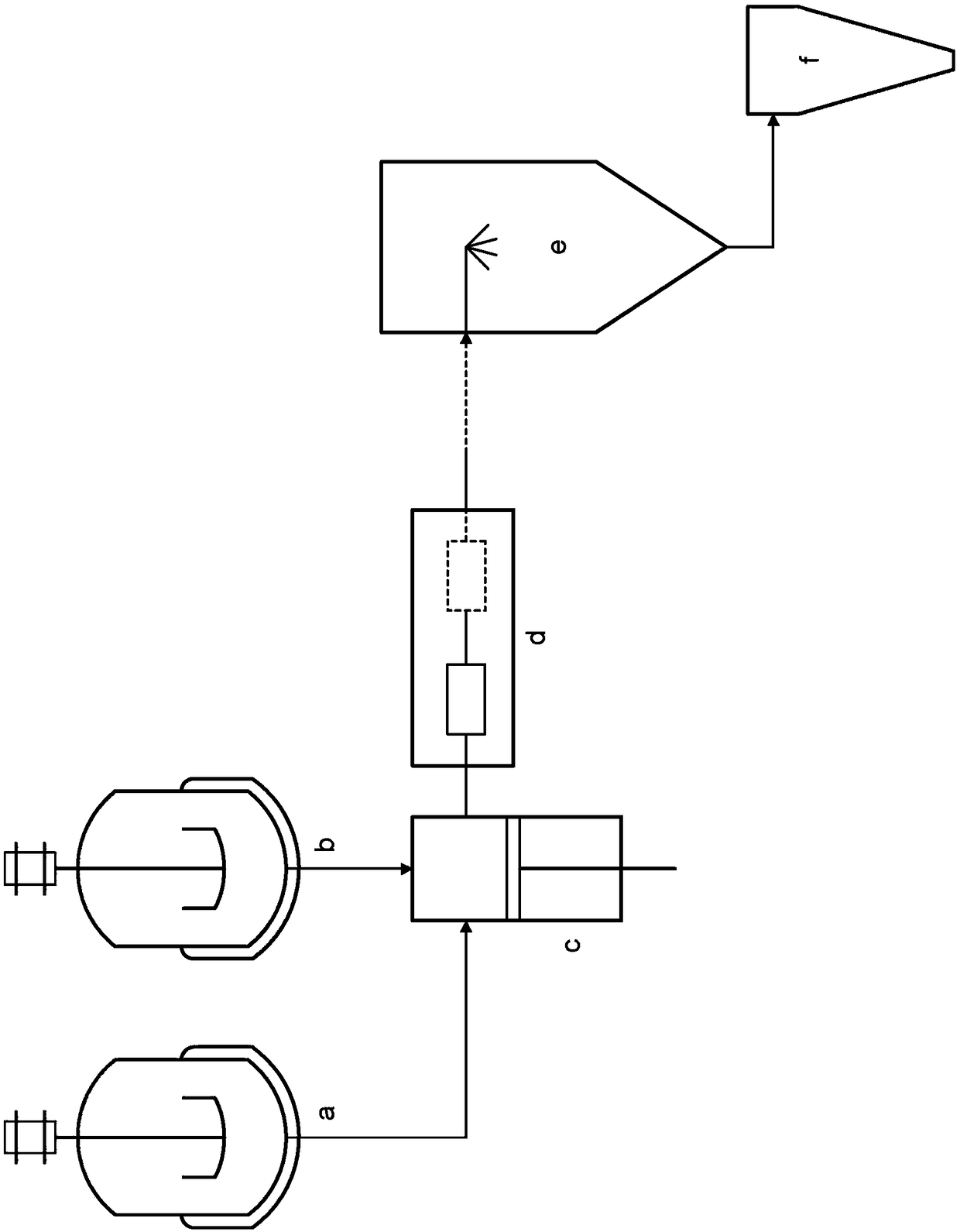
Preparation of drug particles using evaporation precipitation into aqueous solutions
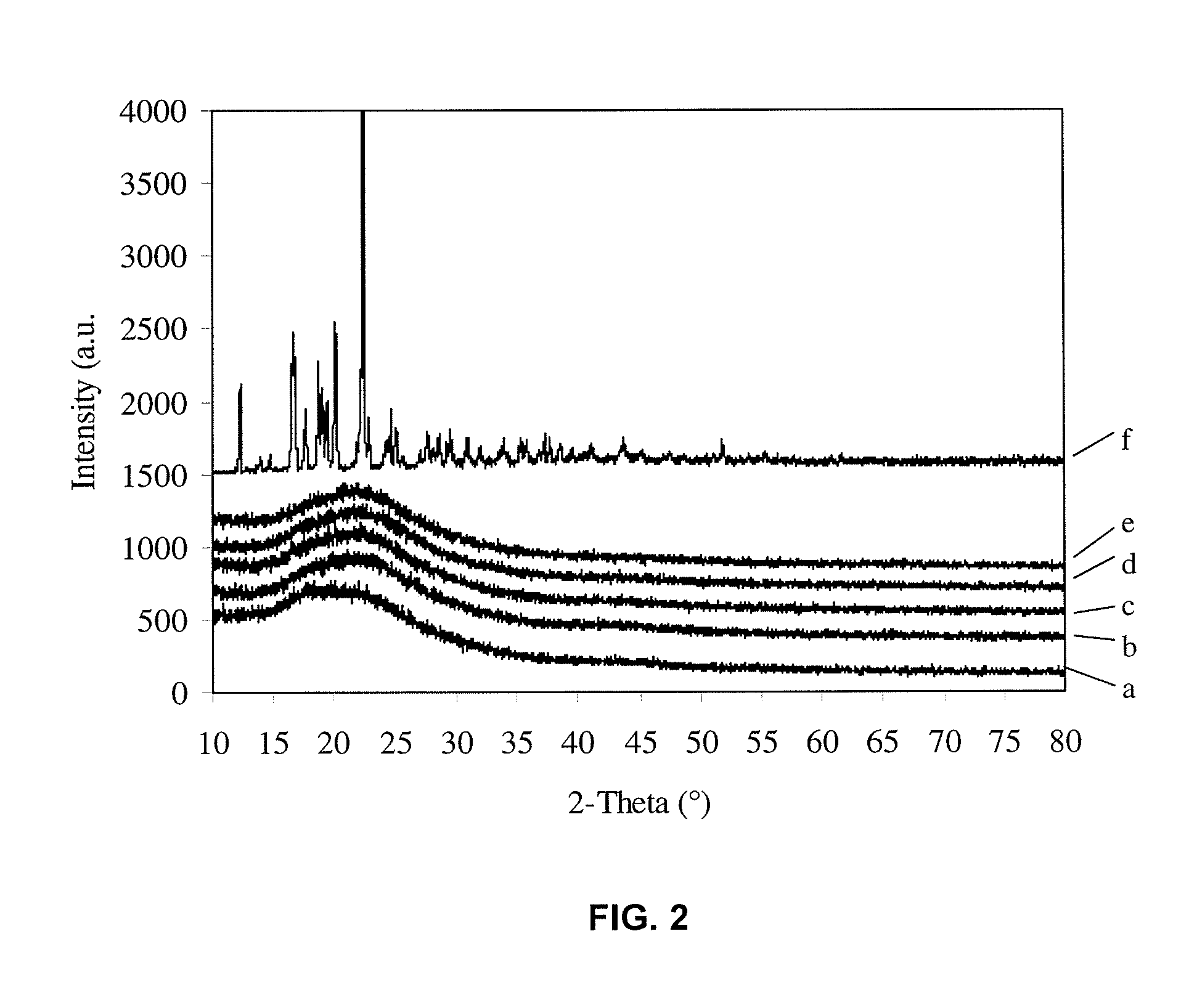
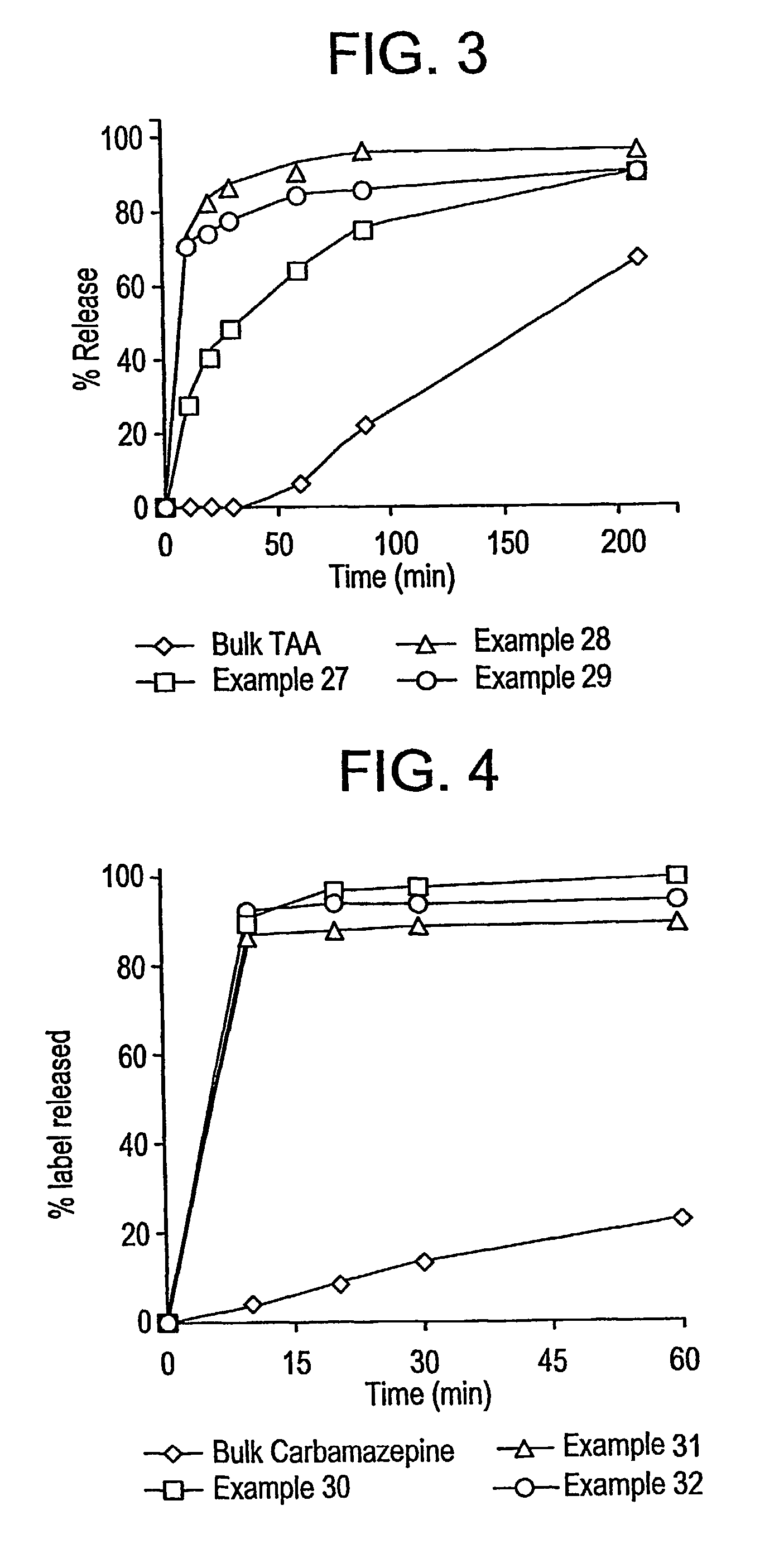
A method for preparing poorly water soluble drug particles is disclosed. The method comprises dissolving a drug in at least one organic solvent to form a drug / organic mixture, spraying the drug / organic mixture into an aqueous solution and concurrently evaporating the organic solvent in the presence of the aqueous solution to form an aqueous dispersion of the drug particles. The resulting drug particles are in the nanometer to micrometer size range and show enhanced dissolution rates and reduced crystallinity when compared to the unprocessed drug. The present invention additionally contemplates products and processes for new drug formulations of insoluble drug particles having high dissolution rates and extremely high drug-to-excipient ratios.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method of Treating Chronic Kidney Disease
InactiveUS20090186939A1Prevent and reverse and maintain and delay progressionAvoid developmentPowder deliveryBiocideSimple Organic CompoundsEnhanced dissolution
