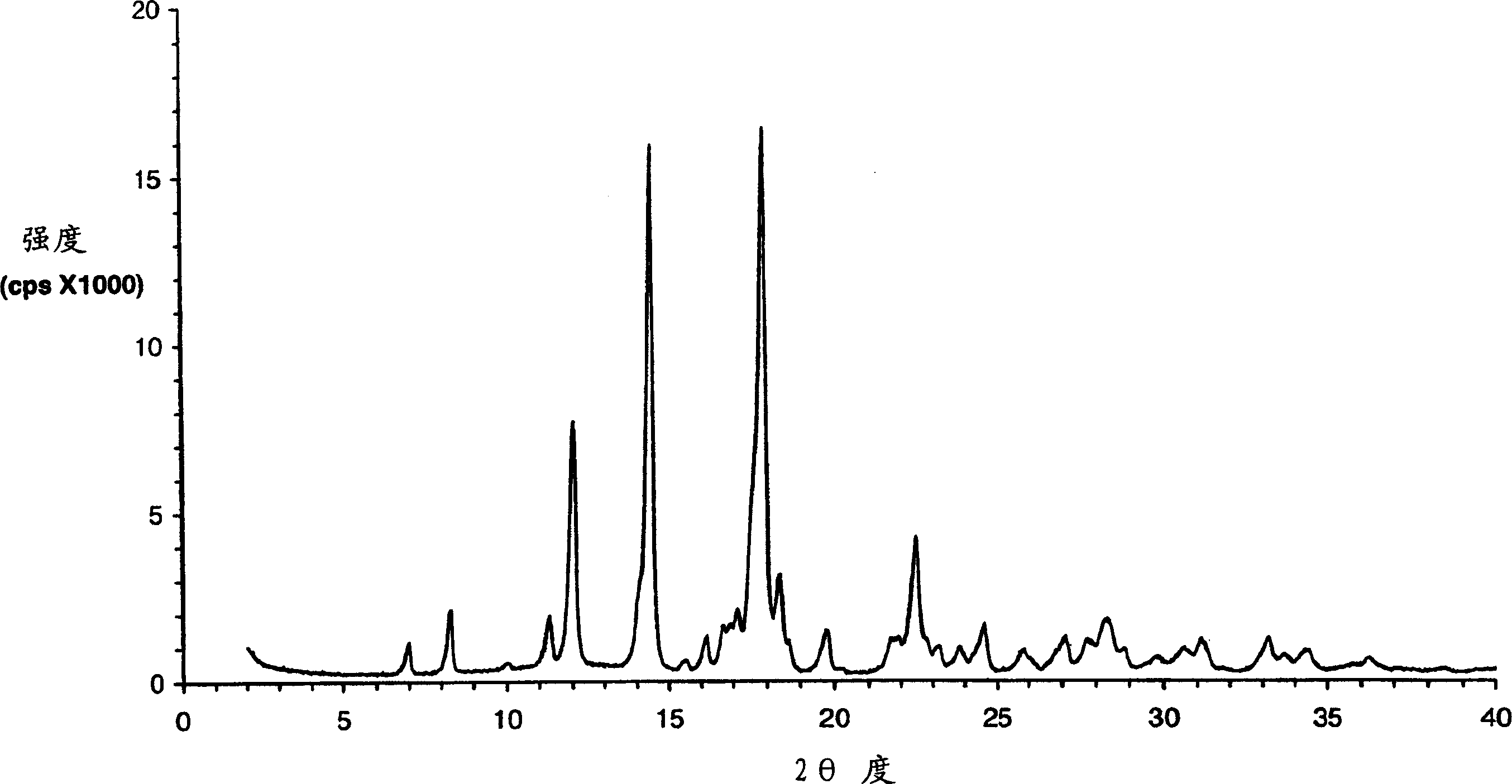
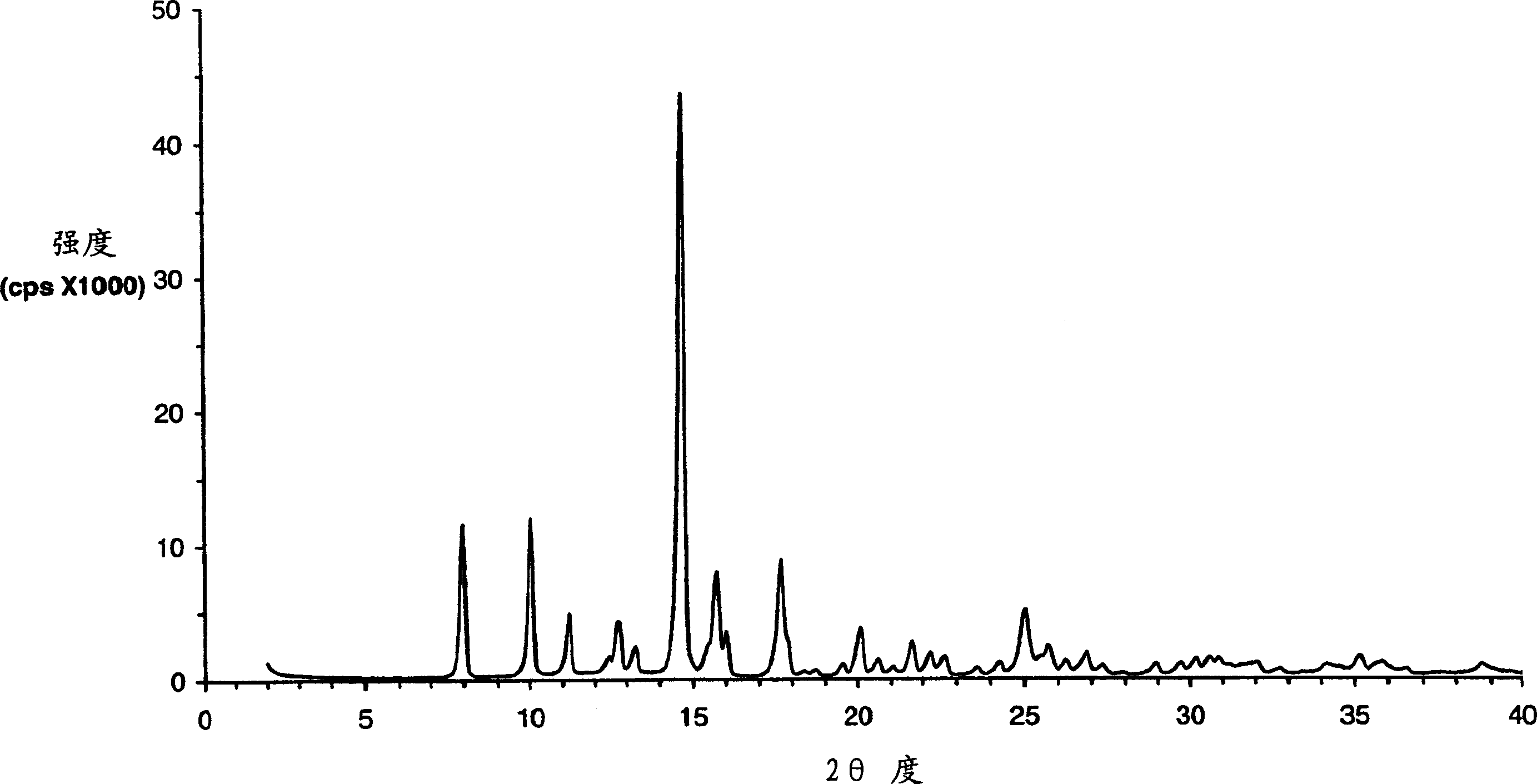
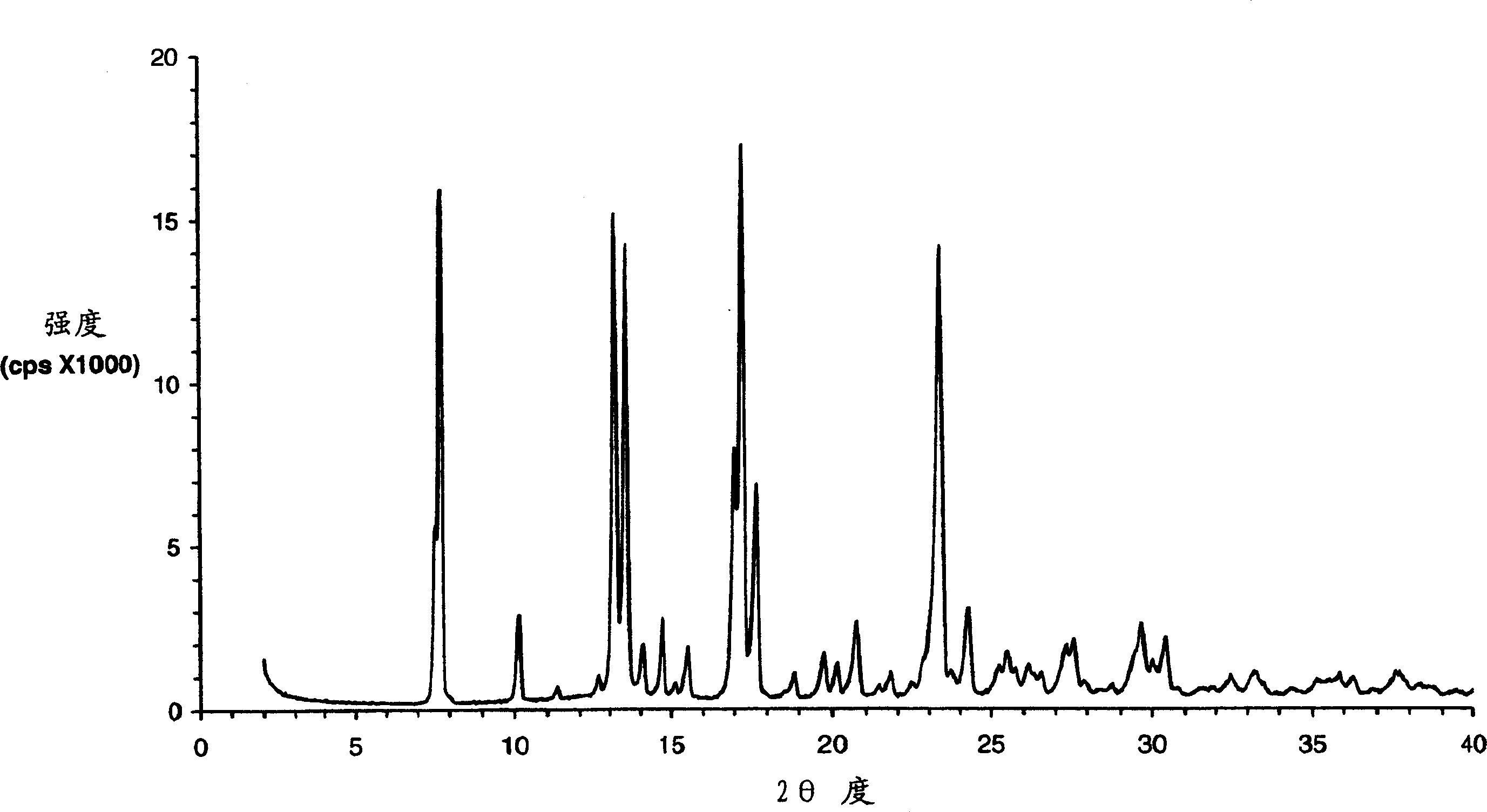
Eplerenone crystalline form exhibiting enhanced dissolution rate
A technology of eplerenone and crystal form, which is applied in the preparation of steroids, medical preparations containing active ingredients, drug combinations, etc., and can solve problems such as gynecomastia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0365] Embodiment 1: prepare butanone compound by high-purity eplerenone raw material and prepare by this solvate Eplerenone Crystalline Form L
[0366] A. Preparation of Butanone Compounds
[0367] Under magnetic stirring at 900 rpm, 437 mg of high-purity eplerenone (purity > 99%, and the total content of diepoxide and 11,12-epoxide < 0.2%) was dissolved by heating to boiling on a hot plate. In 10ml butanone. Under continuous magnetic stirring, the resulting solution was cooled to