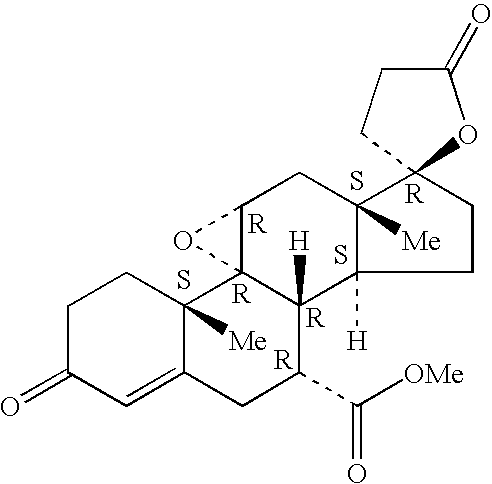
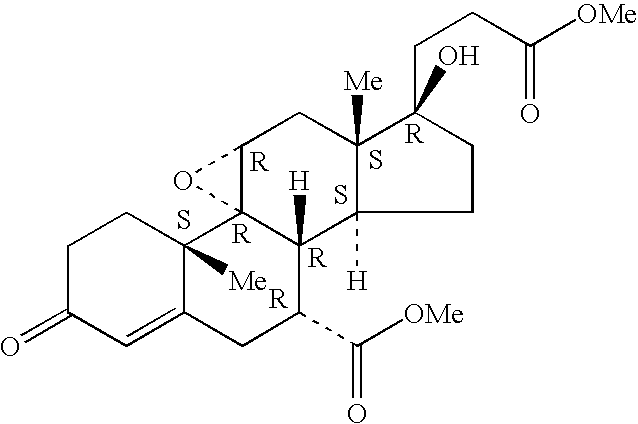
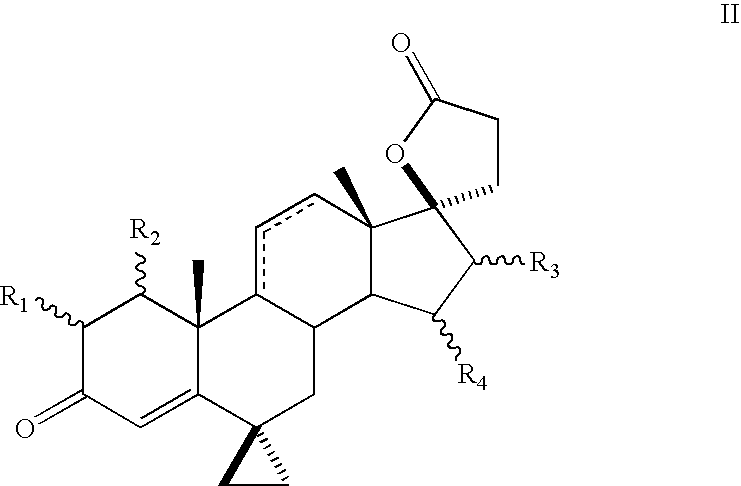