Owner:CSIR +1
Mesoporous material excipients for poorly aqueous soluble ingredients
ActiveUS20110244002A1Easy to scaleInstability concernBiocidePowder deliveryAdditive ingredientEnhanced dissolution
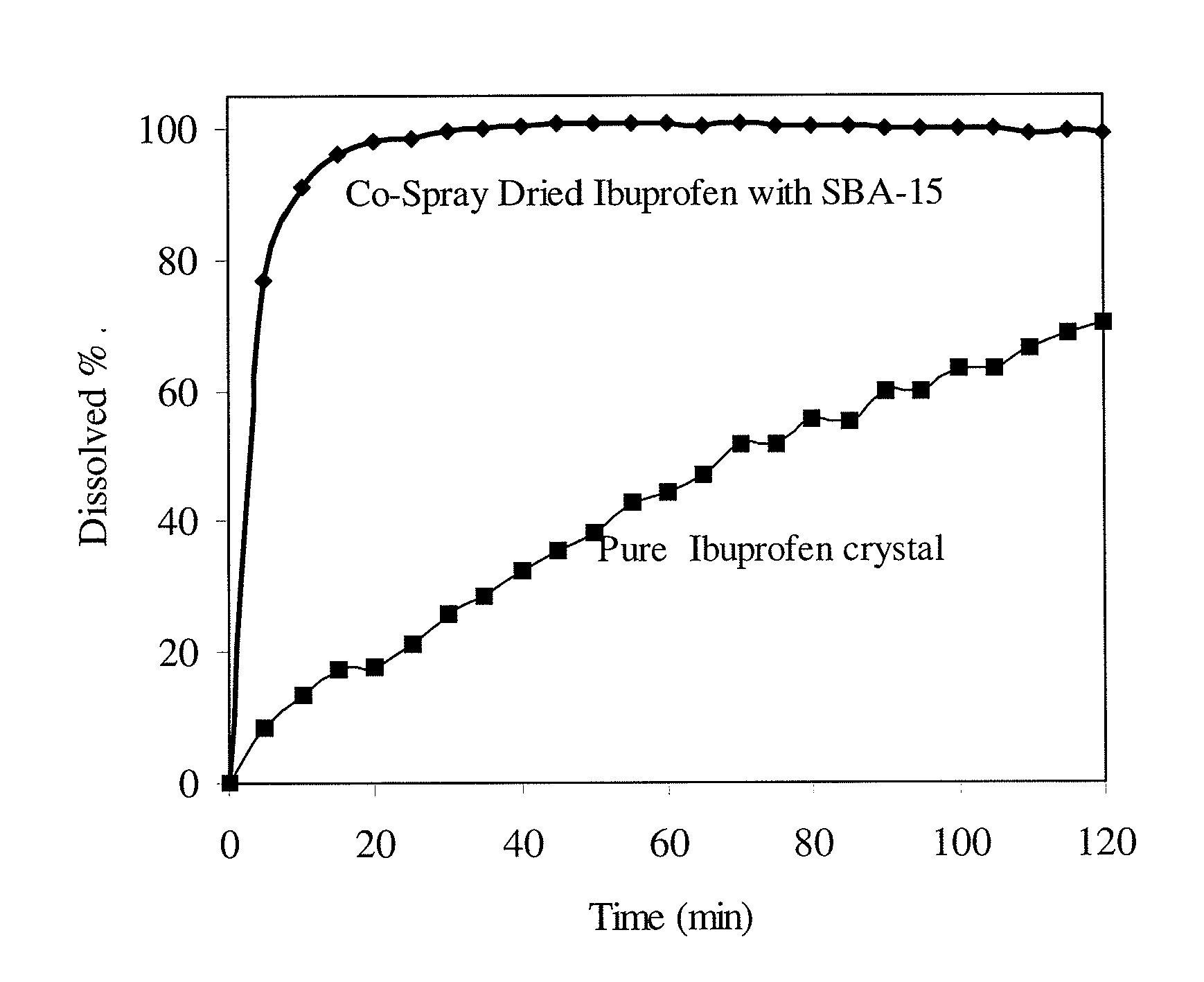
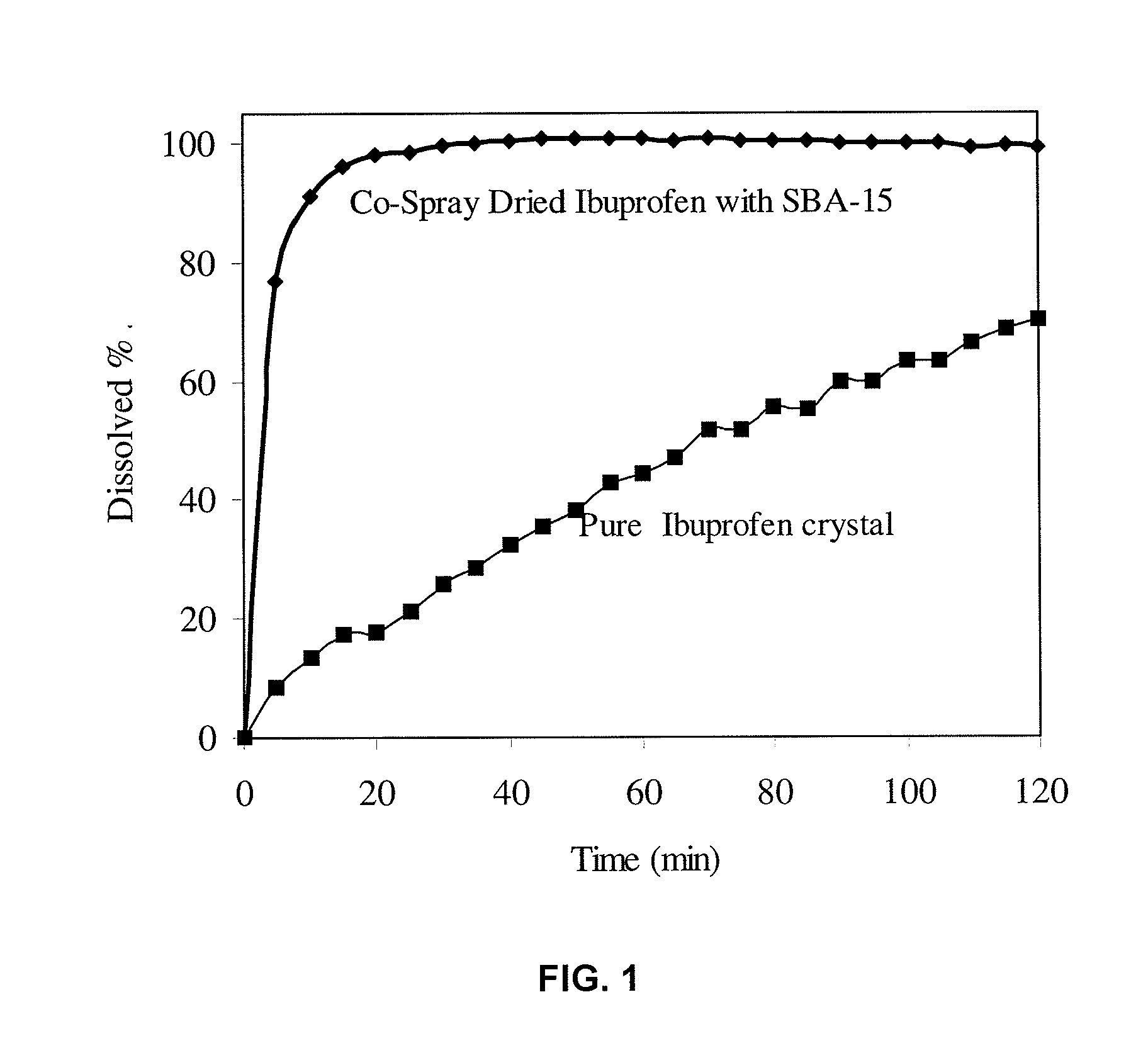
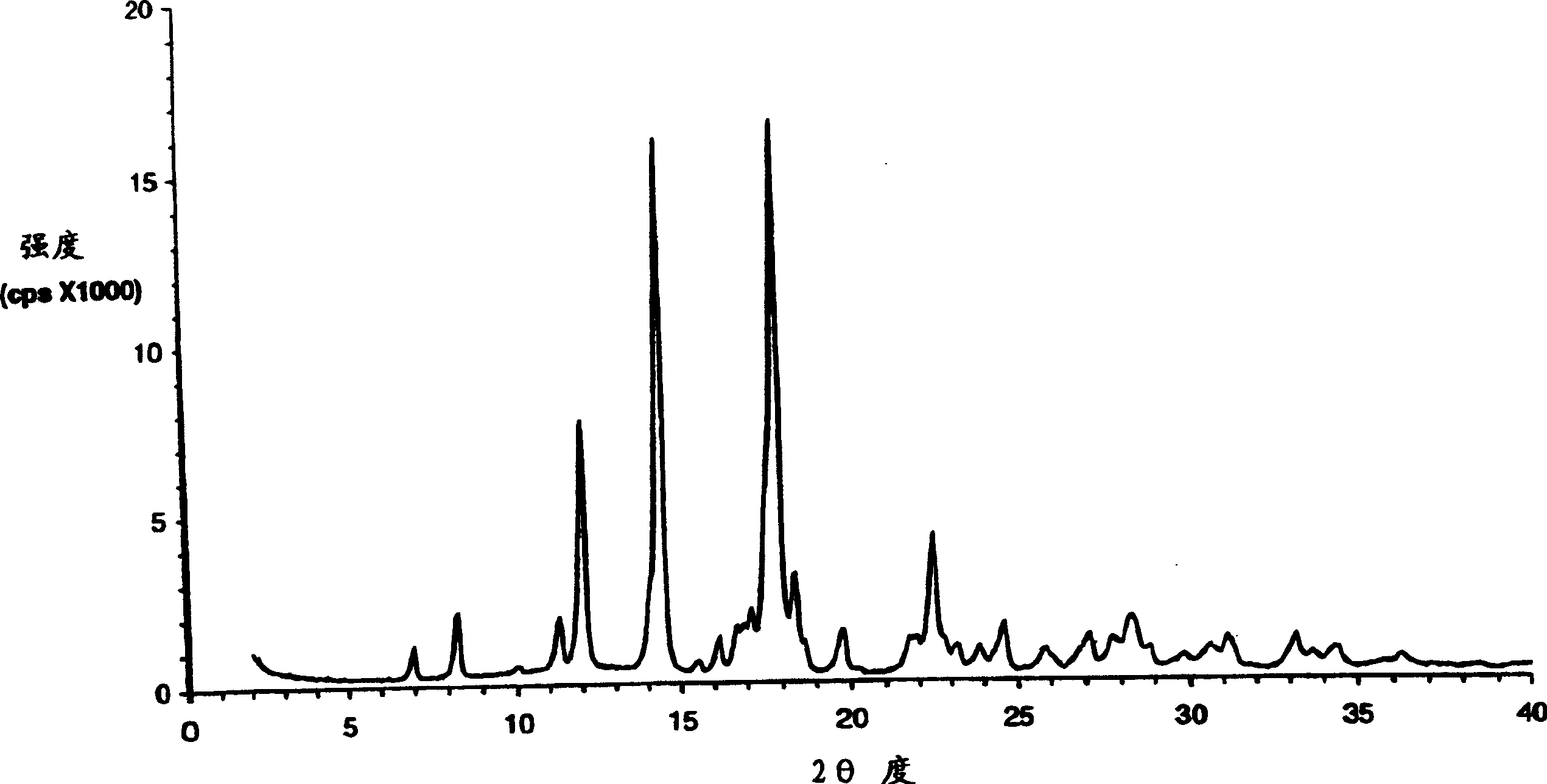
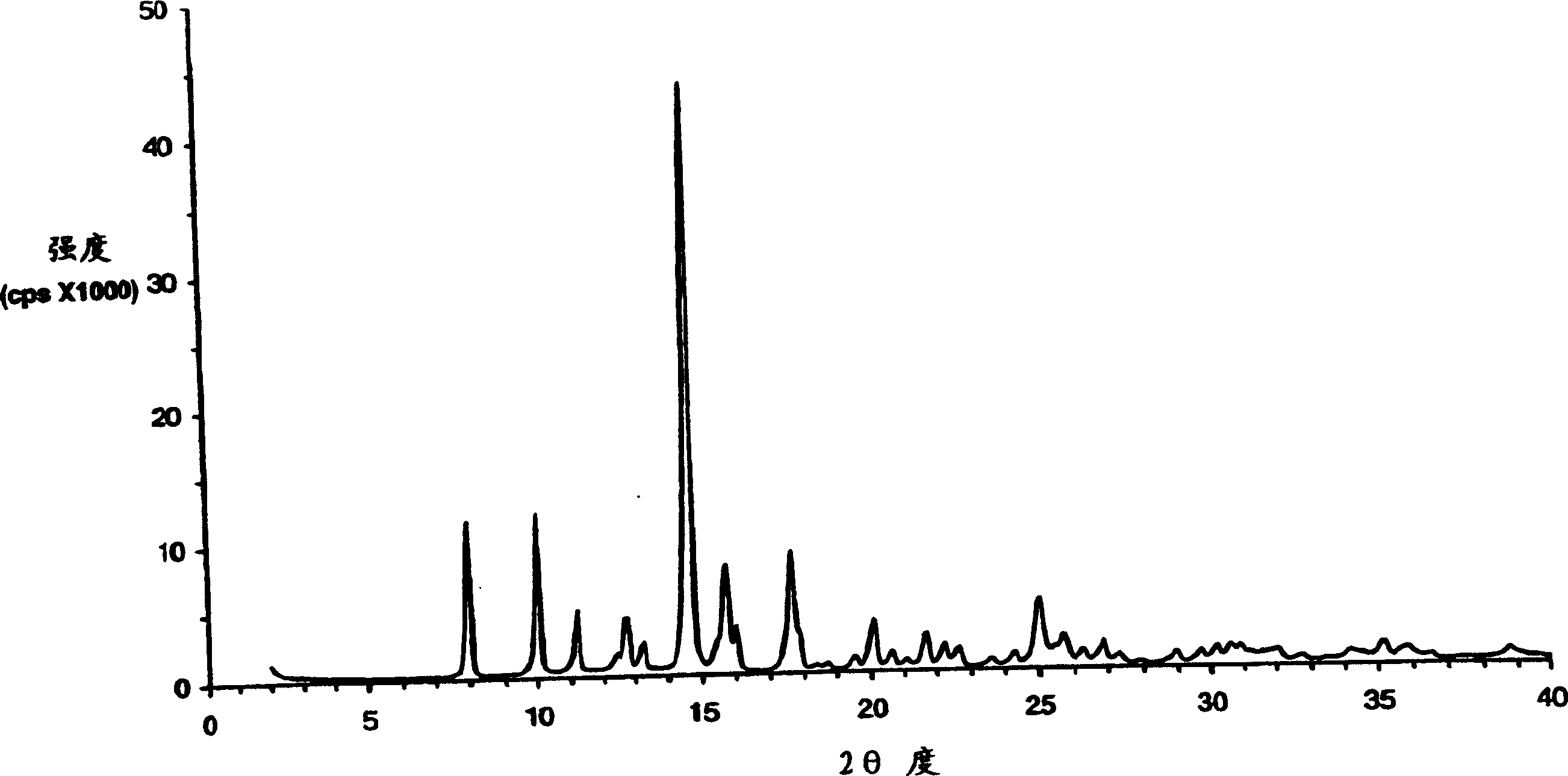
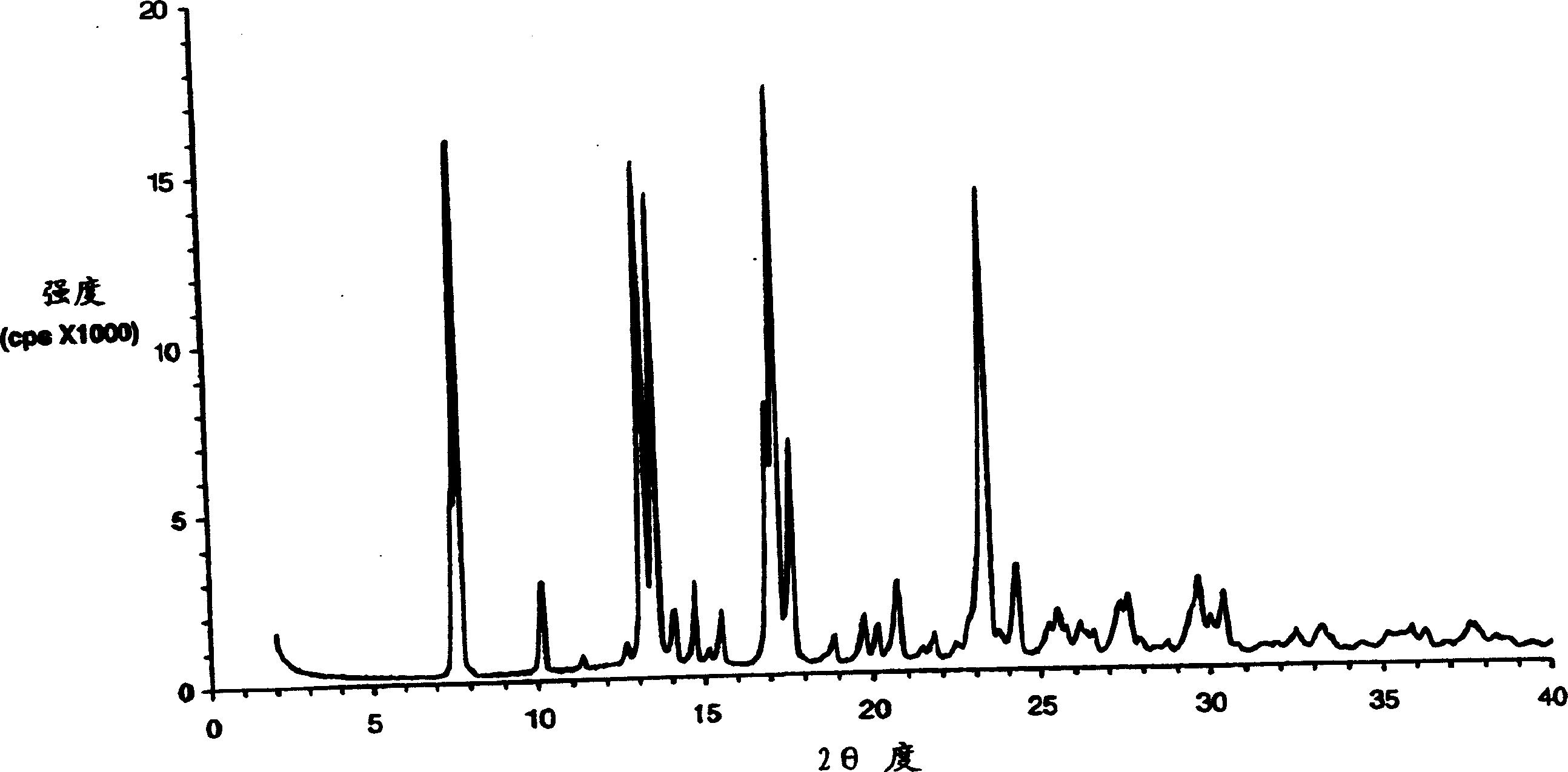
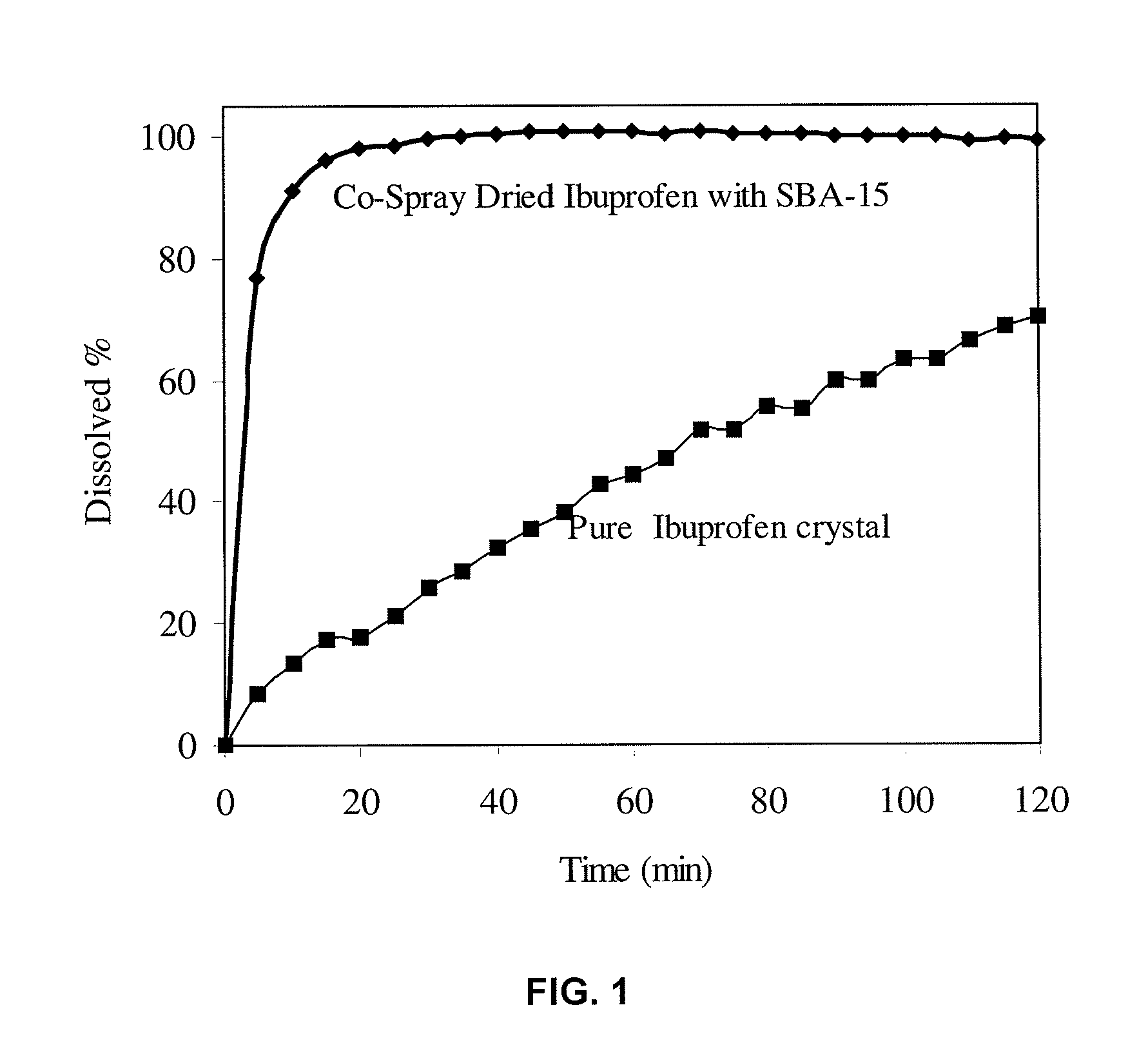
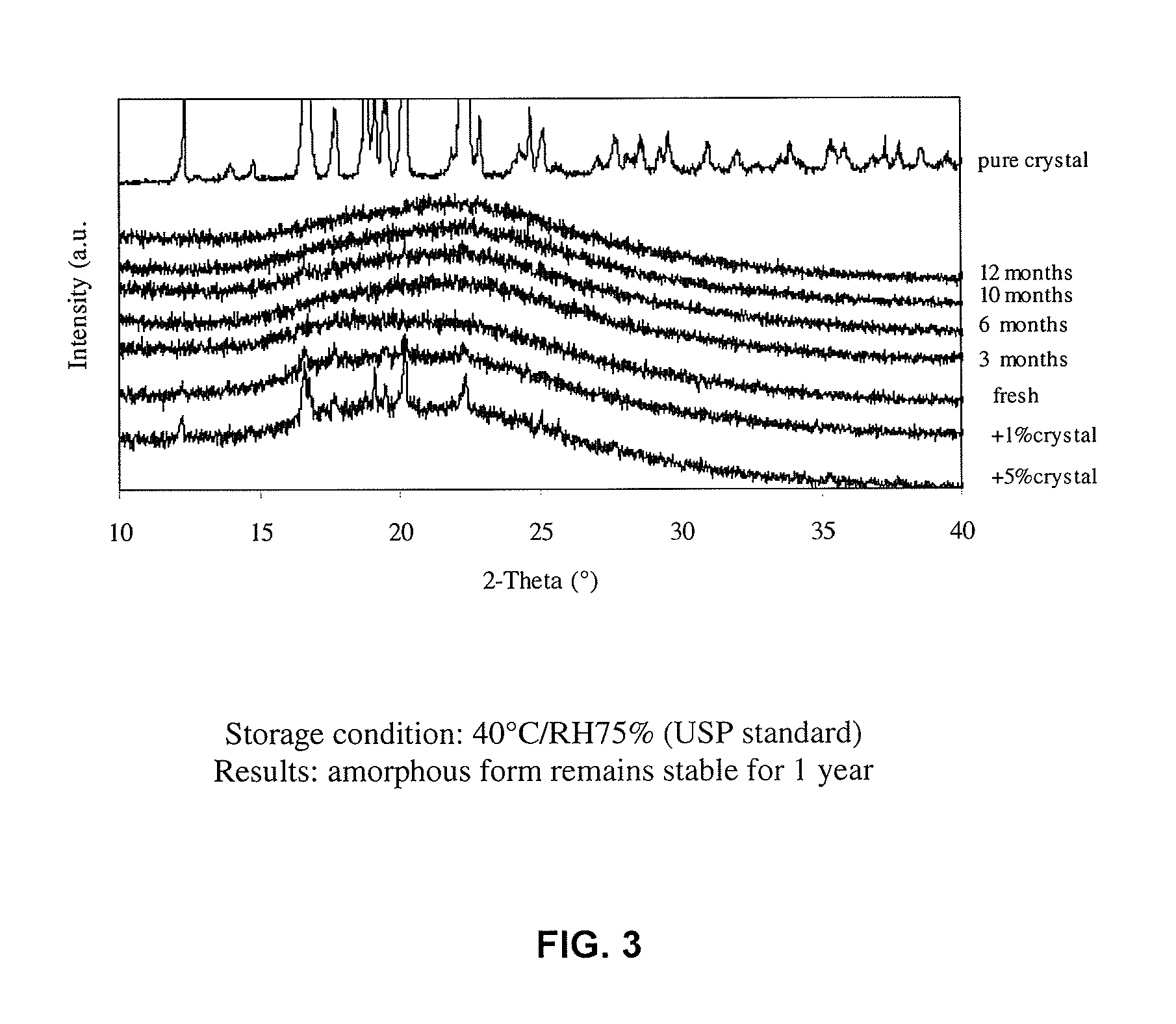
The present invention encompasses formulations and methods for producing solid dispersions comprising mesoporous materials with poorly aqueous soluble active ingredients. The active ingredient is formed in the amorphous state and entrapped in the nanosized pores of the mesoporous excipients using a co-spray drying process. The pore walls of mesoporous channels stabilize the amorphous form of active ingredient against re-crystallization. The amorphous active ingredient entrapped in mesoporous channels exhibits good stability during extended storage under stress test conditions and possesses significantly enhanced dissolution rates.