room temperature. Once at room temperature, the solution was transferred to a 1°C bath and stirring was continued for 1 hour. The butanate solid was collected from the cold solution by vacuum filtration.
[0368] B. Preparation of eplerenone crystalline form L
[0369] The butanate solid prepared as described above was dried in an oven at 100° C. under normal pressure for 4 hours. The dried solid was confirmed to be pure Form L by DSC and XRPD analysis.
Embodiment 2
[0370] Example 2: Preparation of additional solvates from high-purity starting materials
[0371] According to substantially the same method as in Example 1, the following solvents were used instead of butanone to prepare additional solvates: n-propanol, 2-pentanone, acetic acid, acetone, butyl acetate, chloroform, ethanol, isobutanol , isobutyl acetate, isopropanol, methyl acetate, ethyl propionate, n-butanol, n-octanol, propyl acetate, propylene glycol, tert-butanol, tetrahydrofuran, and toluene.
Embodiment 3
[0372] Example 3: Preparation of Butanone Compounds by Vapor Diffusion Growth
[0373] A stock solution was formed by dissolving 400 mg of eplerenone (>99.9% purity) in 20 ml of methyl ethyl ketone by warming on a hot plate. 8 ml of this stock solution was diluted to 10 ml with butanone, and the resulting solution was referred to as an 80% diluted sample. 4 ml of this stock solution was diluted to 10 ml with butanone (40% diluted sample). 2 ml of this stock solution was diluted to 10 ml with butanone (20% diluted sample). Each diluted sample in a 20 ml scintillation vial was transferred to a dry jar containing a small amount of hexane as an anti-solvent. The dry jar was sealed and the hexane vapor was allowed to diffuse into the butanone solution. Crystals of eplerenone butanate grew in 80% of the diluted samples within 24 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com