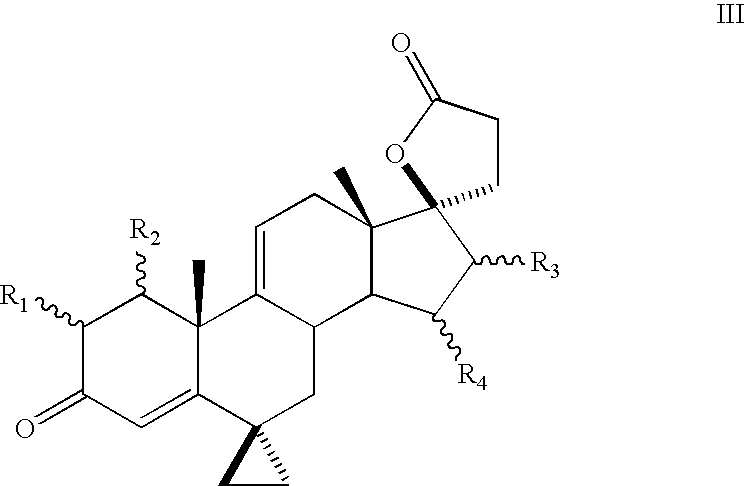
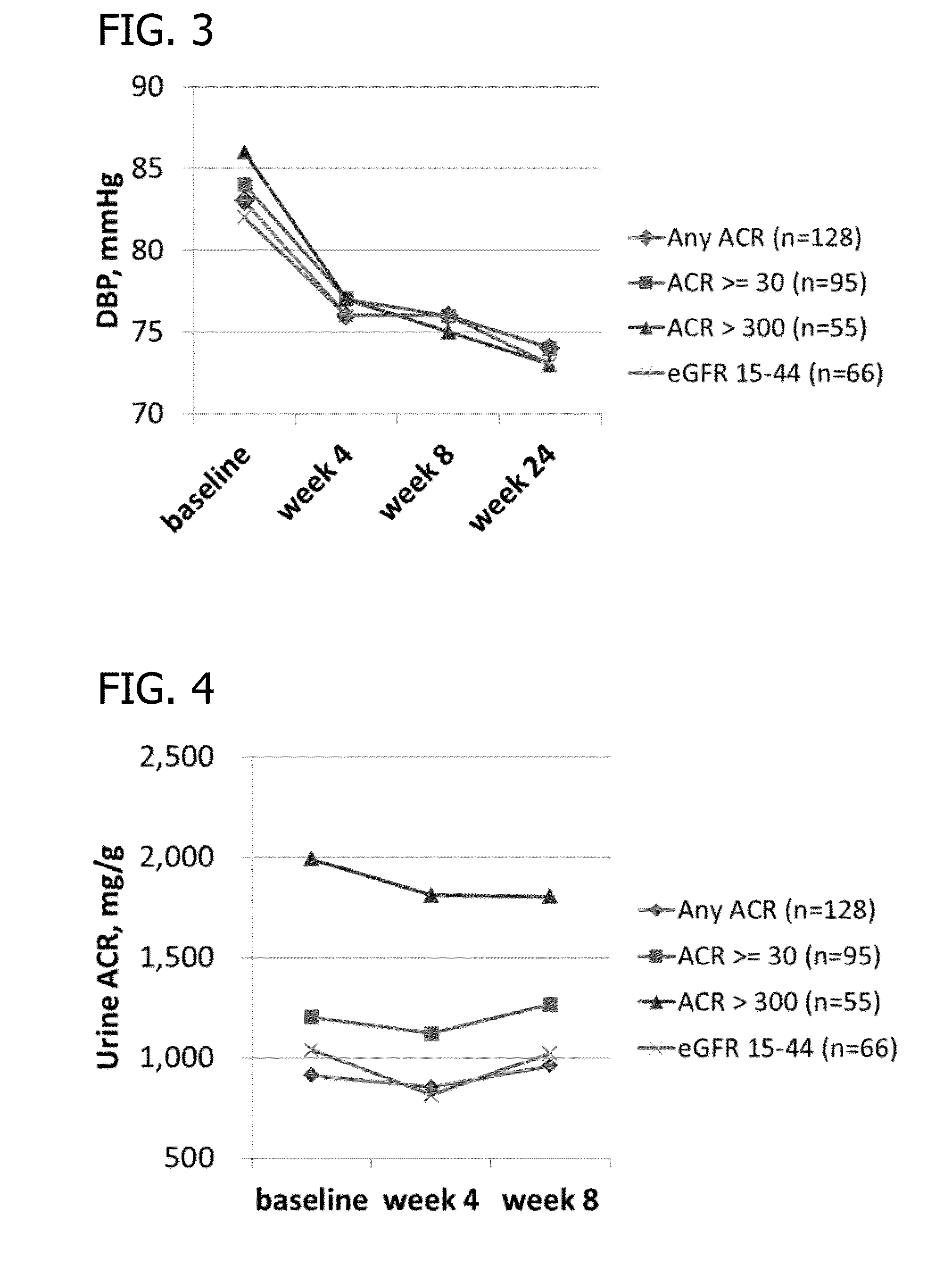
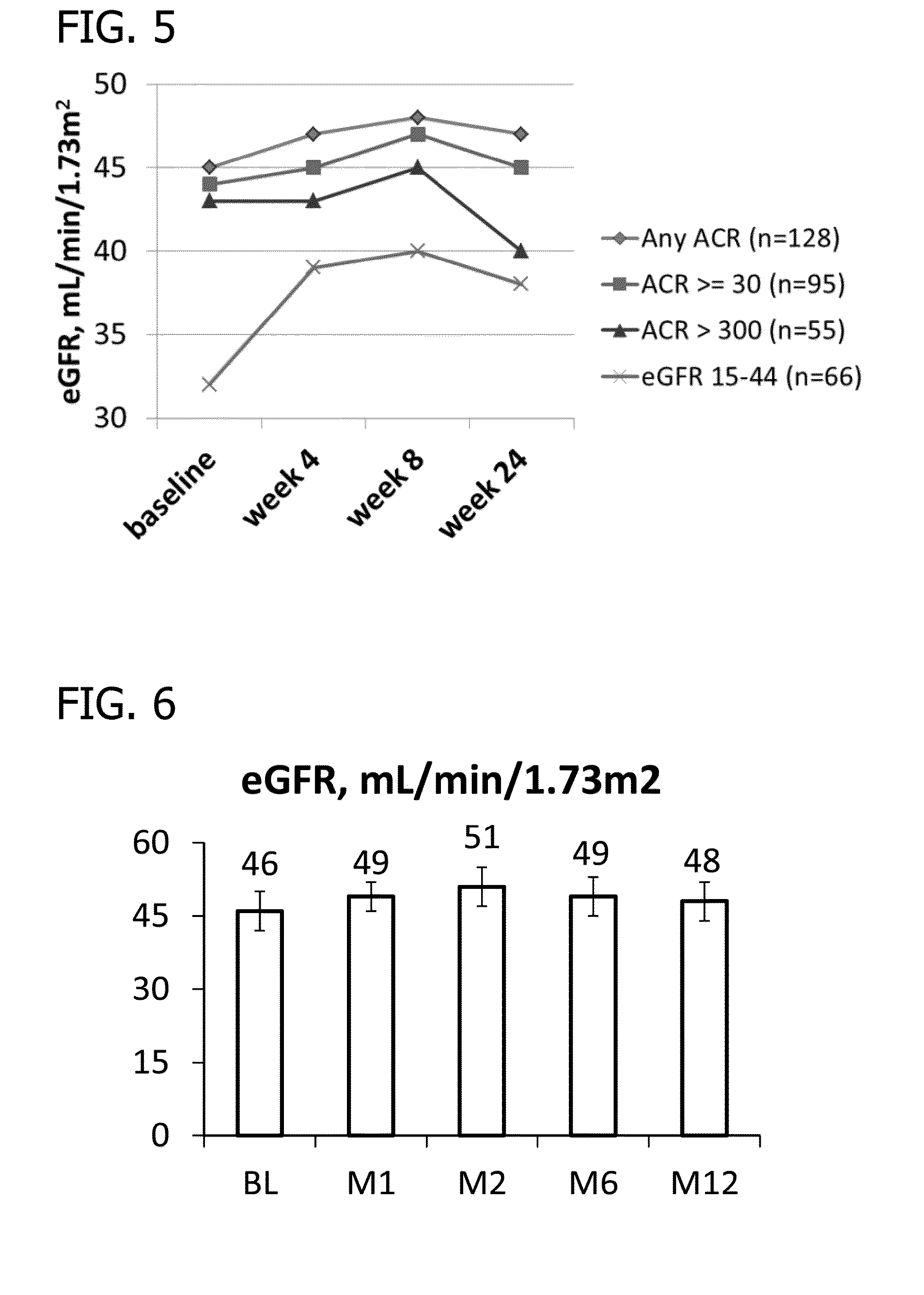