Owner:AGENCY FOR SCI TECH & RES
Positive resist composition
A hydroxystyrene-(meth)acrylate copolymer in which some phenolic hydroxyl groups are crosslinked with acid labile groups is blended as a base resin in a positive resist composition, which has the advantages of enhanced dissolution inhibition and an increased dissolution contrast after exposure.
Owner:SHIN ETSU CHEM IND CO LTD
Method and composition to improve absorption of therapeutic agents
ActiveUS20100286100A1Improve machinabilityFast dissolutionPowder deliverySalicyclic acid active ingredientsAspirinSodium bicarbonate
A tablet with an enhanced dissolution profile for a medicinally active ingredient such as aspirin and methods for making the tablet. The tablet comprises a blend of crystals of the medicinally active ingredient and a dissolution aid such as sodium or calcium carbonate or bicarbonate that coats the crystals upon co-milling. The blend is then compressed to form tablets that have an enhanced dissolution profile for the medicinally active ingredient.
Owner:BAYER HEALTHCARE LLC
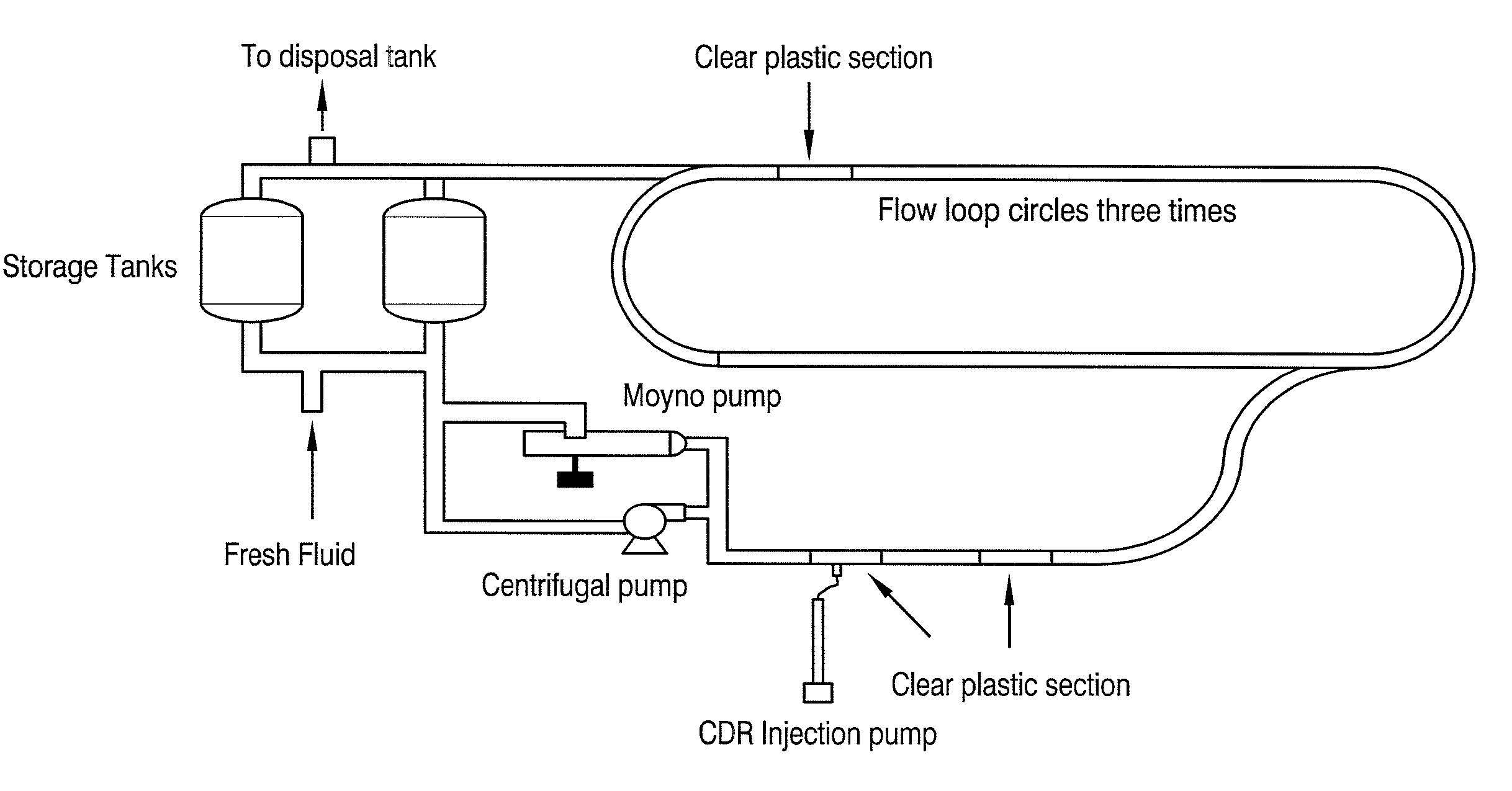
Modified latex drag reducer and processes therefor and therewith
ActiveUS20070240762A1Stable latex drag reducerOther chemical processesFibre treatmentEmulsion polymerizationFriction loss
A modified latex drag reducer and methods of making and using the drag reducer in order to reduce friction losses resulting from turbulent fluid flow through a conduit. Particularly, the modified latex drag reducer is formed from an initial latex which is a product of an emulsion polymerization reaction. The initial latex is then modified, preferably by admixing with at least one low HLB surfactant or at least one solvent, or both, to form a modified latex with an enhanced dissolution rate in a hydrocarbon stream over the initial latex.
Owner:LIQUIDPOWER SPECIALTY PROD INC
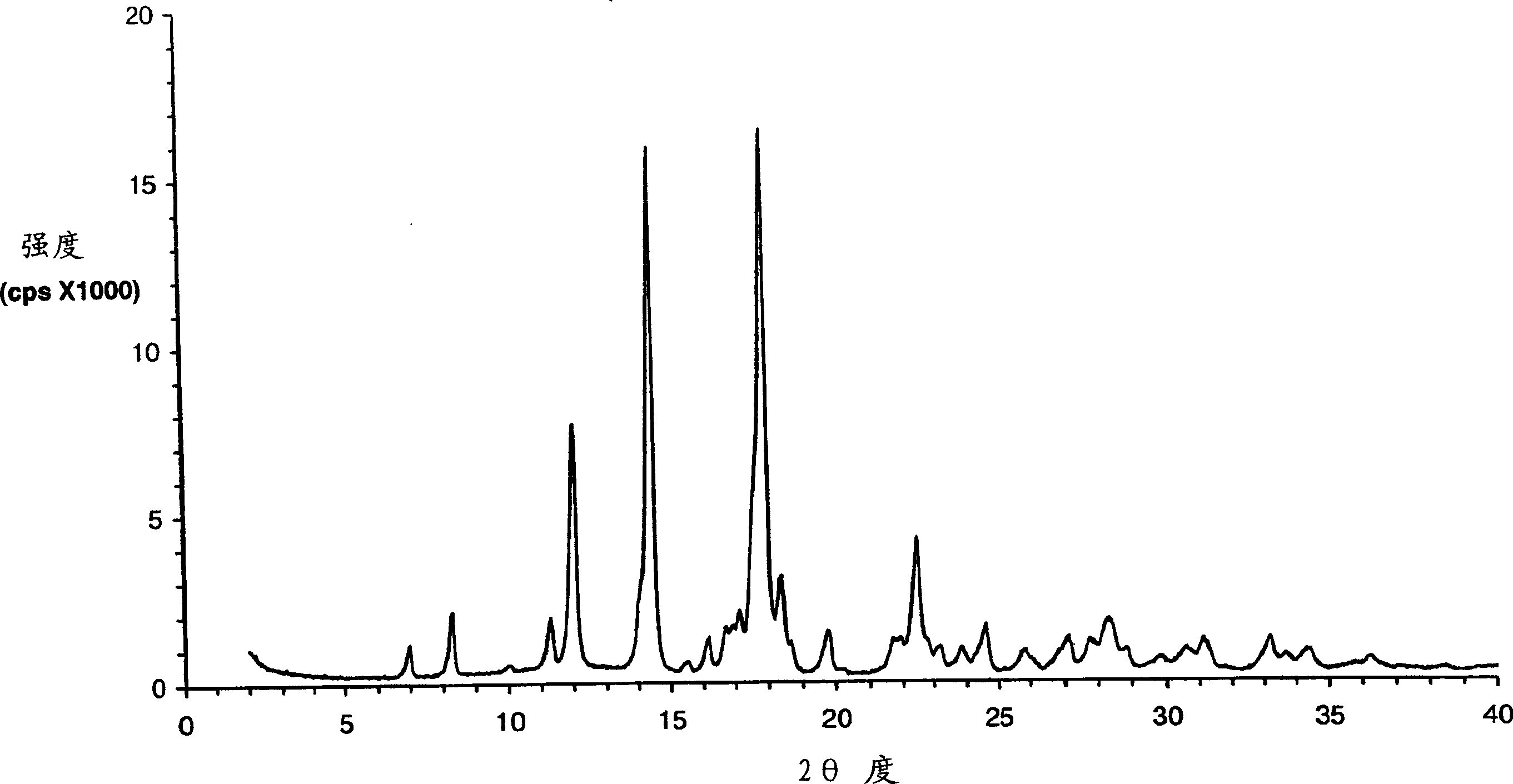
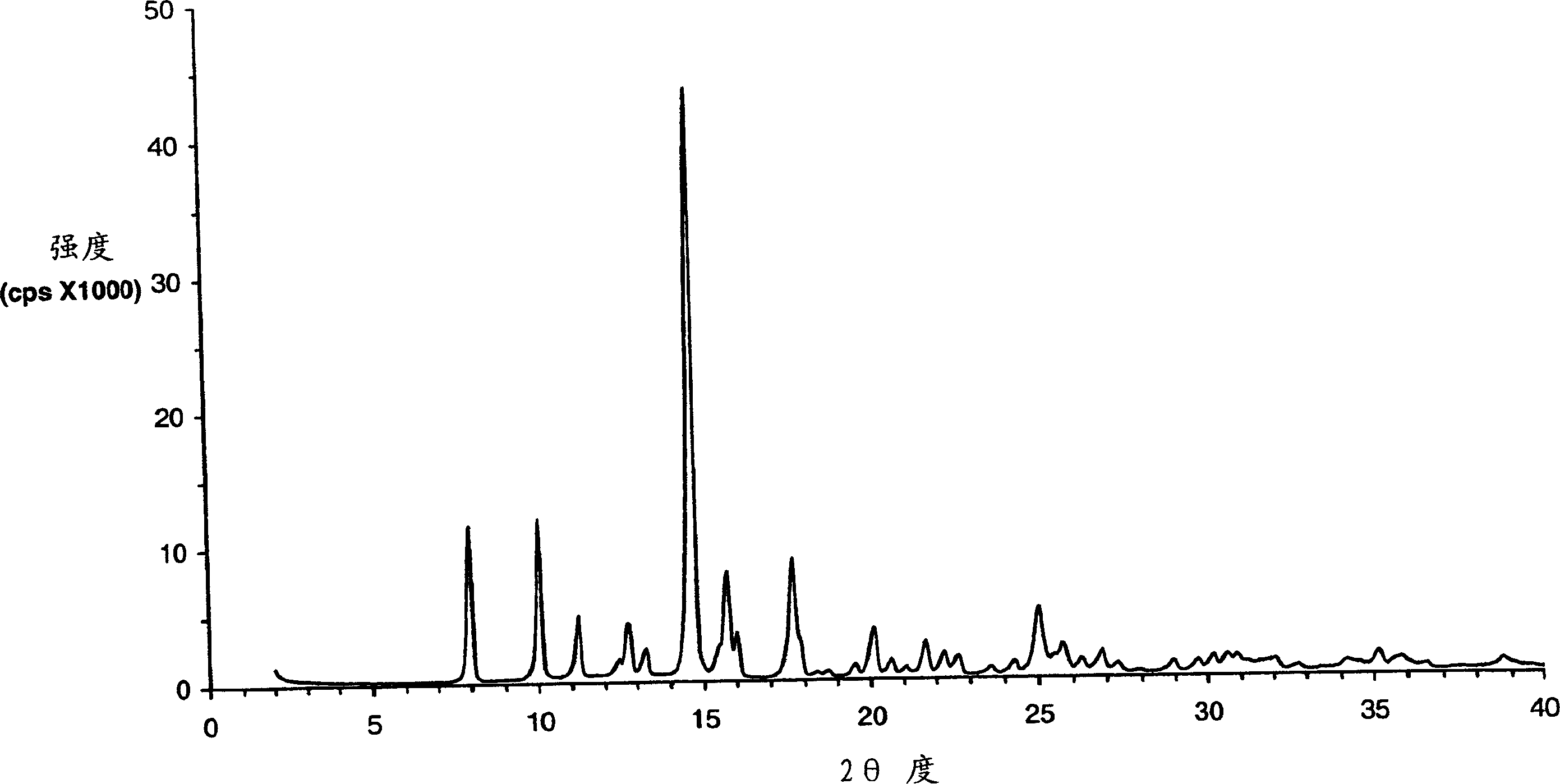
Eplerenone crystalline form exhibiting enhanced dissolution rate
A novel crystalline form (Form H) of the aldosterone receptor antagonist drug eplerenone is provided having a relatively rapid dissolution rate in aqueous media. Also provided are novel solvated crystalline forms of eplerenone that, when desolvated, can yield Form H eplerenone. Also provided is amorphous eplerenone. Pharmaceutical compositions are provided comprising Form H eplerenone, optionally accompanied by one or more other solid state forms of eplerenone, in a total unit dosage amount of eplerenone of about 10 to about 1000 mg, and further comprising one or more pharmaceutically acceptable excipients. Processes are provided for preparing Form H eplerenone and for preparing compositions comprising Form H eplerenone. A method for prophylaxis and / or treatment of an aldosterone-mediated condition or disorder is also provided, comprising administering to a subject a therapeutically effective amount of eplerenone, wherein at least a fraction of the eplerenone present is Form H eplerenone.
Owner:GD SEARLE & CO
Methods for enhancing the release and absorption of water insoluble active agents
InactiveUS8524280B2Improved profilePromote absorptionBiocidePowder deliveryHydrophilic polymersActive agent
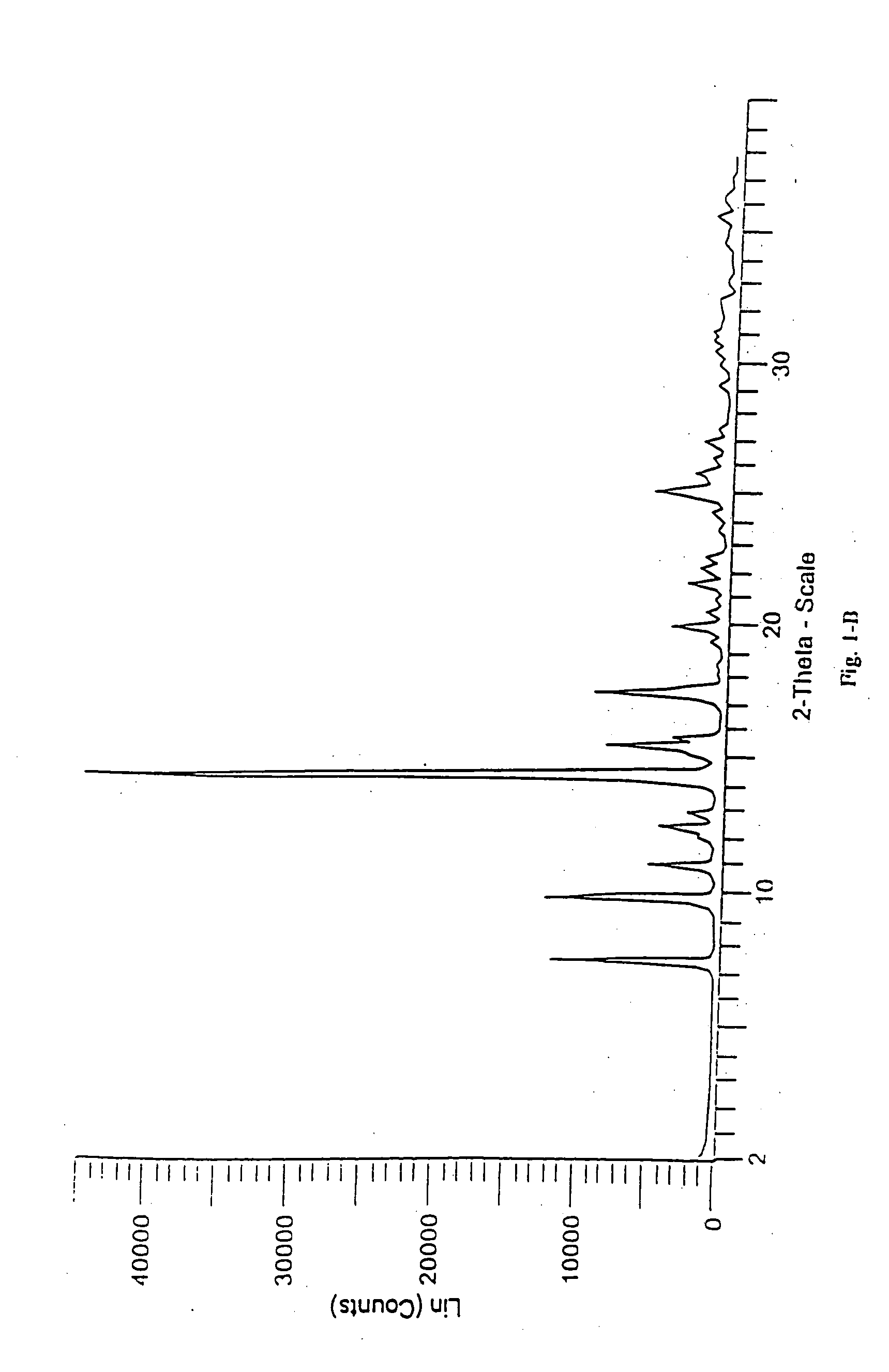
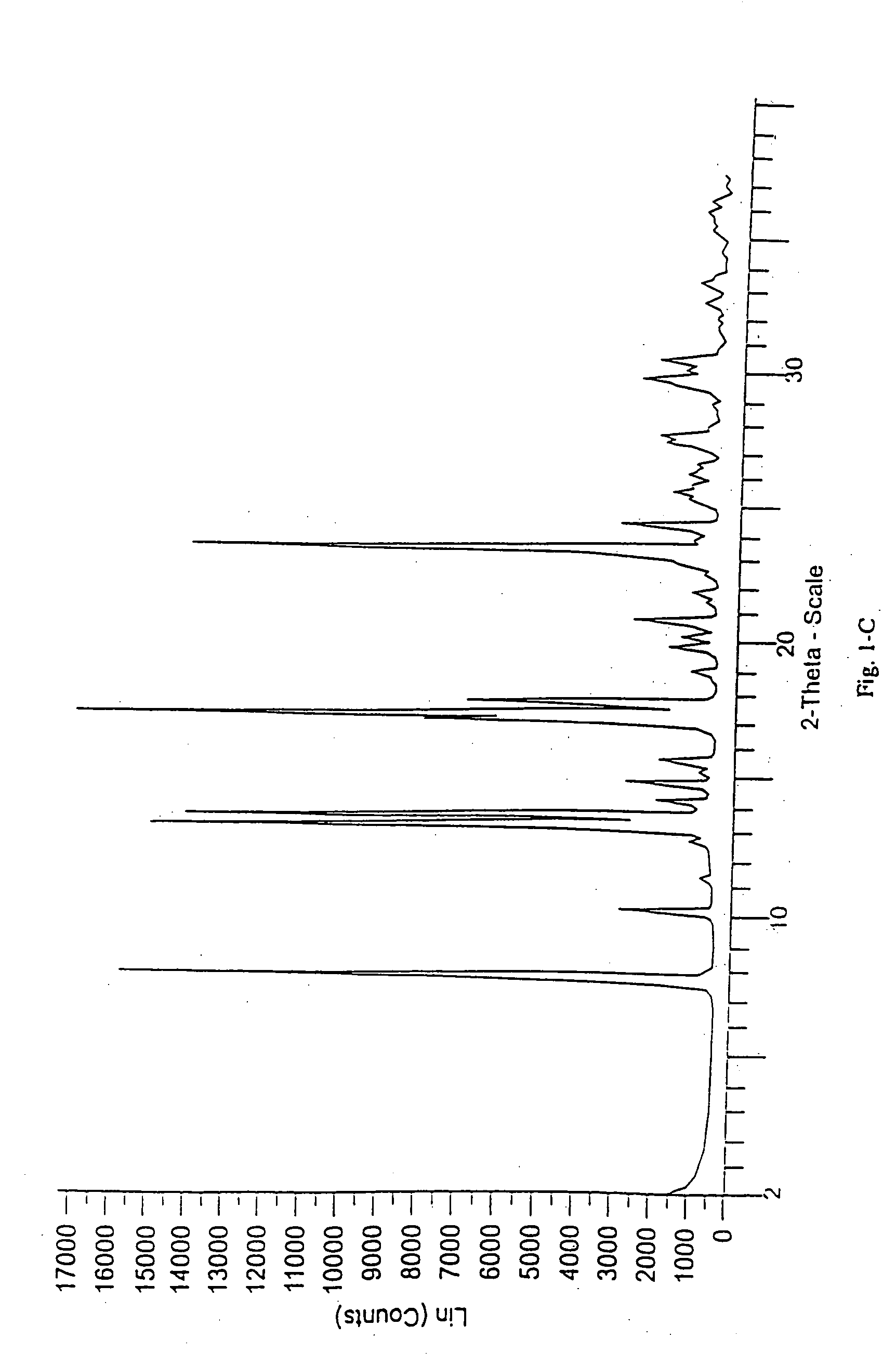
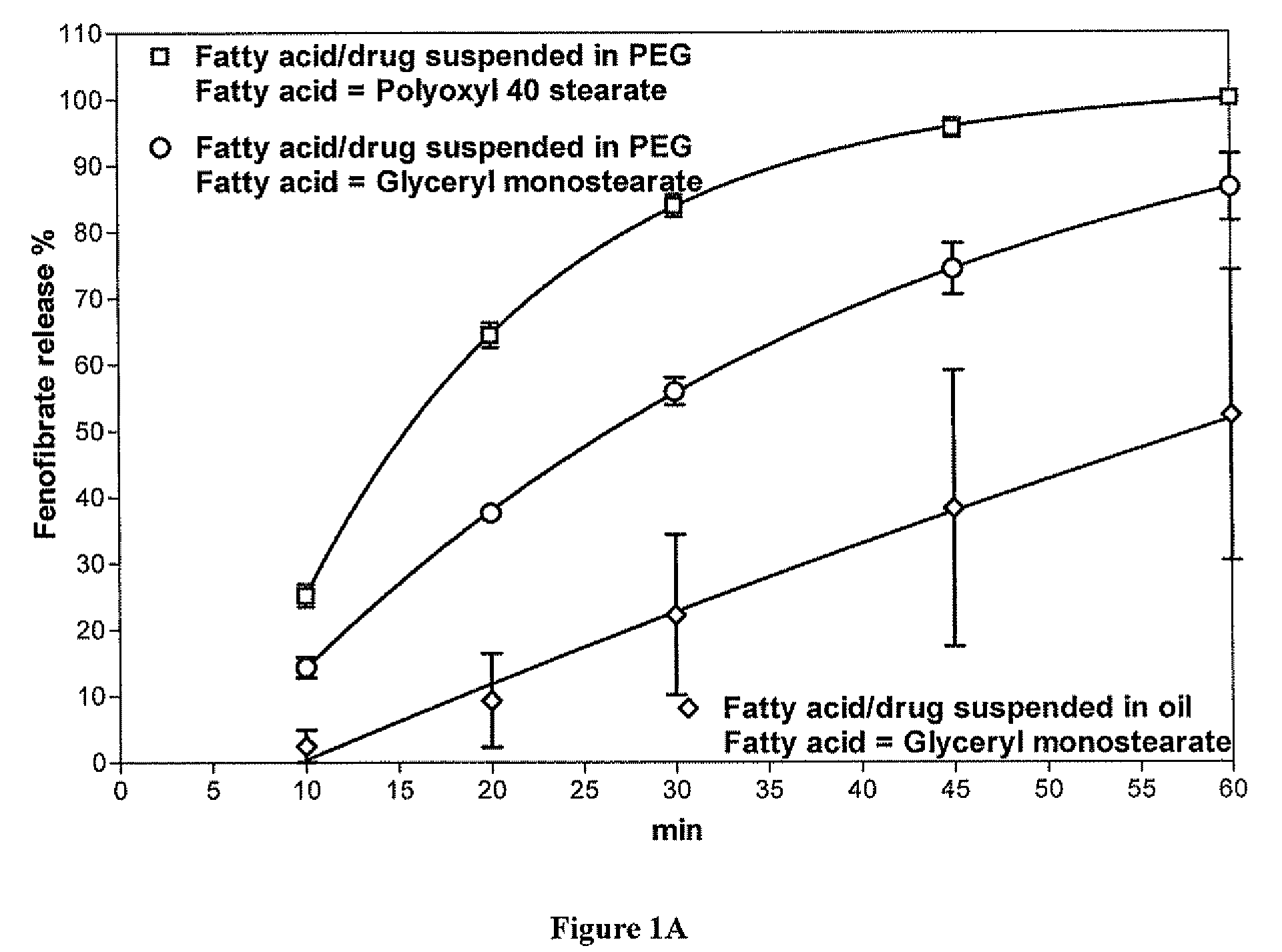
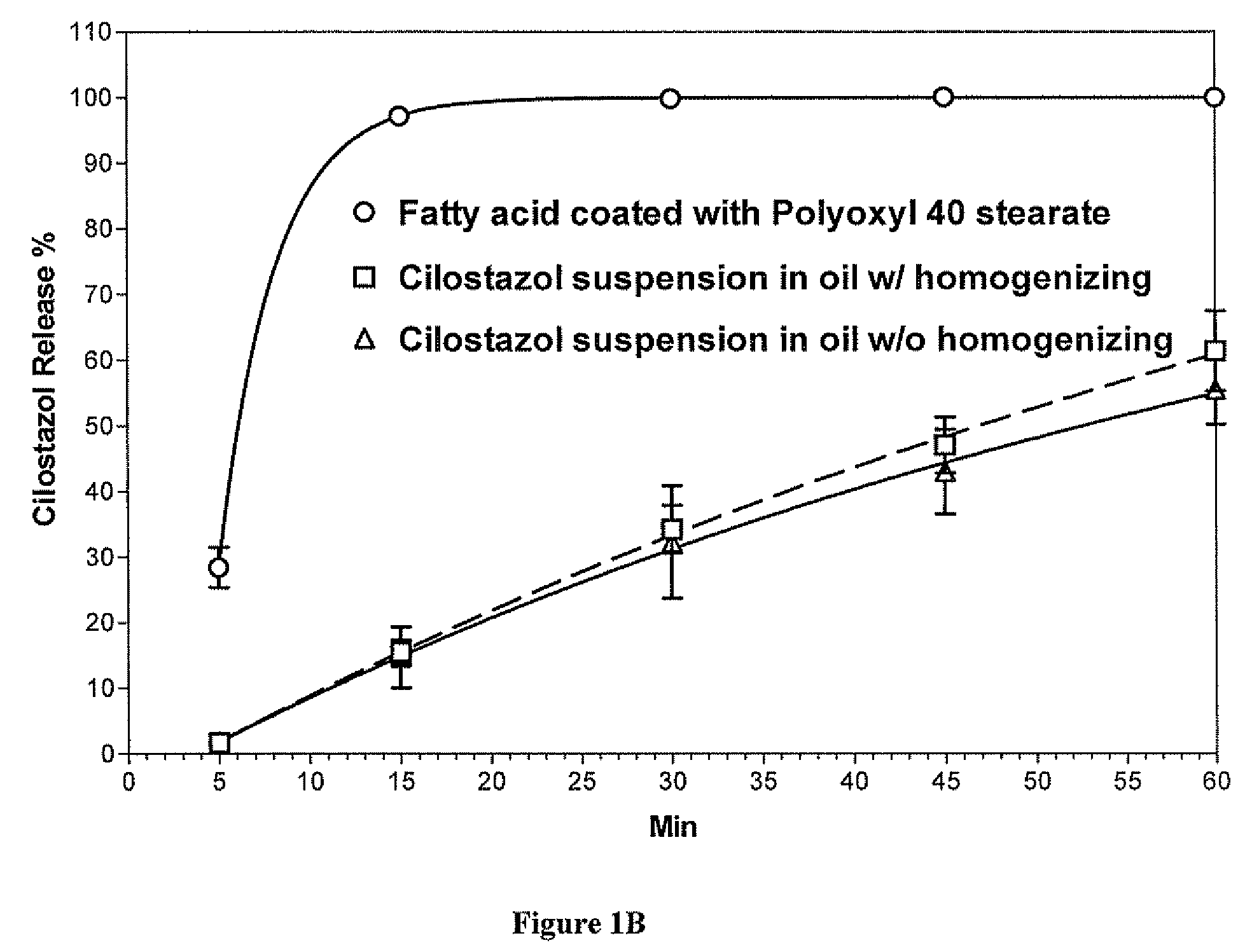
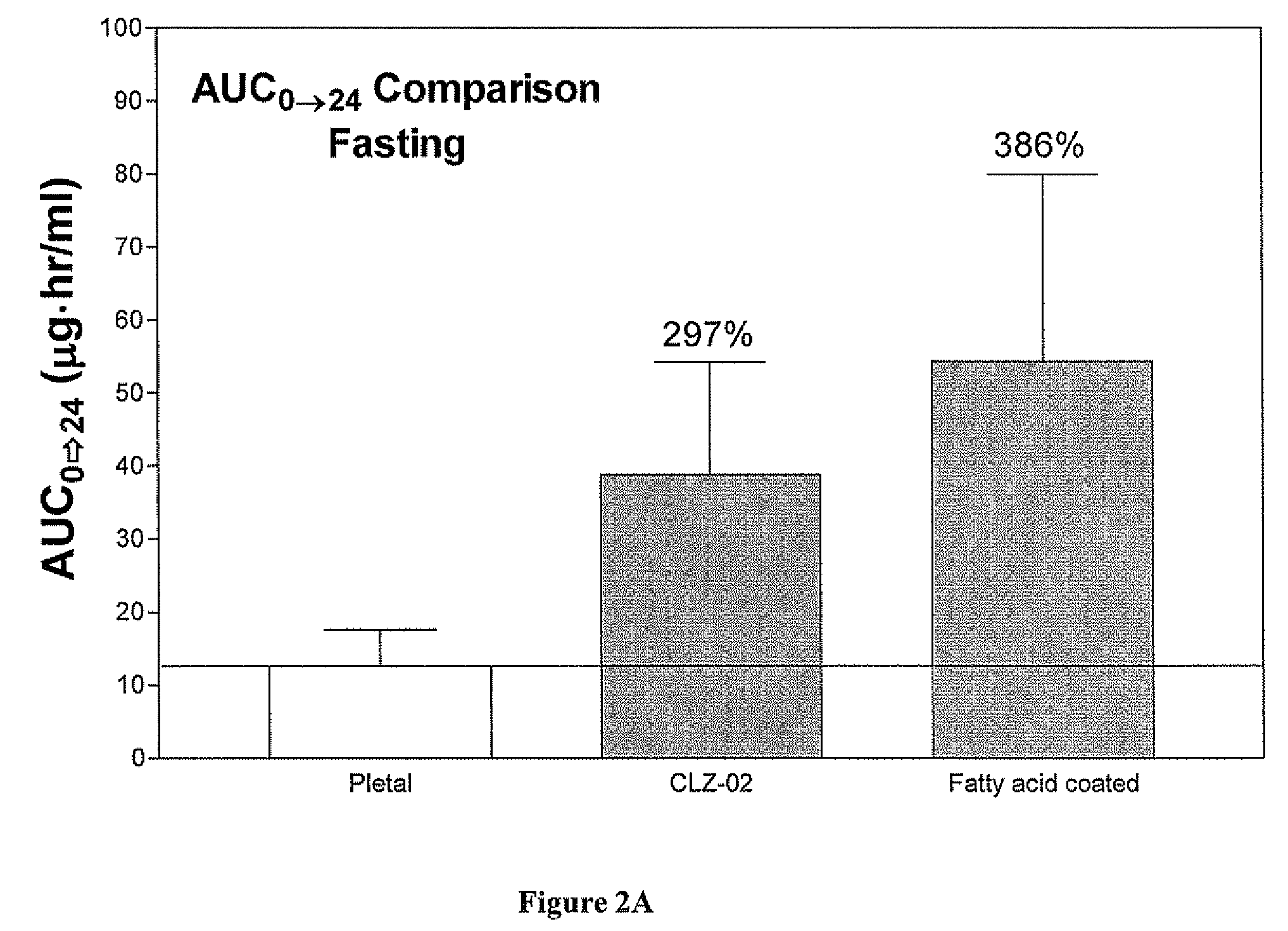
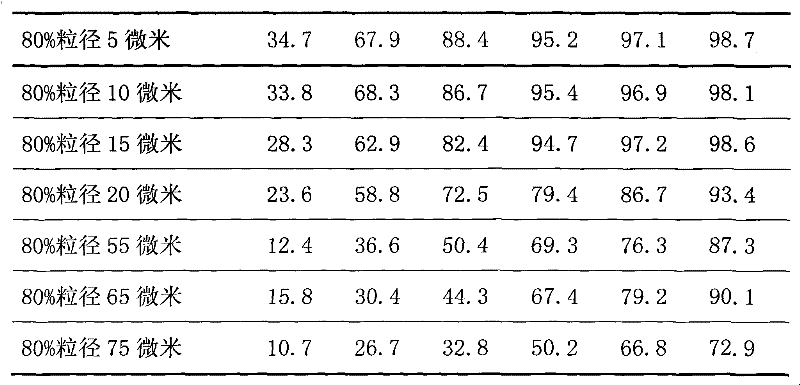
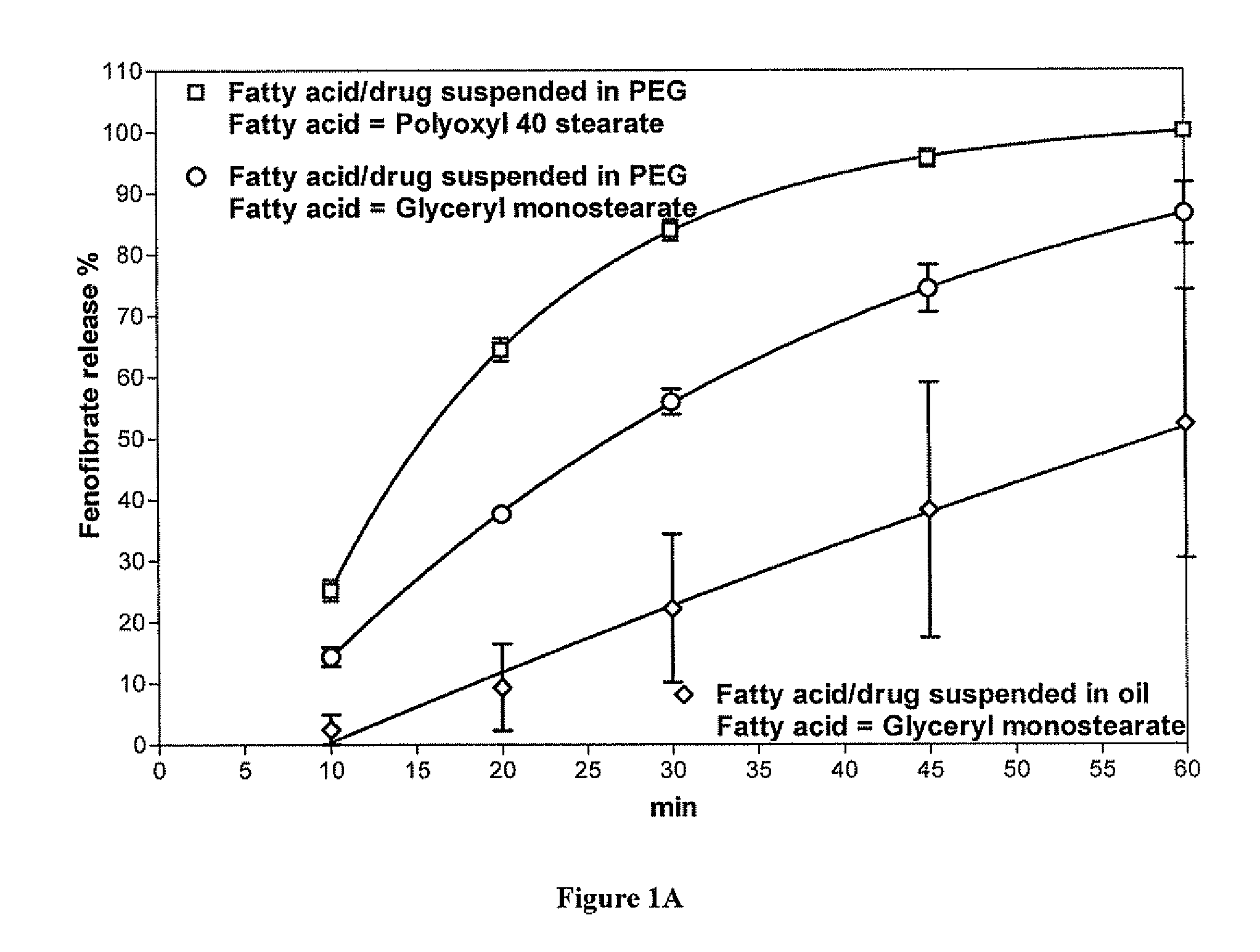
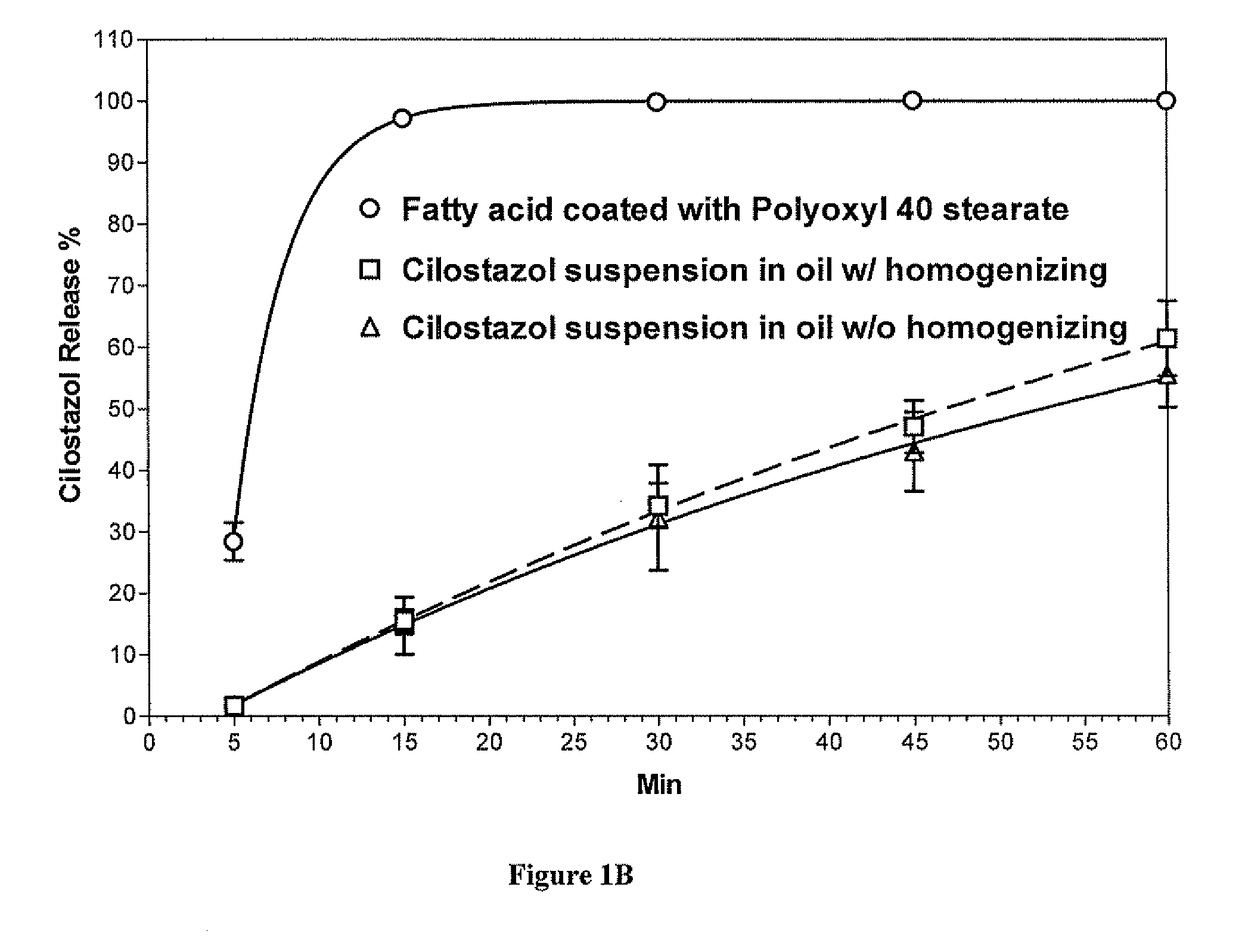
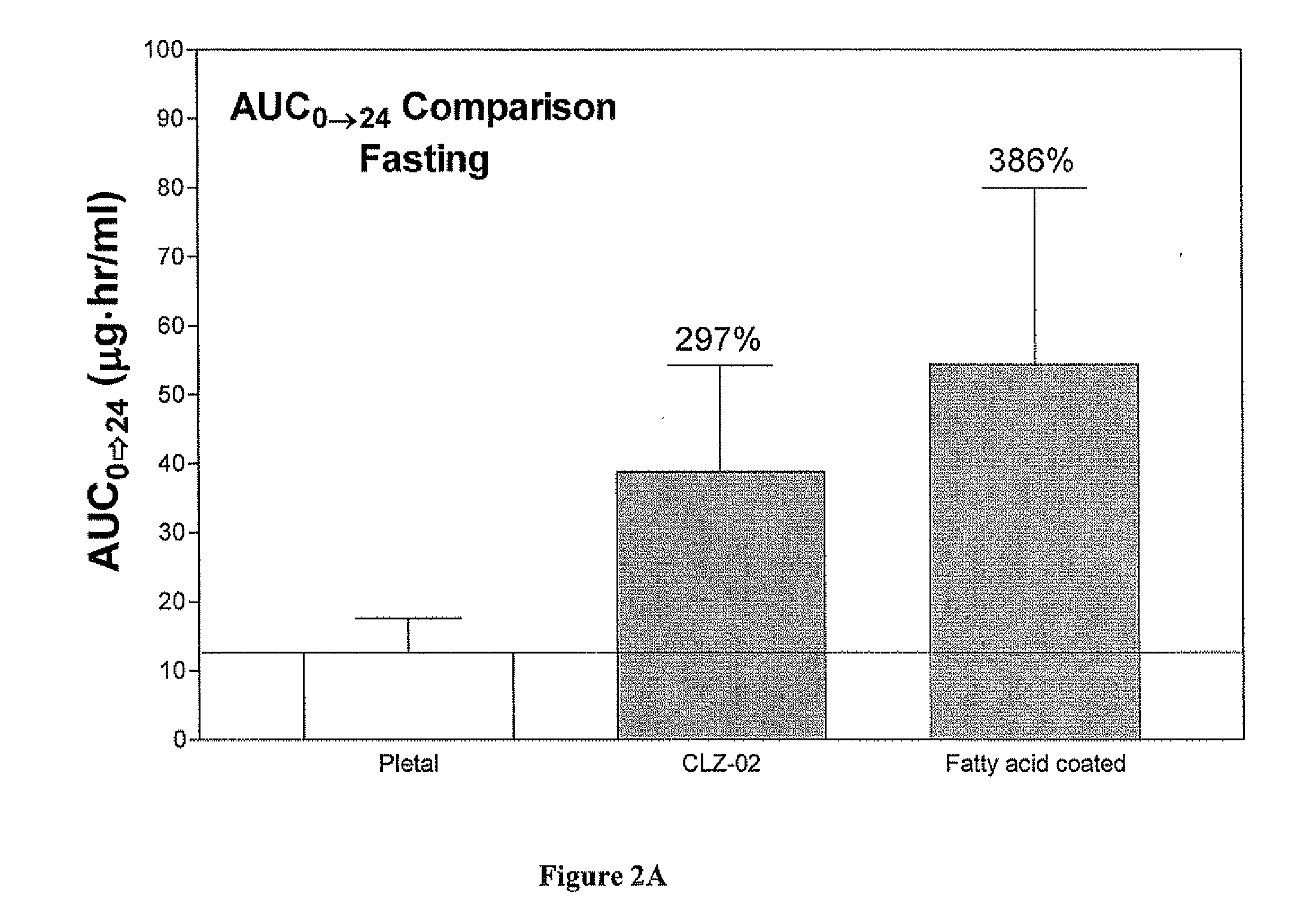
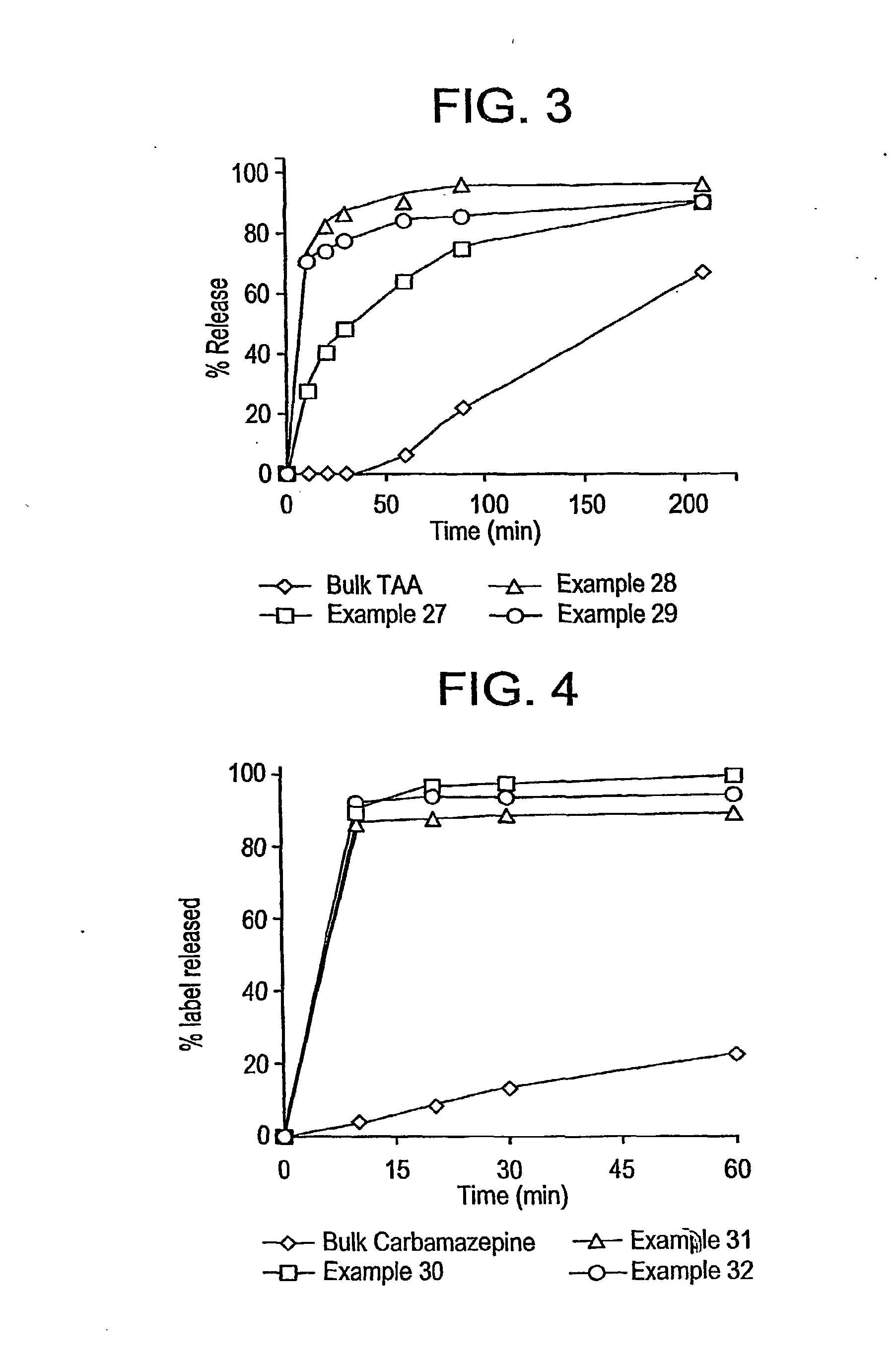
Methods for enhancing the release and / or absorption of poorly water soluble active agents are described herein. The method involves dissolving, melting, or suspending a poorly water soluble active agent in one or more molten fatty acids, conjugated fatty acids, (semi-) solid surfactants of high HLB value, and / or hydrophilic polymers. The molten active agent mixture is then suspended and homogenized in a hydrophilic or lipophilic carrier to form microparticles suspended in the hydrophilic or lipophilic carrier. The particles suspended in the hydrophilic or lipophilic carrier can be encapsulated in a hard or soft gelatin or non-gelatin capsule. It is believed that the microparticles produced by the method described above will exhibit enhanced dissolution profiles. In vitro release studies of formulations containing cilostazol and fenofibrate showed 100% dissolution of cilostazol in 15 minutes and over 90% dissolution of fenofibrate in 35 minutes.
Owner:PATHEON SOFTGELS INC
Method and composition to improve absorption of therapeutic agents
InactiveUS20130122098A1Improve machinabilityFast dissolutionPowder deliveryBiocideSodium bicarbonateAspirin
A tablet with an enhanced dissolution profile for a medicinally active ingredient such as aspirin and methods for making the tablet. The tablet comprises a blend of crystals of the medicinally active ingredient and a dissolution aid such as sodium or calcium carbonate or bicarbonate that coats the crystals upon co-milling. The blend is then compressed to form tablets that have an enhanced dissolution profile for the medicinally active ingredient.
Owner:BAYER HEALTHCARE LLC
Micronized deflazacort oral preparation and preparation method thereof
The invention discloses a deflazacort oral preparation and a preparation method thereof. The deflazacort oral preparation comprises micronized deflazacort, wherein 50 to 100% of deflazacort particles have particle sizes less than or equal to 50 micrometers. The micronized deflazacort oral preparation is prepared from 2 to 40% of micronized deflazacort, 50 to 95% of one or more fillers, 1 to 10% of one or more disintegrants, 1 to 10% of one or more binders and 0.3 to 1% of a lubricant. The micronized deflazacort oral preparation is characterized in that through a process of mixing raw materials and lactose in an equal amount increasing way, a uniformity degree and a dissolution rate of raw materials can be improved; micronized deflazacort can improve a dissolution degree in vitro; and selected and combined raw materials are suitable for drug molding and have a high stability and good drug effects.
Owner:SHANGHAI ANHANTE BIOPHARM TECH
Fenofibrate compositions
The present invention relates to a fenofibrate composition comprising: fenofibrate, a surfactant, a hydrophilic polymer and one or more anti-foaming agents. The invention also relates to a novel process for preparing said composition that has enhanced dissolution and absorption characteristics.
Owner:RANBAXY LAB LTD
Methods for enhancing the release and absorption of water insoluble active agents
InactiveUS20110052682A1Improved dissolution profilePromote absorptionBiocidePowder deliveryHydrophilic polymersActive agent
Methods for enhancing the release and / or absorption of poorly water soluble active agents are described herein. The method involves dissolving, melting, or suspending a poorly water soluble active agent in one or more molten fatty acids, conjugated fatty acids, (semi-) solid surfactants of high HLB value, and / or hydrophilic polymers. The molten active agent mixture is then suspended and homogenized in a hydrophilic or lipophilic carrier to form microparticles suspended in the hydrophilic or lipophilic carrier. The particles suspended in the hydrophilic or lipophilic carrier can be encapsulated in a hard or soft gelatin or non-gelatin capsule. It is believed that the microparticles produced by the method described above will exhibit enhanced dissolution profiles. In vitro release studies of formulations containing cilostazol and fenofibrate showed 100% dissolution of cilostazol in 15 minutes and over 90% dissolution of fenofibrate in 35 minutes.
Owner:PATHEON SOFTGELS INC
Efficient scleroglucan extraction and purification method and application thereof
The invention discloses an efficient scleroglucan extraction and purificatiodn method and an application thereof. The method includes: 1), performing microwave enhanced dissolution after dilute alkali dissolution by a mycelium contained scleroglucan crude product or a high-concentration scleroglucan fermentation liquid raw material; 2), performing separation on the scleroglucan solution obtained form the step 1) to remove the insoluble mycelium with frame filter or centrifugal separation and merging a scrubbing solution into the scleroglucan solution by adopting the mycelium obtained from the scrubbing solution; 3), neutralizing the scleroglucan solution after bacterial removal; 4), treating the scleroglucan solution through an ultrafiltration membrane prior to desalting and concentrating, and washing the scleroglucan concentrated solution is washed through water and prior to desalting; 5), precipitating and washing the scleroglucan with ethyl alcohol to obtain the product after drying. Through treatment of a series of physical methods, the stable and efficient scleroglucan preparation process is established, chemical reaction is not needed, impurity components in the finished product are reduced, and the purity of the targeted scleroglucan product is up to 90%.