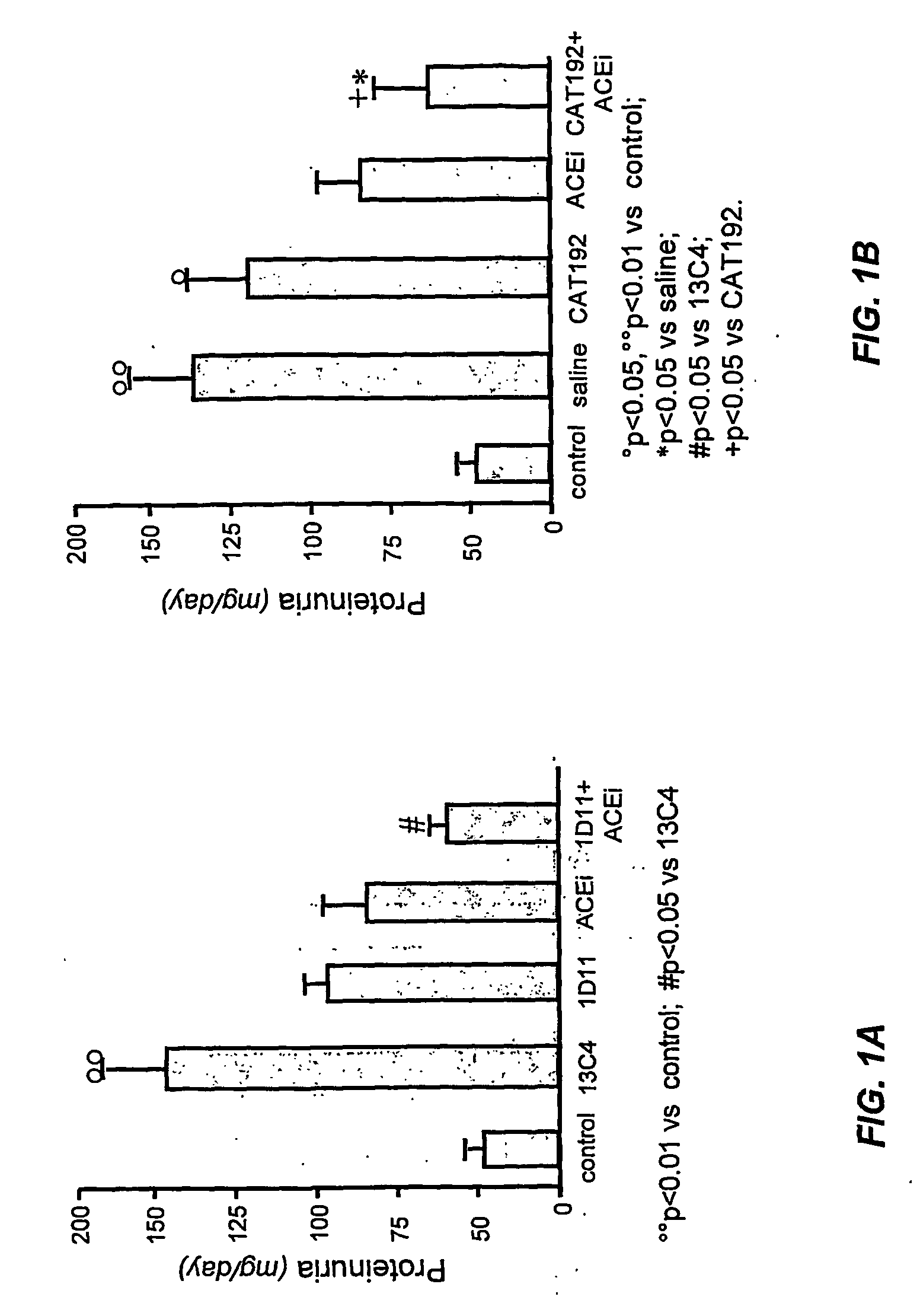
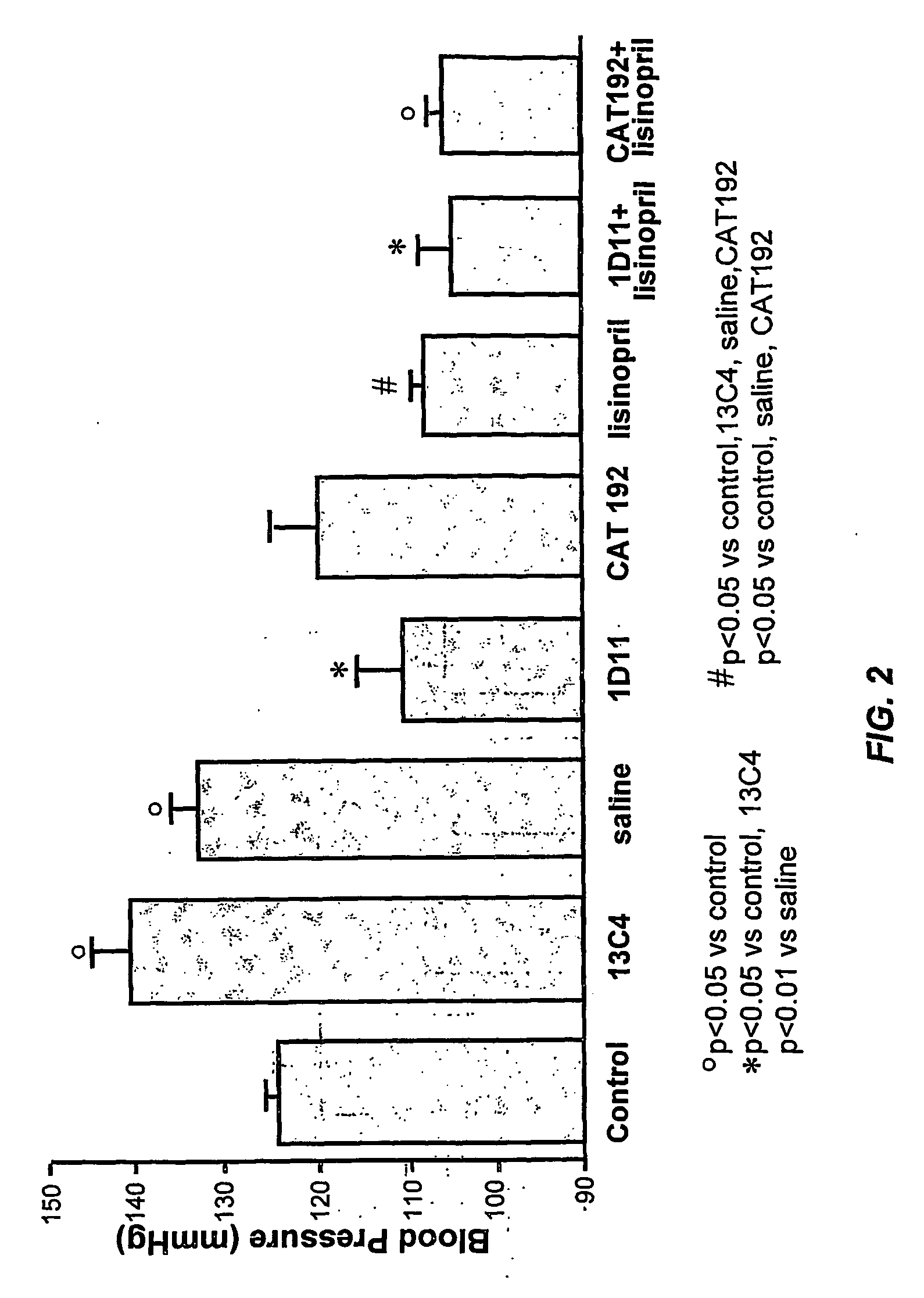
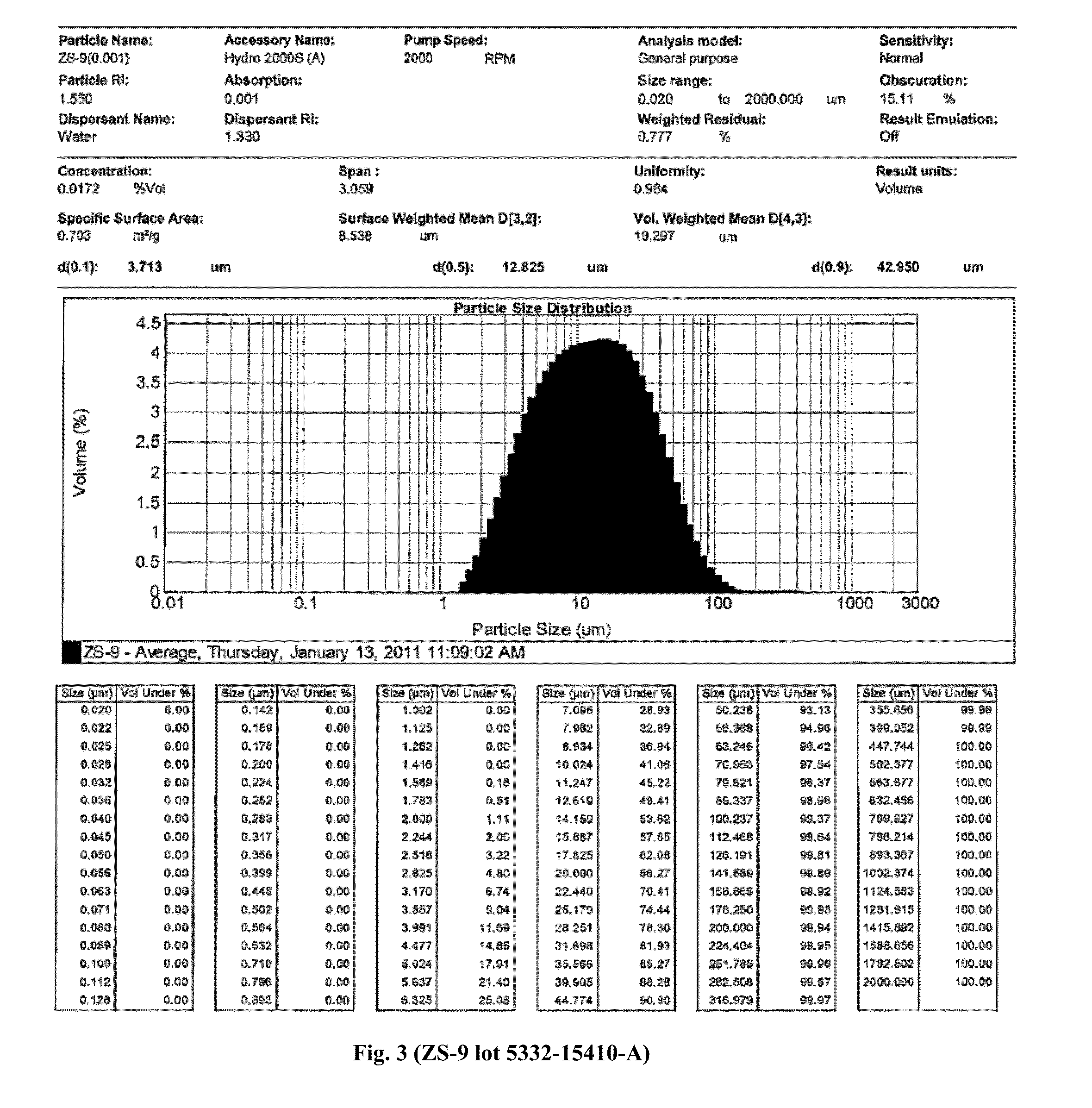
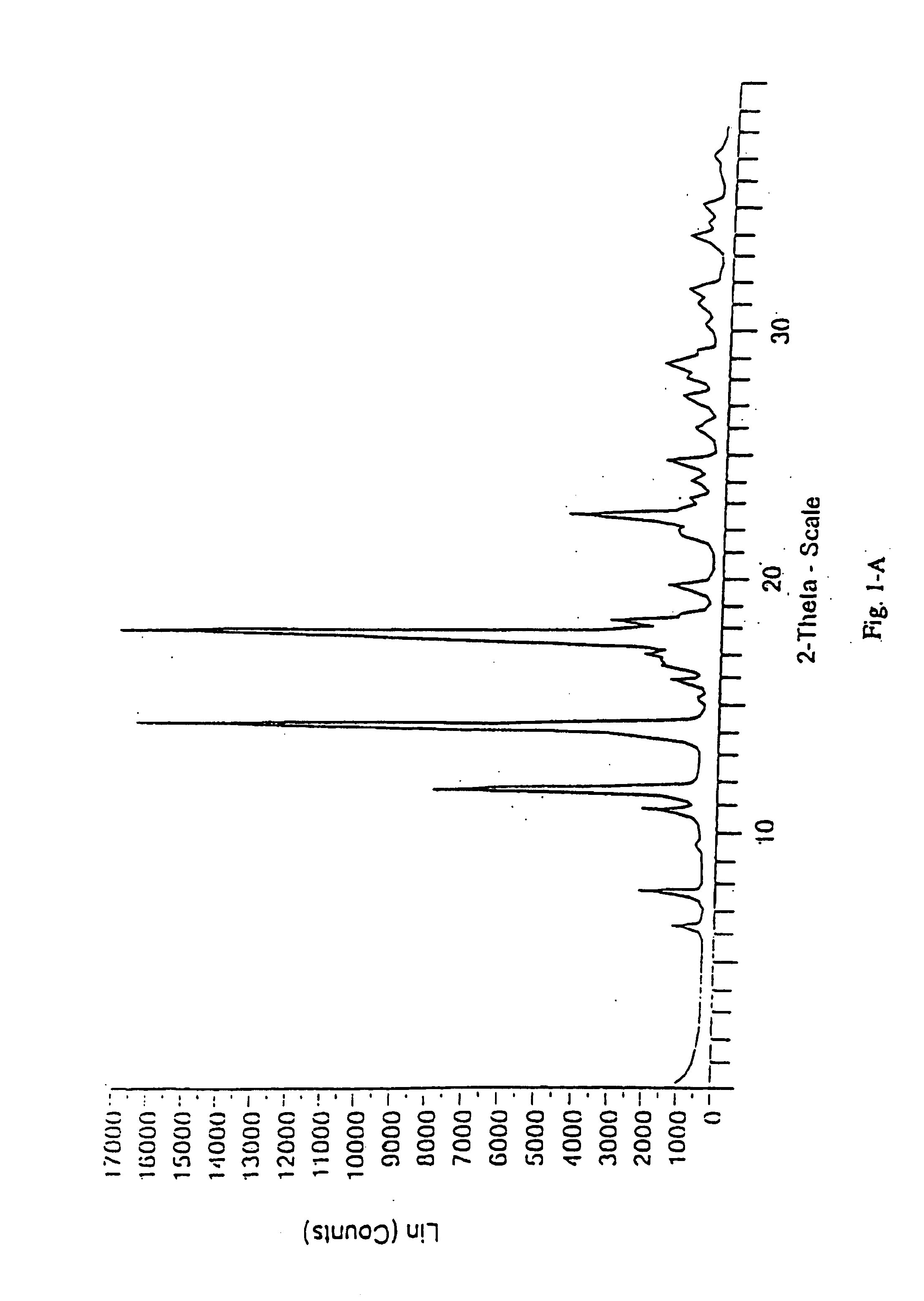