Owner:SHANDONG FOOD & FERMENT IND RES & DESIGN INST
Eplerenone crystalline form exhibiting enhanced dissolution rate
InactiveCN1891209AHigh chemical purityOrganic active ingredientsNervous disorderEnhanced dissolutionSolvation
A novel crystalline form (Form H) of the aldosterone receptor antagonist drug eplerenone is provided having a relatively rapid dissolution rate in aqueous media. Also provided are novel solvated crystalline forms of eplerenone that, when desolvated, can yield Form H eplerenone. Also provided is amorphous eplerenone. Pharmaceutical compositions are provided comprising Form H eplerenone, optionally accompanied by one or more other solid state forms of eplerenone, in a total unit dosage amount of eplerenone of about 10 to about 1000 mg, and further comprising one or more pharmaceutically acceptable excipients. Processes are provided for preparing Form H eplerenone and for preparing compositions comprising Form H eplerenone. A method for prophylaxis and / or treatment of an aldosterone-mediated condition or disorder is also provided, comprising administering to a subject a therapeutically effective amount of eplerenone, wherein at least a fraction of the eplerenone present is Form H eplerenone.
Owner:PHARMACIA CORP
Mesoporous material excipients for poorly aqueous soluble ingredients
ActiveUS8778401B2Easy to scaleInstability concernBiocidePowder deliveryAdditive ingredientMesoporous material
The present invention encompasses formulations and methods for producing solid dispersions comprising mesoporous materials with poorly aqueous soluble active ingredients. The active ingredient is formed in the amorphous state and entrapped in the nanosized pores of the mesoporous excipients using a co-spray drying process. The pore walls of mesoporous channels stabilize the amorphous form of active ingredient against re-crystallization. The amorphous active ingredient entrapped in mesoporous channels exhibits good stability during extended storage under stress test conditions and possesses significantly enhanced dissolution rates.
Owner:AGENCY FOR SCI TECH & RES
Method and composition to improve absorption of therapeutic agents
ActiveUS11135188B2Improve machinabilityFast dissolutionPowder deliverySalicyclic acid active ingredientsAspirinEnhanced dissolution
A tablet with an enhanced dissolution profile for a medicinally active ingredient such as aspirin and methods for making the tablet. The tablet comprises a blend of crystals of the medicinally active ingredient and a dissolution aid such as sodium or calcium carbonate or bicarbonate that coats the crystals upon co-milling. The blend is then compressed to form tablets that have an enhanced dissolution profile for the medicinally active ingredient.
Owner:BAYER HEALTHCARE LLC
Crystalline drug particles prepared using a controlled precipitation process
Drug particles which are essentially crystalline and have a mean particle size below about 2 microns, when dispersed in water, are described. When added to an aqueous medium at 25-95% of the equilibrium solubility of the drug substance, the drug particles show complete dissolution, as characterized by a 95% reduction in turbidity, in less than 5 minutes. Using a controlled precipitation process to prepare such drug particles is also described. Such drug particles exhibit an enhanced dissolution rate and better stability as compared to particles prepared according to processes described in the prior art.
Owner:TUCKER CHRISTOPHER J +3
Preparation of drug particles using evaporation precipitation into aqueous solutions
InactiveUS8551526B2Raise the ratioHigh dissolution ratePowder deliveryCosmetic preparationsEvaporationWater soluble drug
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Eplerenone crystalline form exhibiting enhanced dissolution rate
InactiveCN1557833AHigh chemical purityOrganic active ingredientsNervous disorderPharmaceutical drugEnhanced dissolution
A novel crystalline form (Form H) of the aldosterone receptor antagonist drug eplerenone is provided having a relatively rapid dissolution rate in aqueous media. Also provided are novel solvated crystalline forms of eplerenone that, when desolvated, can yield Form H eplerenone. Also provided is amorphous eplerenone. Pharmaceutical compositions are provided comprising Form H eplerenone, optionally accompanied by one or more other solid state forms of eplerenone, in a total unit dosage amount of eplerenone of about 10 to about 1000 mg, and further comprising one or more pharmaceutically acceptable excipients. Processes are provided for preparing Form H eplerenone and for preparing compositions comprising Form H eplerenone. A method for prophylaxis and / or treatment of an aldosterone-mediated condition or disorder is also provided, comprising administering to a subject a therapeutically effective amount of eplerenone, wherein at least a fraction of the eplerenone present is Form H eplerenone.