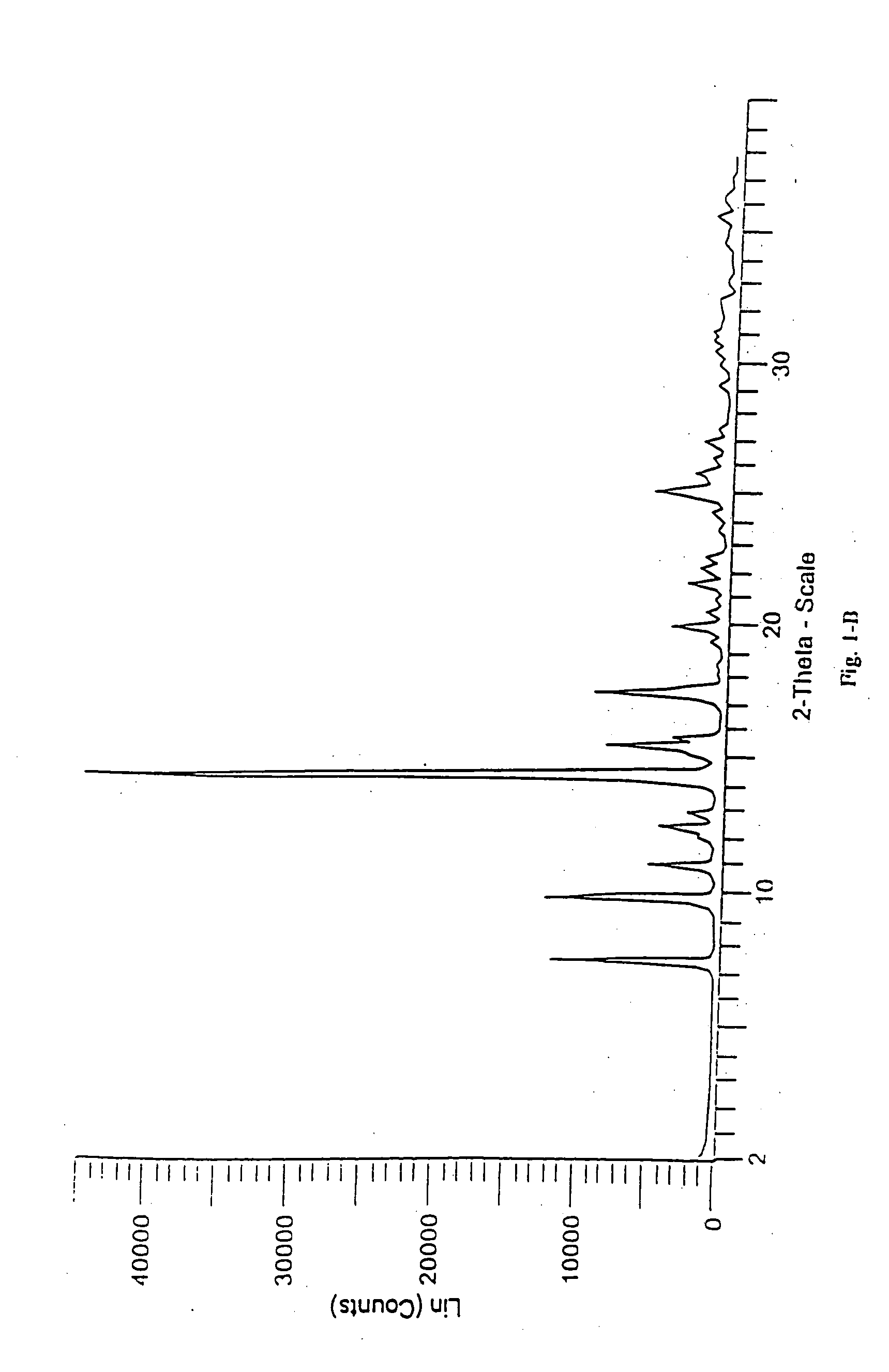
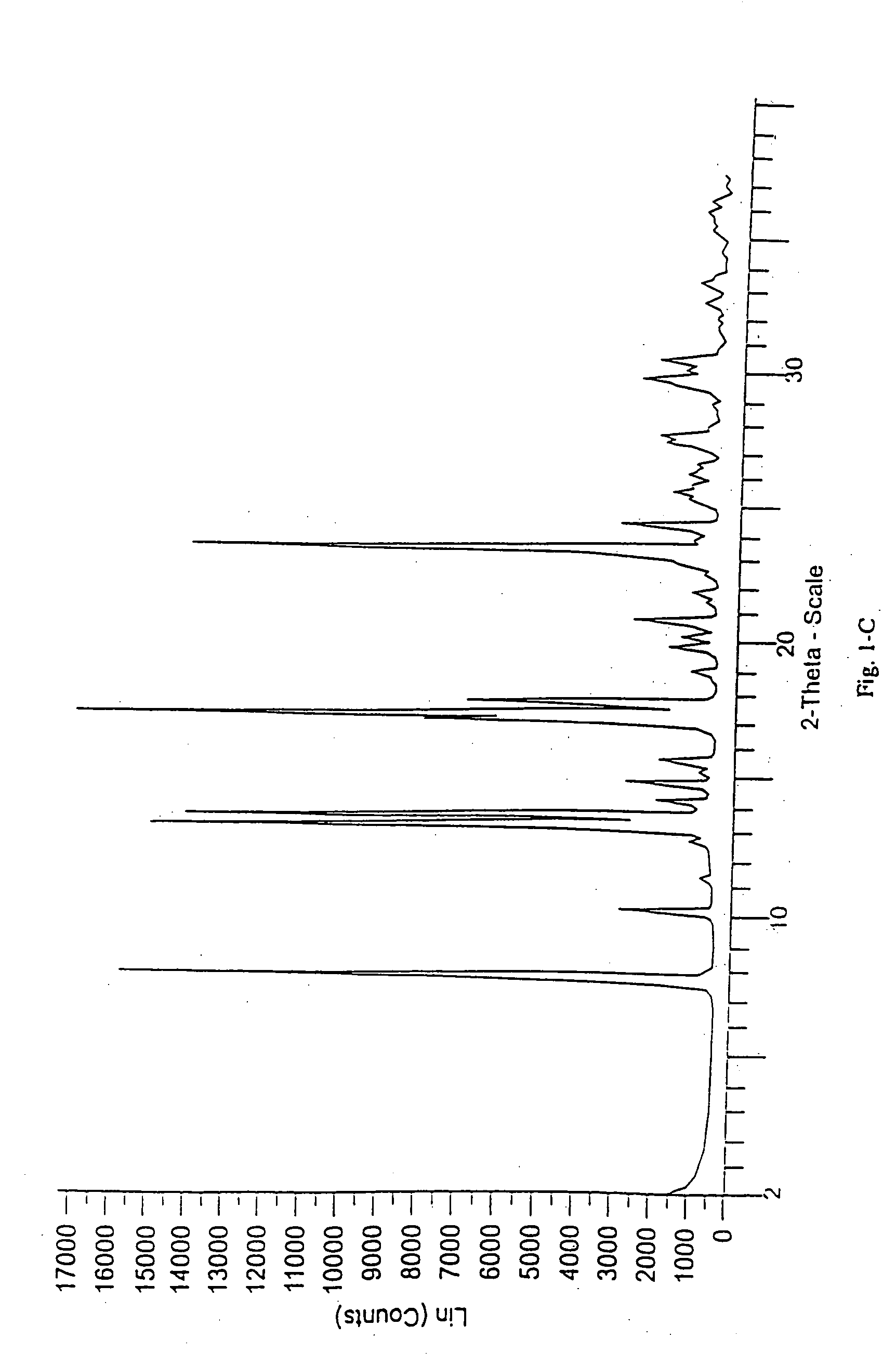
Patents
Literature
63 results about "Increased aldosterone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Aldosterone release causes sodium and water retention, which causes increased blood volume, and a subsequent increase in blood pressure, which is sensed by the baroreceptors. To maintain normal homeostasis these receptors also detect low blood pressure or low blood volume, causing aldosterone to be released.