Owner:PHARMACIA CORP
A high-efficiency extraction and purification method of Sclerotin and its application
Owner:SHANDONG FOOD & FERMENT IND RES & DESIGN INST
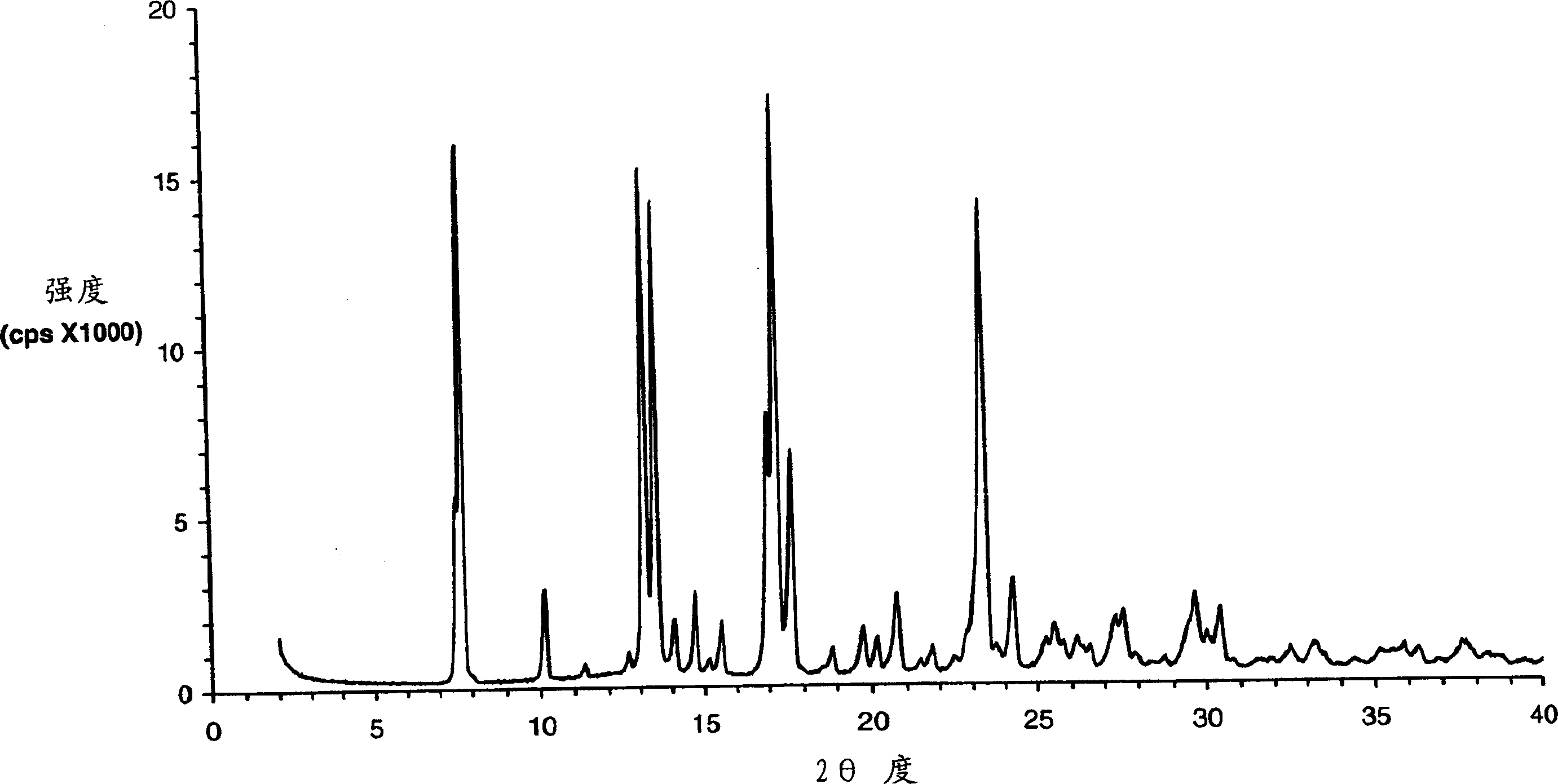
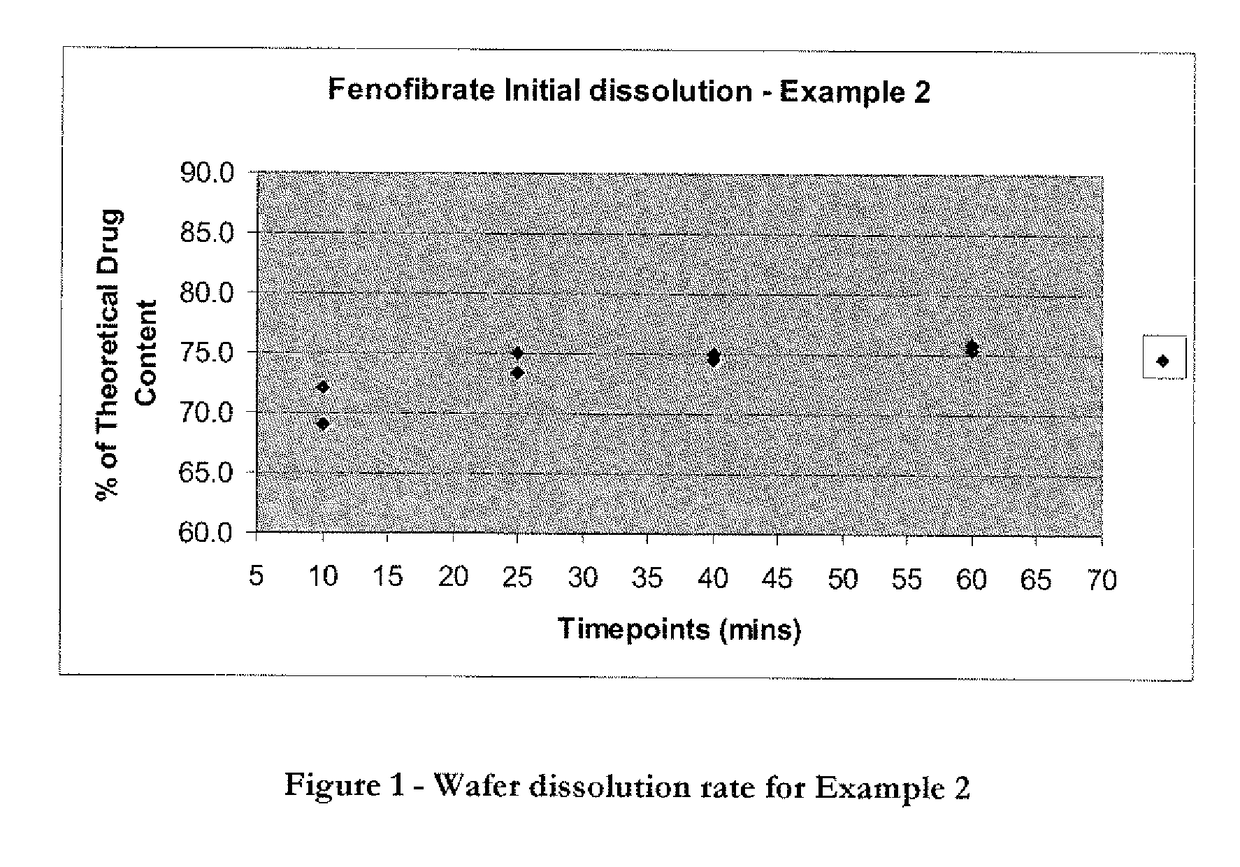
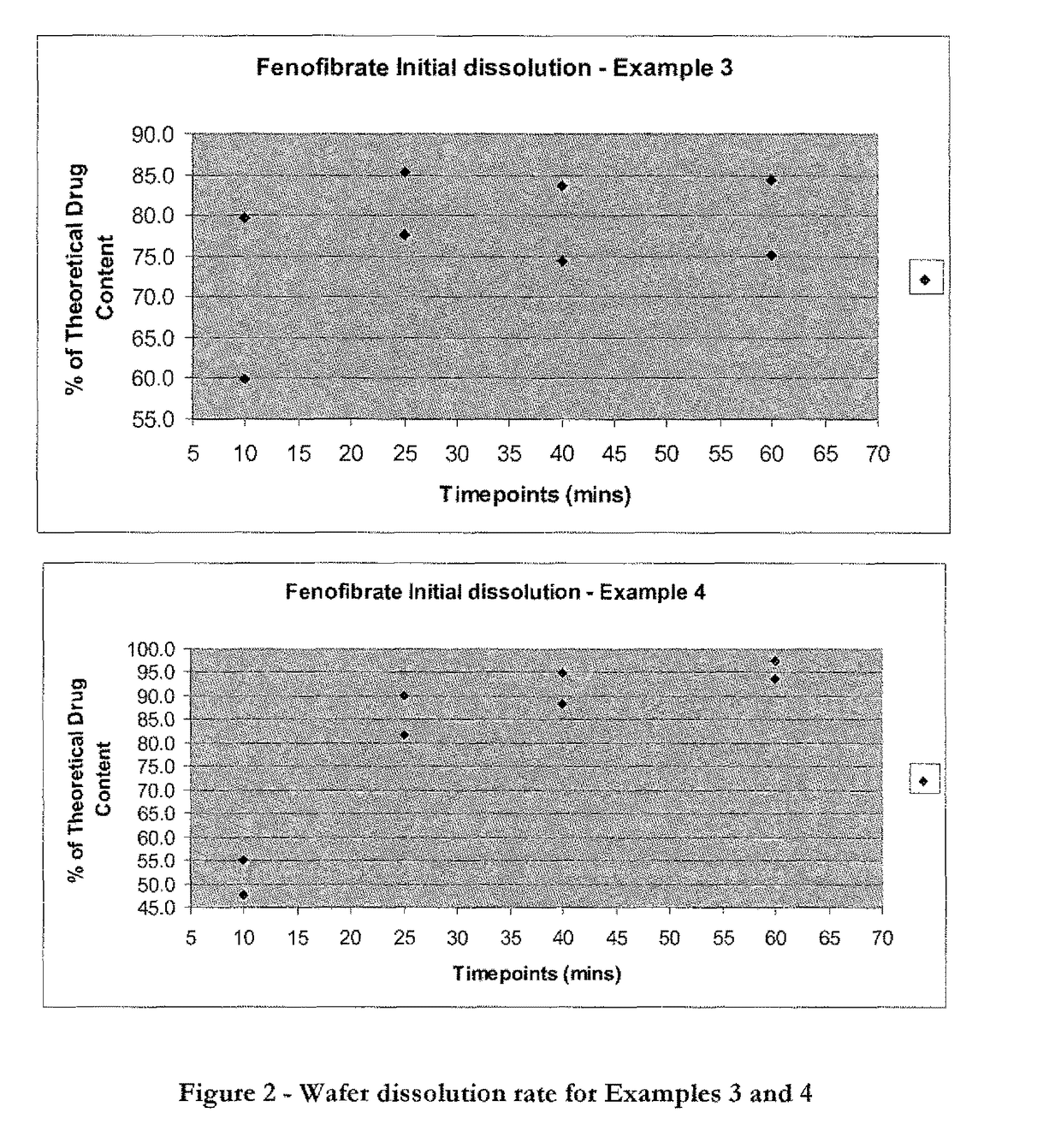
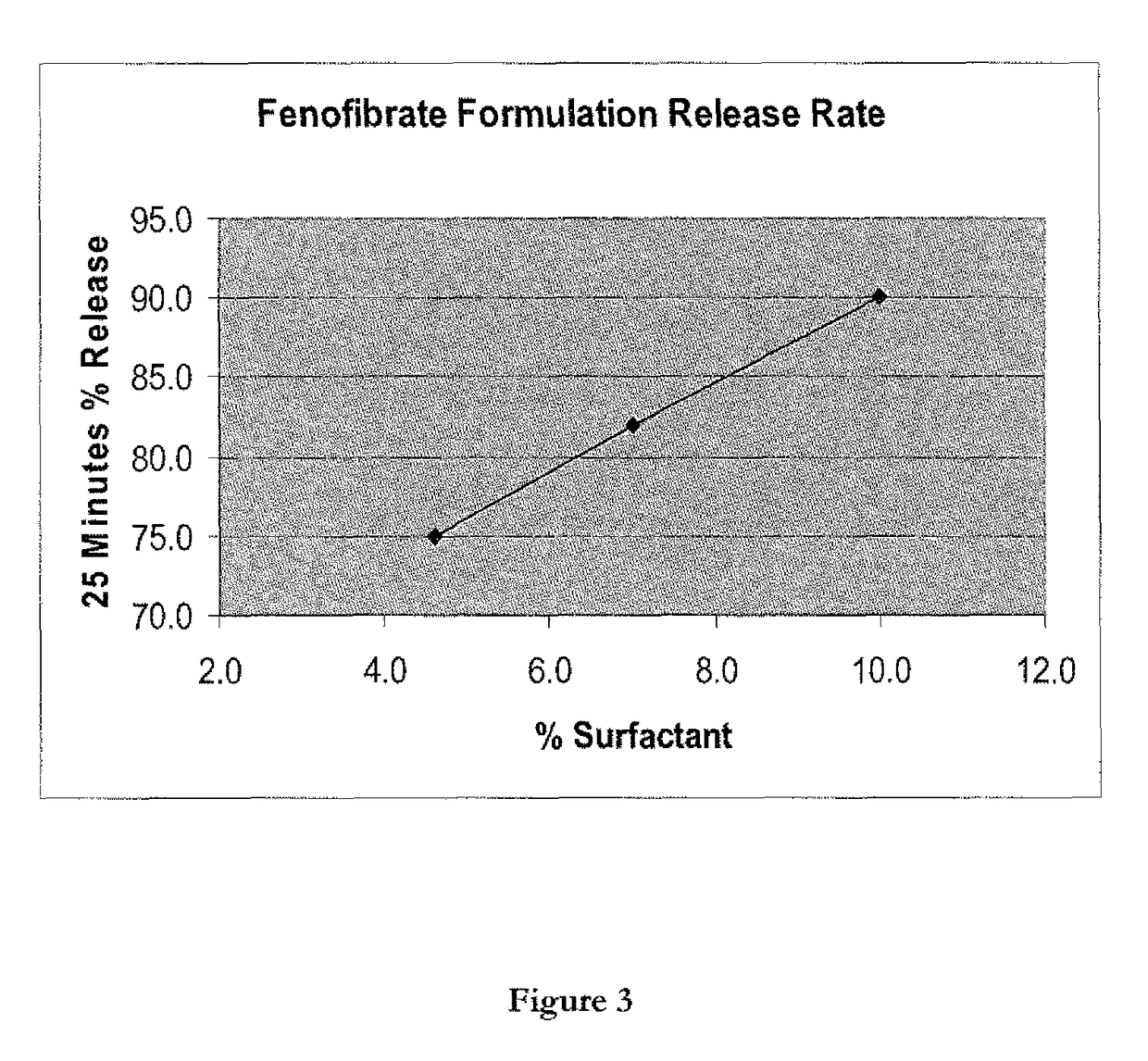
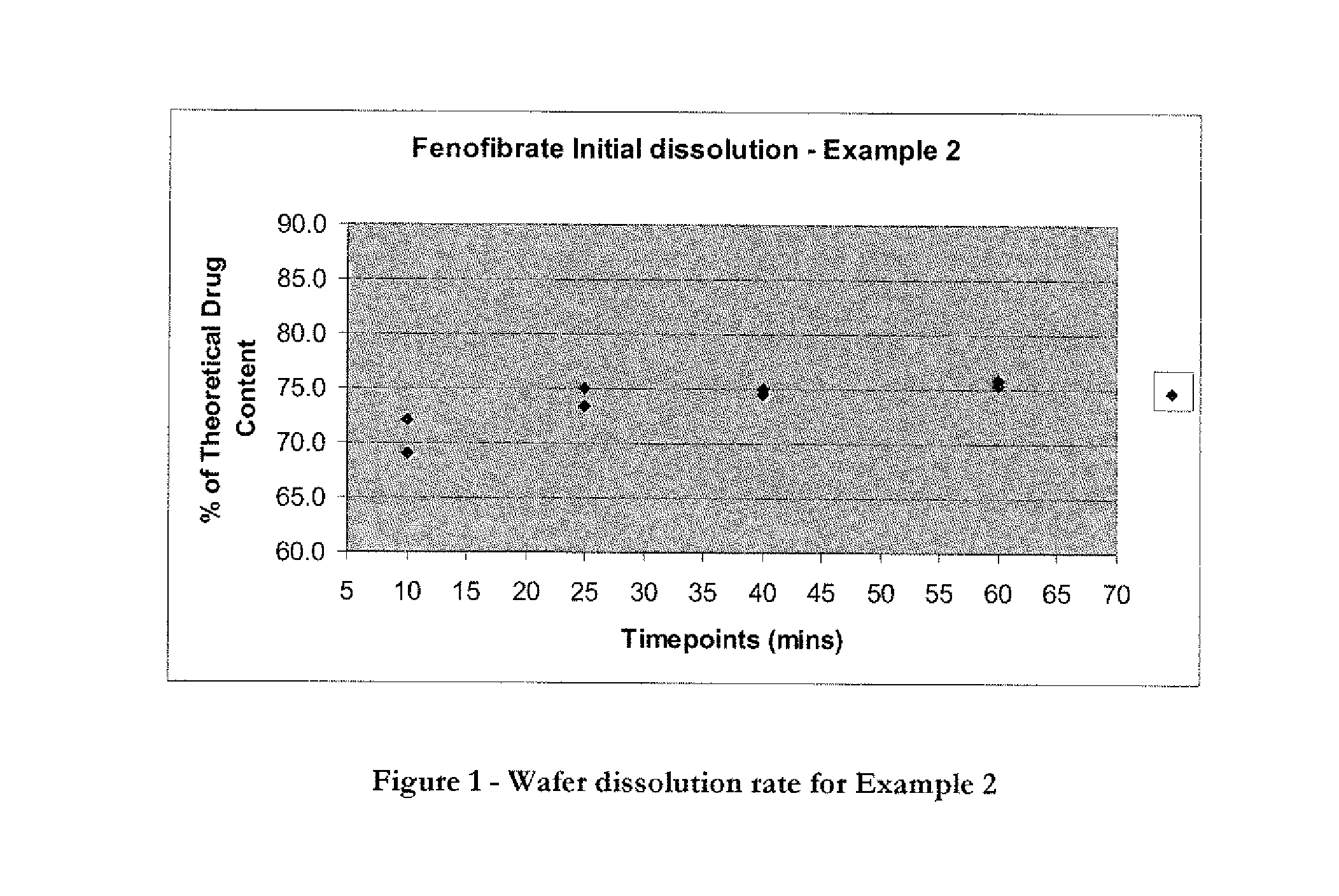
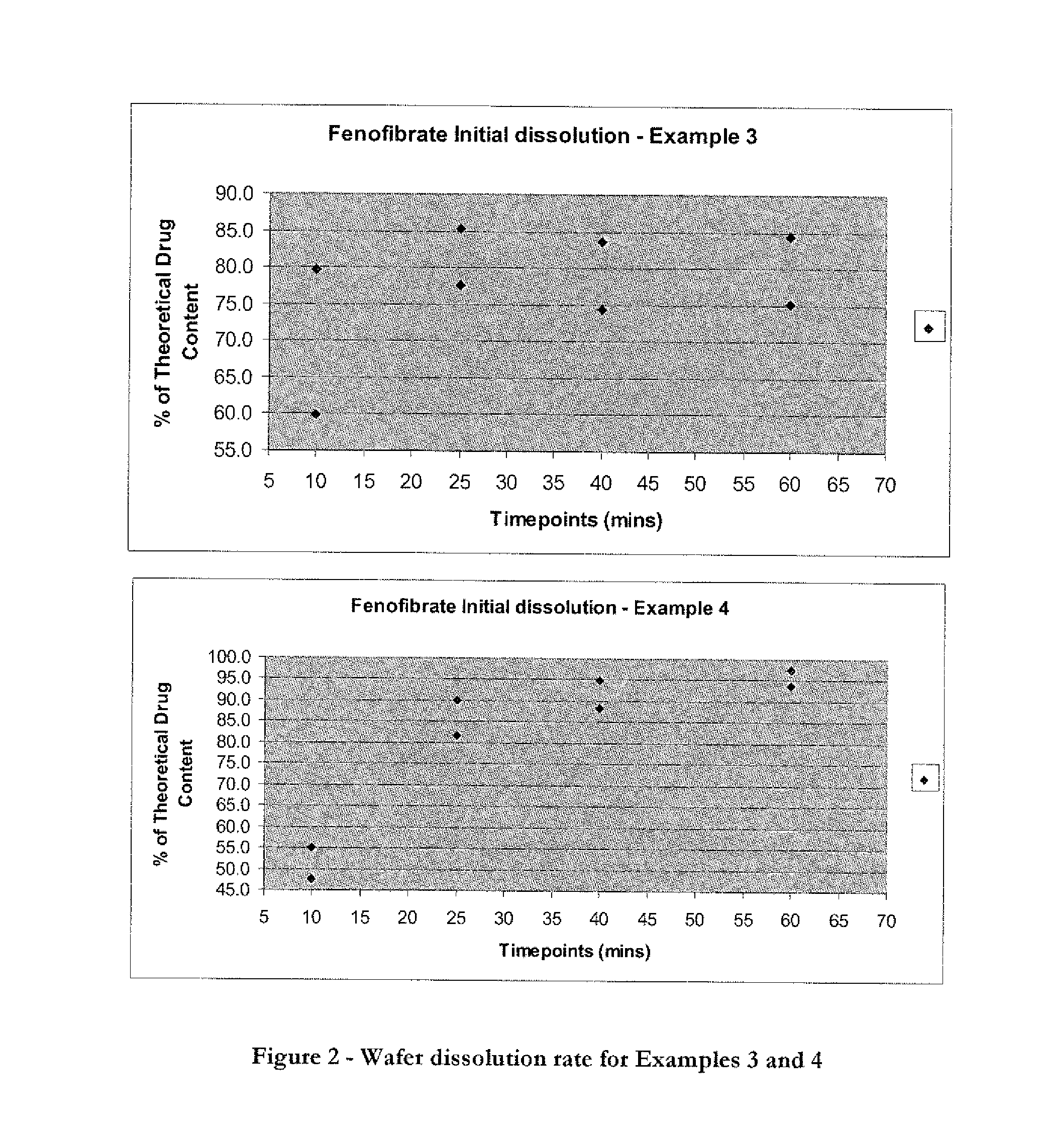
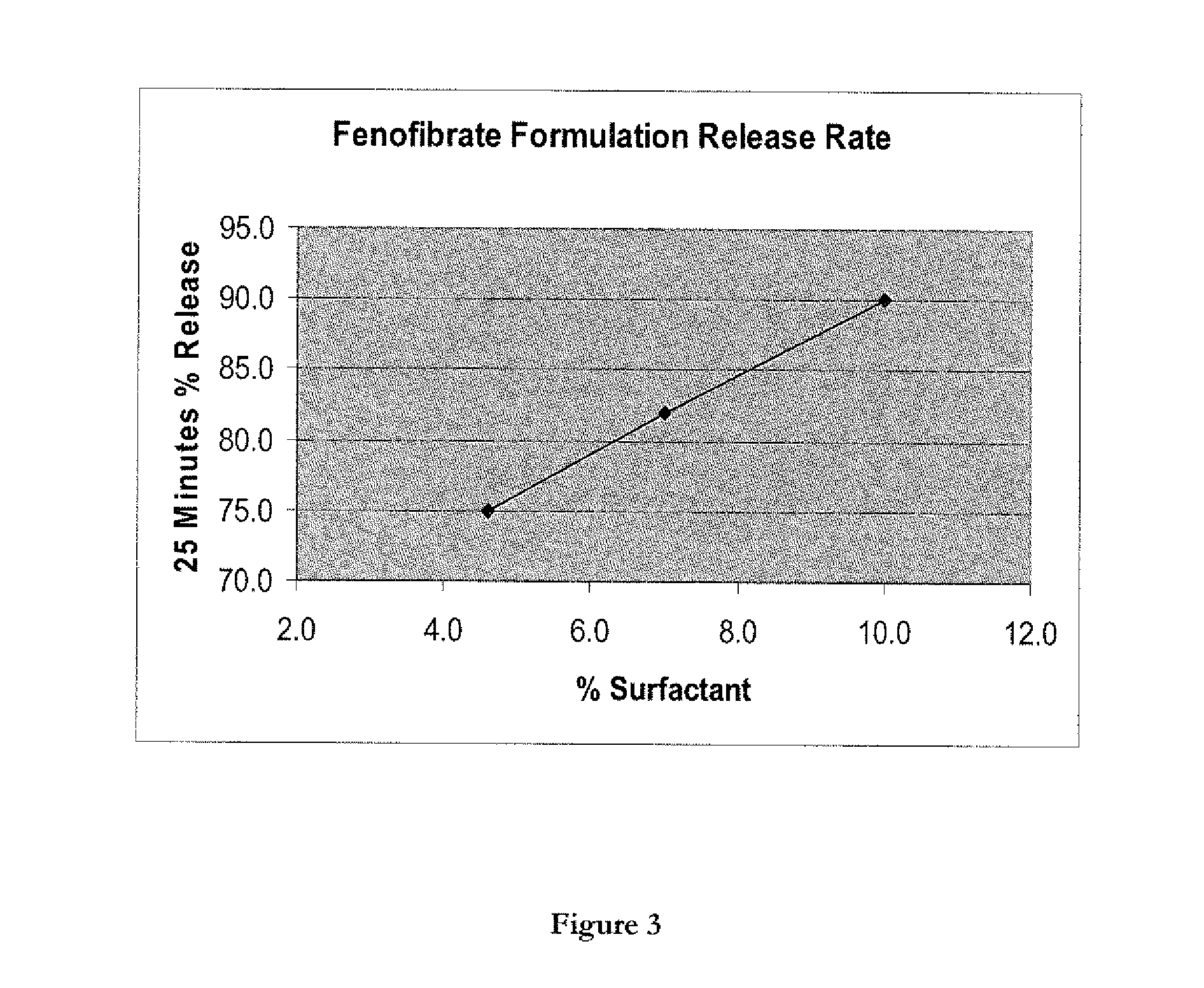
Wafer and capsule formulations with enhanced dissolution rates for fenofibrate
Owner:LTS LOHMANN THERAPIE-SYST AG
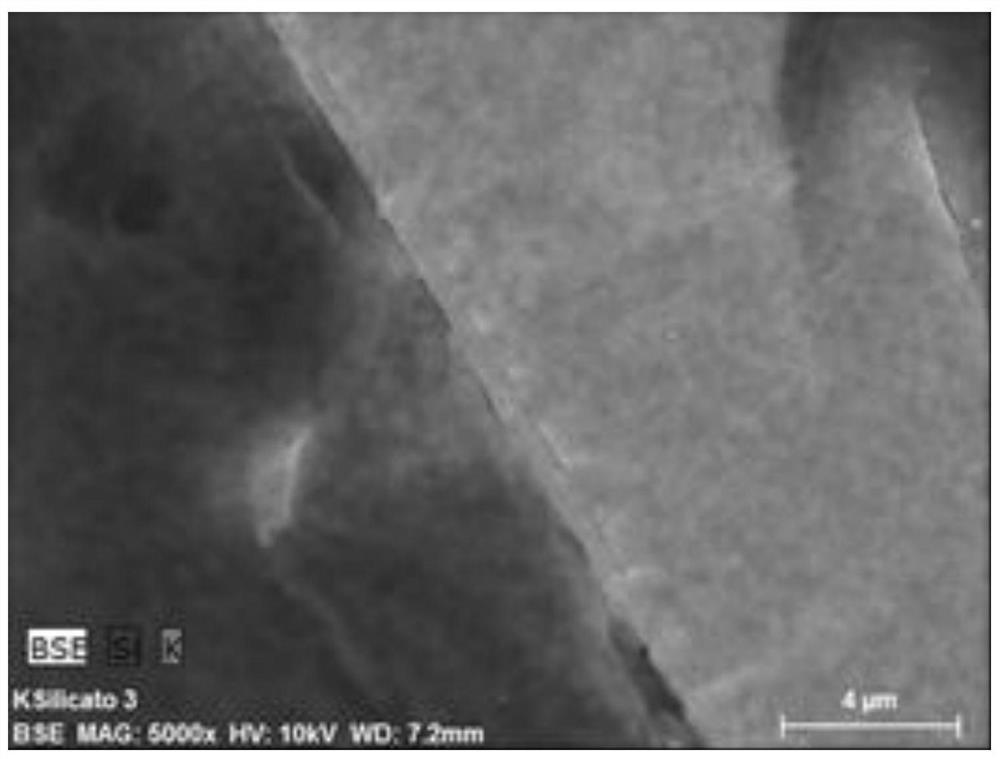
A zeolite molecular sieve modified silicon source prepared by enhanced dissolution method
ActiveCN112919488BEasy to prepareImprove efficiencyCrystalline aluminosilicate zeolitesMolecular sieveEnhanced dissolution
The invention relates to a superior silicon source prepared by an enhanced dissolution method, and ultra-small nanocrystals of zeolite molecular sieve prepared by using the silicon source, and specifically discloses a preparation method thereof, including 1) immersing a dense silicon source in an acid solution to remove impurities; 2. ) crushing the material obtained in step 1) by ball milling; 3) preparing an aqueous solution of methanol, adjusting the pH value to 12-14, adding the silicon material crushed by ball-milling, and dissolving by solvothermal method at 100-300° C. to obtain a silicon solution; 4) prepare an alkali metal source solution and mix with the silicon solution in (3); 5) remove the solvent quickly and uniformly to obtain a silicon-alkali metal binary uniform embedding mixture; 6) use the silicon-alkali metal prepared in (5) The alkali metal binary homogeneous intercalation mixture is used as an improved silicon source for the synthesis of zeolite molecular sieves, and it can be crystallized with other components required for the synthesis, which can improve the crystallization rate and obtain ultra-small nanocrystalline products of zeolite molecular sieves.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Detergent composition
InactiveCN103003404ASurface-active detergent compositionsDetergent compounding agentsMagnesium saltFast release
The present invention is in the field of detergent powders. The invention particularly relates to detergent granules exhibiting improved dissolution behaviour for faster release of active to a wash liquor. A detergent particle that provides further improved dissolution, even after storage, remains to be desired. This applies not only to detergent powder composition, but also to compositions in tablet form. It is an object of the present invention to provide a detergent composition having enhanced dissolution properties. Surprisingly it has been found that a granule comprising a mixture of a magnesium salt of linear alkylbenzene sulphonic acid (Mg(LAS)
Owner:UNILEVER NV
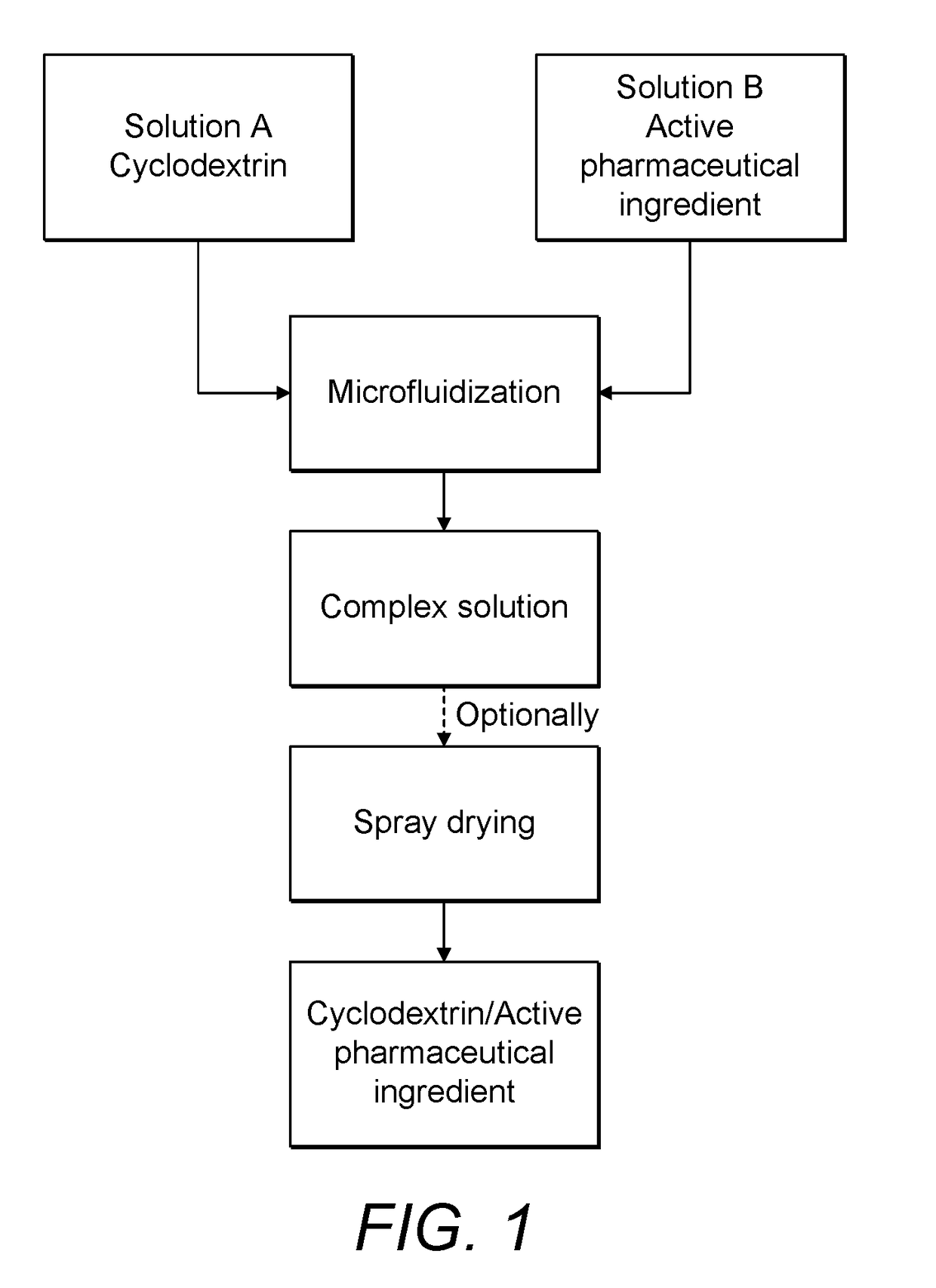
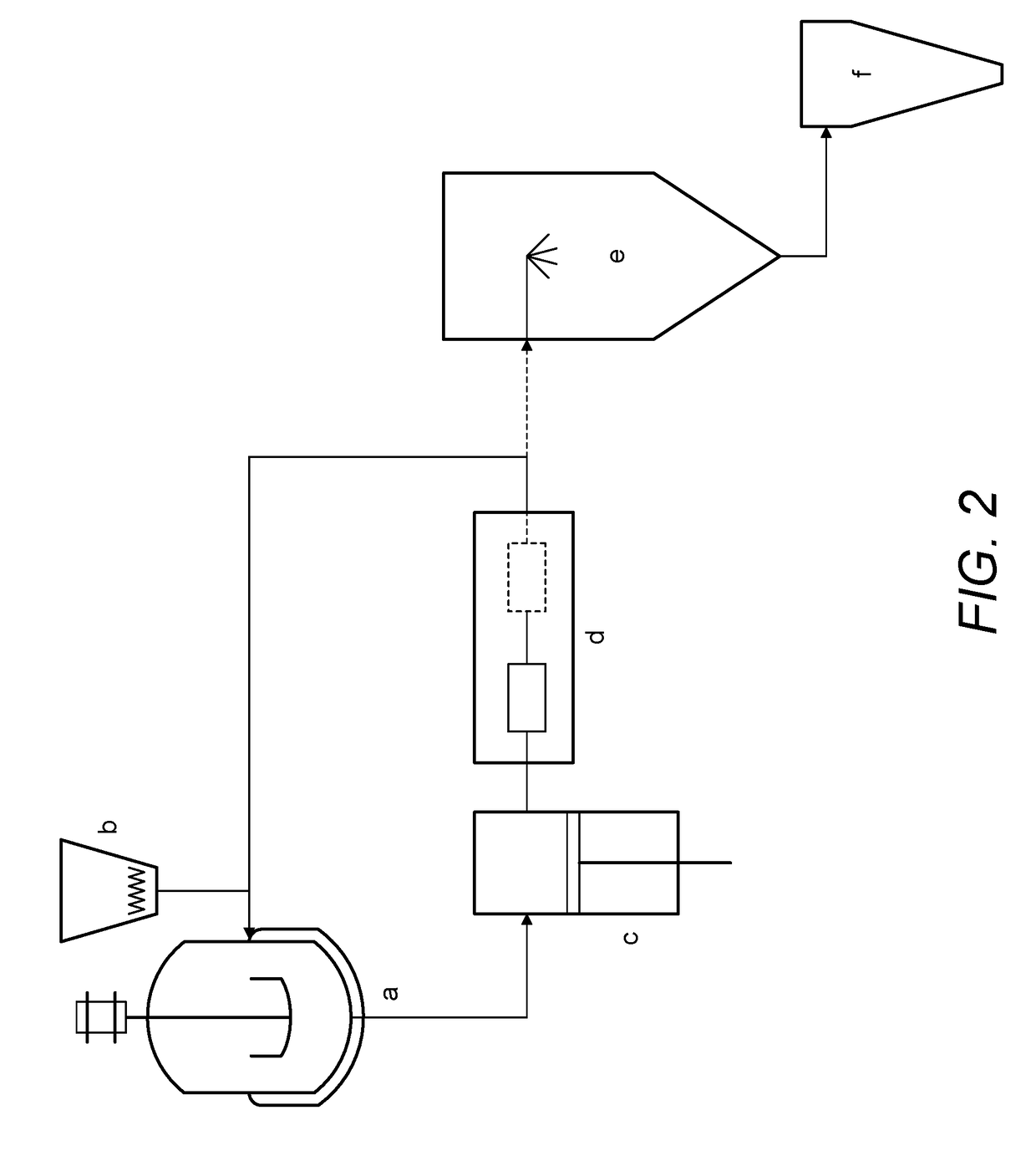
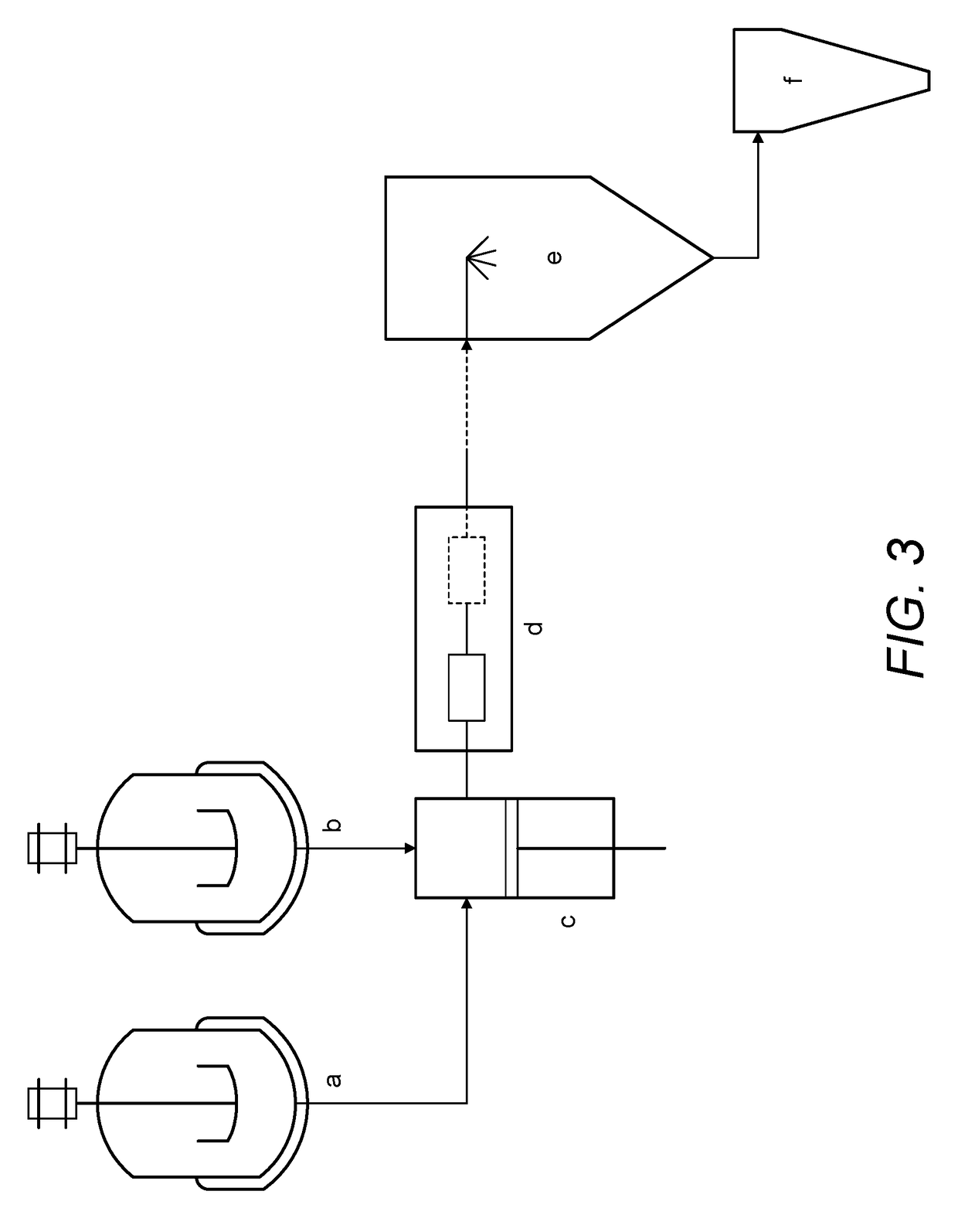
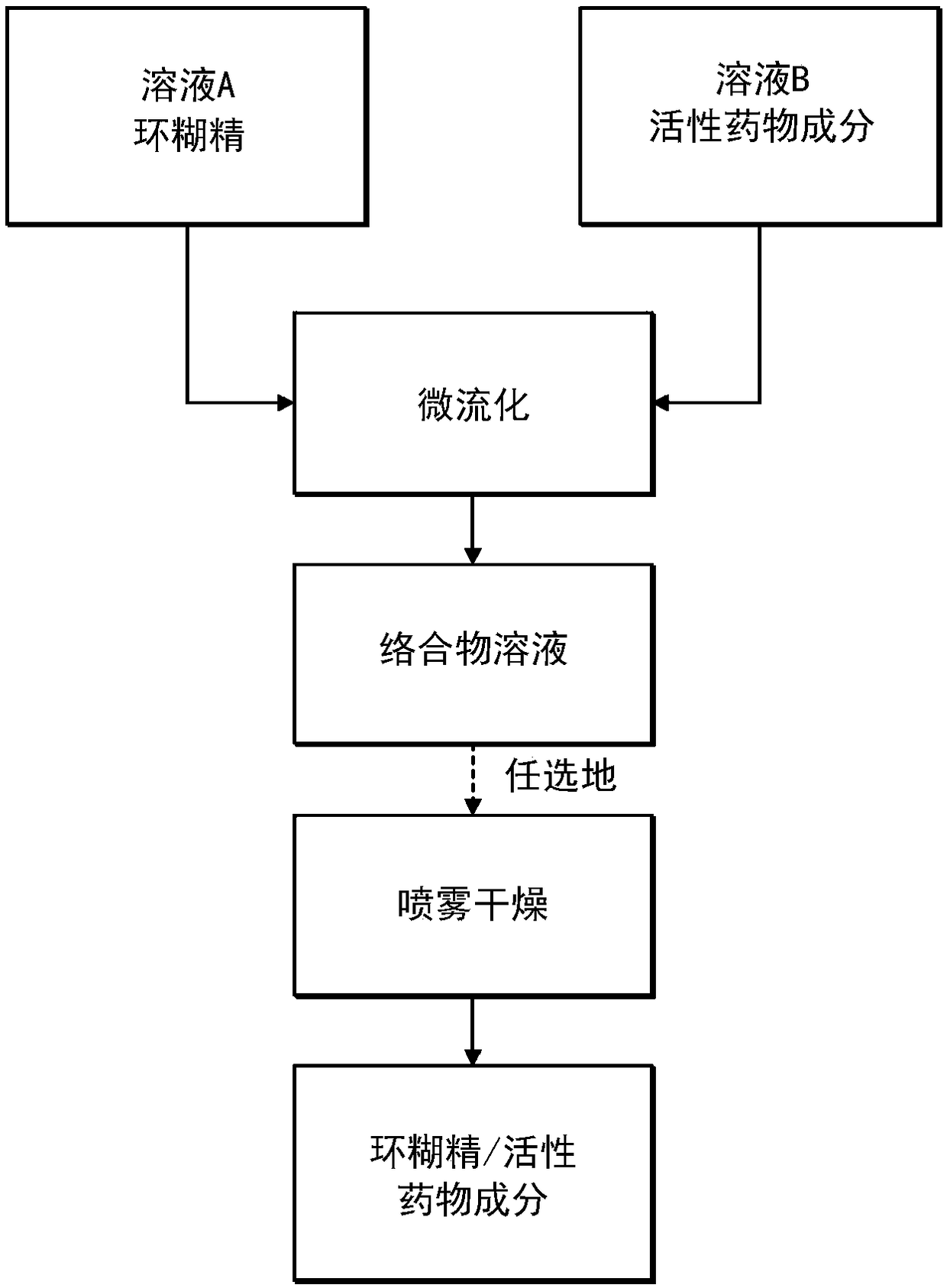
Continuous Complexation of Active Pharmaceutical Ingredients
ActiveUS20190060486A1Shorten the timeImprove bioavailabilityPowder deliveryOrganic active ingredientsAdditive ingredientCyclodextrin
A complexation process between a cyclodextrin and active pharmaceutical ingredients is disclosed, and comprises a process for preparing a complex of at least one cyclodextrin and at least one active pharmaceutical ingredient comprising the steps ofa. Preparing a first solution (solution A) comprising at least one cyclodextrin and at least one solvent;b. Preparing a second solution (solution B) comprising at least one dissolved, partially dissolved or suspended API;c. Mixing said solution A and solution B by means of a microfludization system to produce a solution and / or suspension of at least one of said complex;d. Isolating said solution and / or suspension and / or optionally drying it; ande. Optionally collecting a powdered form of the complex.The described process has high throughput with higher yields of complexation in less time than prior art methods.The complexes obtained by the invention are characterized by having enhanced dissolution and / or bioavailability of the active pharmaceutical ingredient in body fluids.
Owner:HOVIONE SCIENTIA
Gut-protective compositions comprising boswellic acid
PendingUS20220362184A1High dissolution rateImprove bioavailabilityGranular deliveryAnhydride/acid/halide active ingredientsBiotechnologyMicroorganism
The invention describes a gut-protective composition comprising boswellic acid, at least one pH modifier, wherein the composition exhibits enhanced dissolution. The composition as described herein may be comprised of about 5 to 95% of boswellic acid. The said composition is stable and exhibits improved bioavailability. The invention also provides a process for preparation of the said composition, wherein boswellic acid and pH modifier are mixed well by optionally adding at least one or more excipient and processed to get granular powder. The composition may be further formulated into solid, semi solid or liquid dosage forms, for administration to human or animals. The compositions described herein exhibit gut-protective effect in conditions of colitis as well as microbial infections.
Owner:SUVARNAPATHAKI DR RUPALI +1
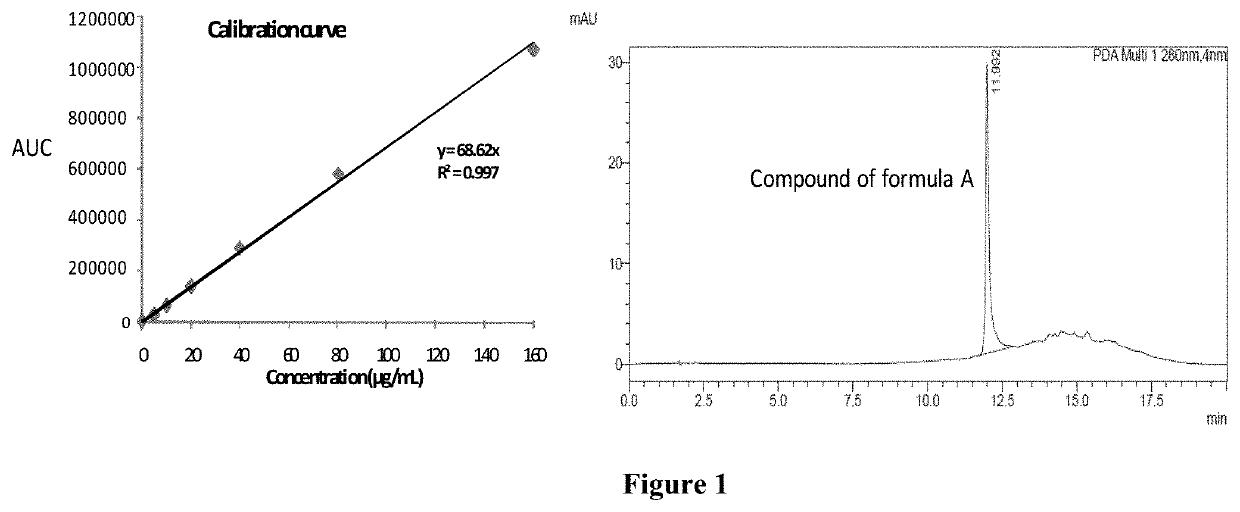
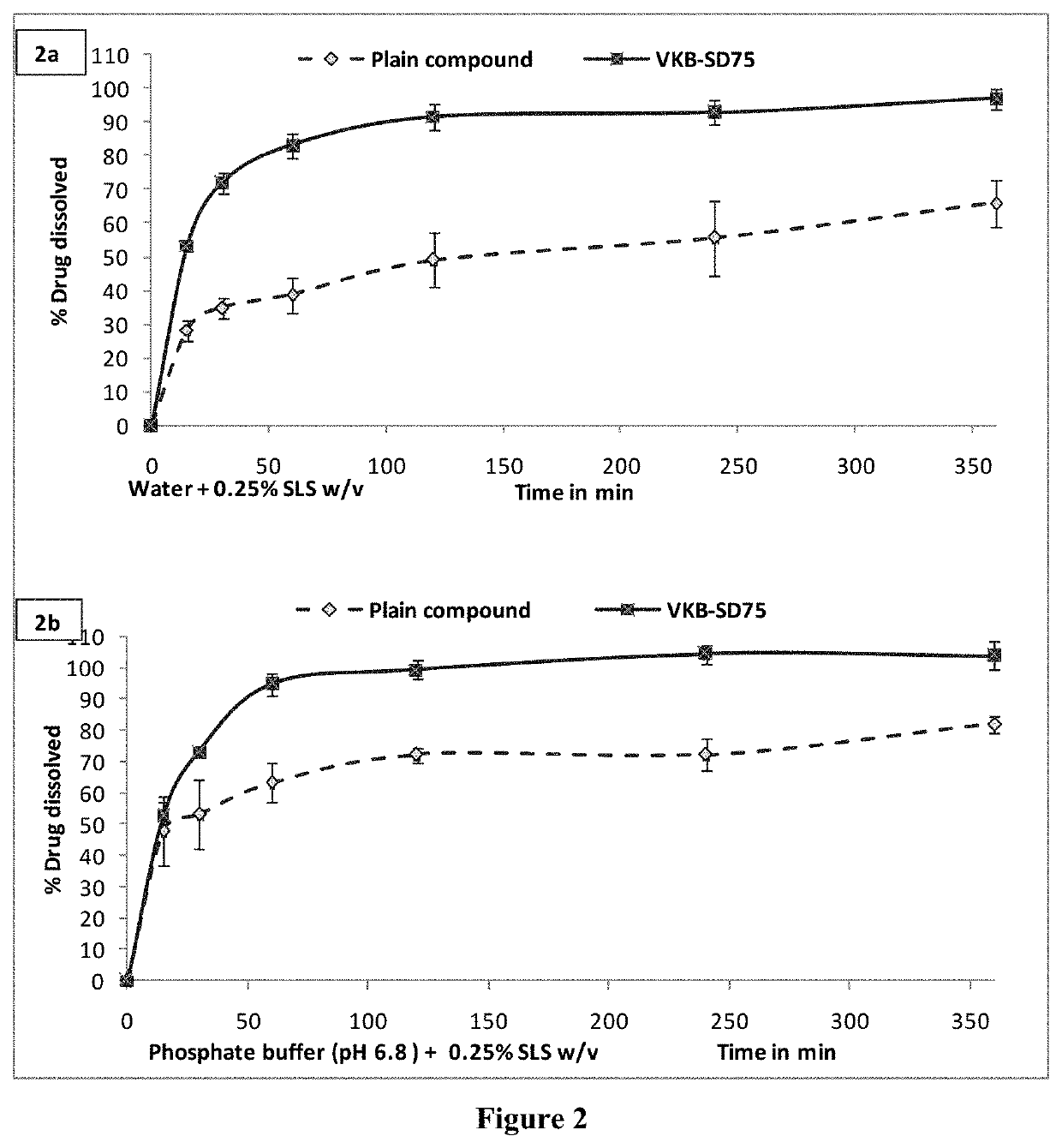
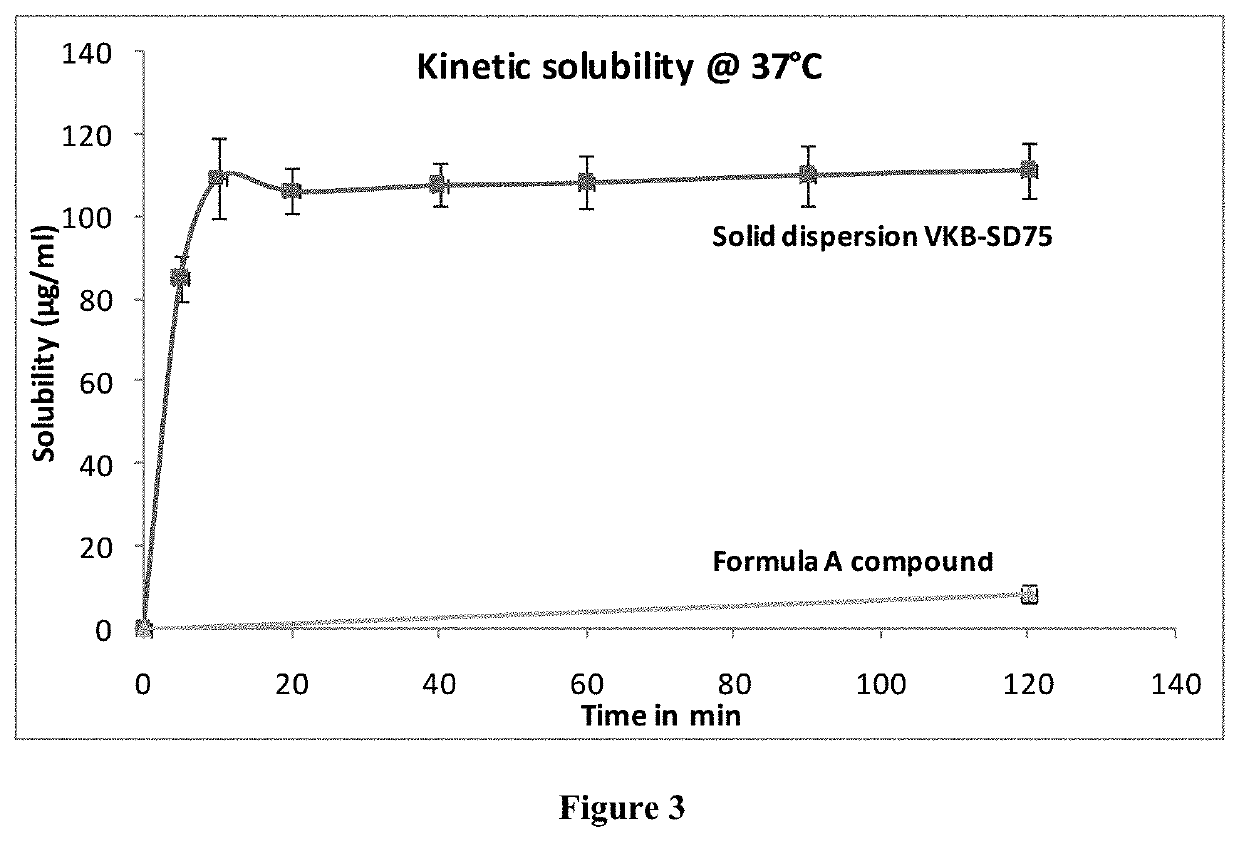
Solid dispersion comprising an anticancer compound for improved solubility and efficacy
PendingUS20210308116A1Enhance in-vivo efficacyOrganic active ingredientsOrganic chemistryChemical compoundEnhanced dissolution
The present invention is related to the novel solid dispersion formulations of an anticancer compound of formula A and a process for preparing the same wherein the anticancer compound is formulated with hydrophilic polymer. The said formulation showed enhanced dissolution of the anticancer compound and improved in-vivo anticancer activity. The said formulations are useful for the treatment of various types of cancer.
Owner:COUNCIL OF SCI & IND RES
Wafer and capsule formulations with enhanced dissolution rates for fenofibrate
The objective of this invention is to develop a novel wafer and capsule formulations using fenofibrate which is difficult to dissolve and control its release rate in vitro.
Owner:LTS LOHMANN THERAPIE-SYST AG
Eplerenone crystalline form exhibiting enhanced dissolution rate
InactiveCN1152886CHigh chemical purityOrganic active ingredientsNervous disorderEnhanced dissolutionSolvation
A novel crystalline form (Form H) of the aldosterone receptor antagonist drug eplerenone is provided having relatively hich physical stability at normal temperatures of storage and use. Pharmaceutical compositions are also provided comprising Form H eplerenone, optionally accompanied by one or more other solid state forms of eplerenone, in a total unit dosage amount of eplerenone of about 10 to about 1000 mg, and further comprising one or more pharmaceutically acceptable excipients. Processes are provided for preparing Form H eplerenone and for preparing compositions comprising Form H eplerenone. A method for prophylaxis and / or treatment of an aldosterone-mediated condition or disorder is also provided, comprising administering to a subject a therapeutically effective amount of eplerenone, wherein at least a fraction of the eplerenone present is Form H eplerenone.
Owner:PHARMACIA CORP
Preparation of drug particles using evaporation precipitation into aqueous solutions
InactiveUS20140030340A1Raise the ratioHigh dissolution ratePowder deliveryBiocideOrganic solventMicrometer
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Continuous complexation of active pharmaceutical ingredients
A complexation process between a cyclodextrin and active pharmaceutical ingredients is disclosed, and comprises a process for preparing a complex of at least one cyclodextrin and at least one active pharmaceutical ingredient comprising the steps of: a. preparing a first solution (solution A) comprising at least one cyclodextrin and at least one solvent; b. preparing a second solution (solution B)comprising at least one dissolved, partially dissolved or suspended API; c.Mixing said solution A and solution B by means of a microfludization system to produce a solution and / or suspension of at least one of said complex; d. isolating said solution and / or suspension and / or optionally drying it; and e. optionally collecting a powdered form of the complex. The described process has high throughputwith higher yields of complexation in less time than prior art methods. The complexes obtained by the invention are characterized by having enhanced dissolution and / or bioavailability of the activepharmaceutical ingredient in body fluids.
Owner:HOVIONE SCIENTIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com