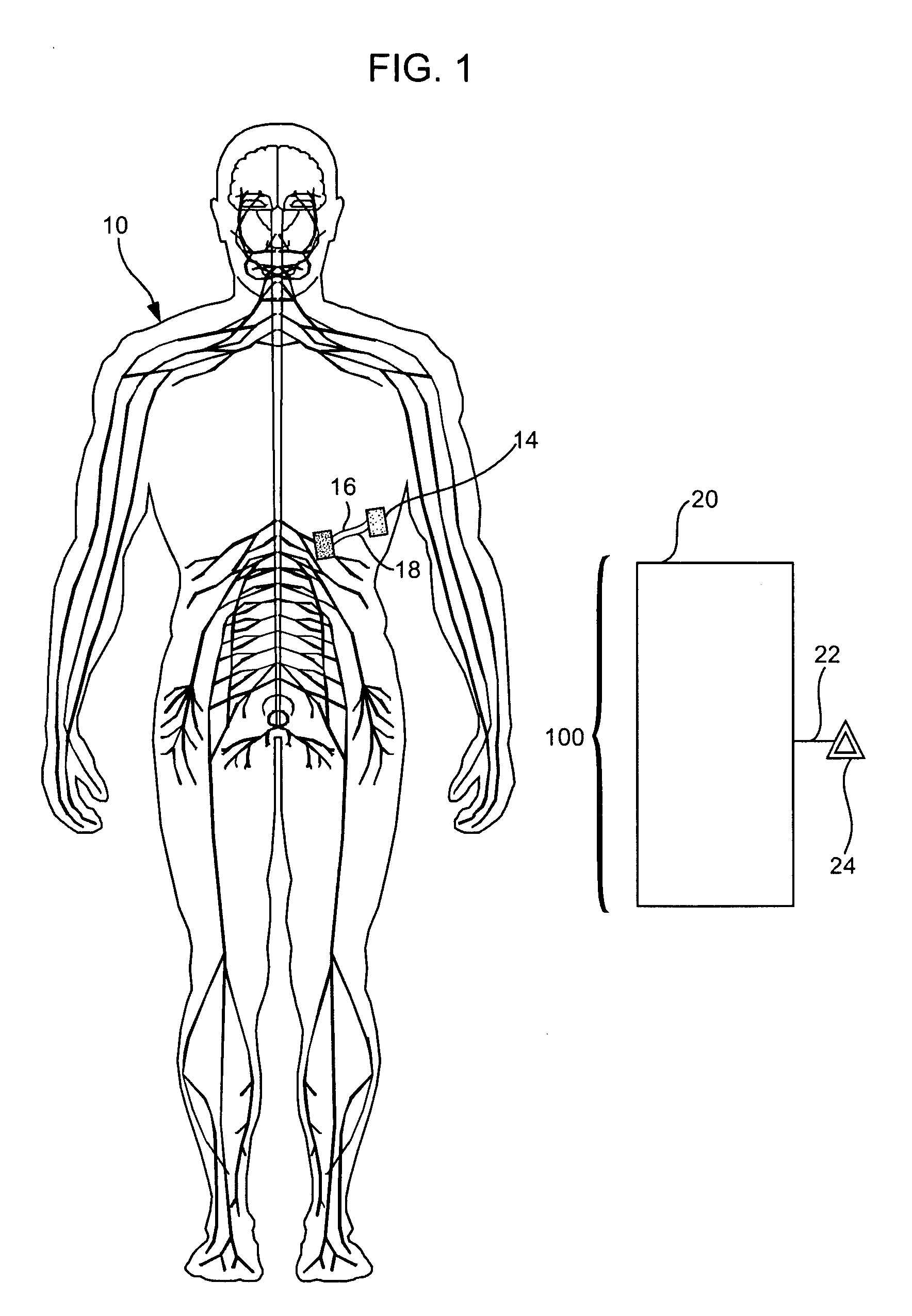
Treatment of conditions through modulation of the autonomic nervous system
InactiveUS20050153885A1Effective treatmentOrganic active ingredientsNervous disorderNervous systemMedicine
Methods are provided for treating a subject for a condition caused by an abnormality in the subject's autonomic nervous system. In accordance with the subject methods, at least a portion of a subject's autonomic nervous system is pharmacologically modulated with at least one aldosterone antagonist in a manner that is effective to treat the subject for the condition. Also provided are systems and kits for use in practicing the subject methods.
Owner:PALO ALTO INVESTORS
Amido compounds and their use as pharmaceuticals
The present invention relates to inhibitors of 11-β hydroxyl steroid dehydrogenase type 1, antagonists of the mineralocorticoid receptor (MR), and pharmaceutical compositions thereof. The compounds of the invention can be useful in the treatment of various diseases associated with expression or activity of 11-β hydroxyl steroid dehydrogenase type 1 and / or diseases associated with aldosterone excess.
Owner:INCYTE CORP
Amido compounds and their use as pharmaceuticals
The present invention relates to inhibitors of 11-β hydroxyl steroid dehydrogenase type 1, antagonists of the mineralocorticoid receptor MR, and pharmaceutical compositions thereof. The compounds of the invention can be useful in the treatment of various diseases associated with expression or activity of 11-β hydroxyl steroid dehydrogenase type 1 and / or diseases associated with aldosterone excess.
Owner:INCYTE
2-Methylprop anamides and their use as pharmaceuticals
The present invention relates to inhibitors of 11-β hydroxyl steroid dehydrogenase type 1, antagonists of the mineralocorticoid receptor (MR), and pharmaceutical compositions thereof. The compounds of the invention can be useful in the treatment of various diseases associated with expression or activity of 11-β hydroxyl steroid dehydrogenase type 1 and / or diseases associated with aldosterone excess.
Owner:INCYTE HLDG CORP
Amido compounds and their use as pharmaceuticals
The present invention relates to inhibitors of 11-β hydroxyl steroid dehydrogenase type 1, antagonists of the mineralocorticoid receptor (MR), and pharmaceutical compositions thereof. The compounds of the invention can be useful in the treatment of various diseases associated with expression or activity of 11-β hydroxyl steroid dehydrogenase type 1 and / or diseases associated with aldosterone excess.
Owner:INCYTE
Amido compounds and their use as pharmaceuticals
InactiveUS20060122197A1Improve actionReduce releaseBiocideSenses disorderDiseaseMineralocorticoid receptor
The present invention relates to inhibitors of 11-β hydroxyl steroid dehydrogenase type 1, antagonists of the mineralocorticoid receptor (MR), and pharmaceutical compositions thereof. The compounds of the invention can be useful in the treatment of various diseases associated with expression or activity of 11-β hydroxyl steroid dehydrogenase type 1 and / or diseases associated with aldosterone excess.
Owner:INCYTE
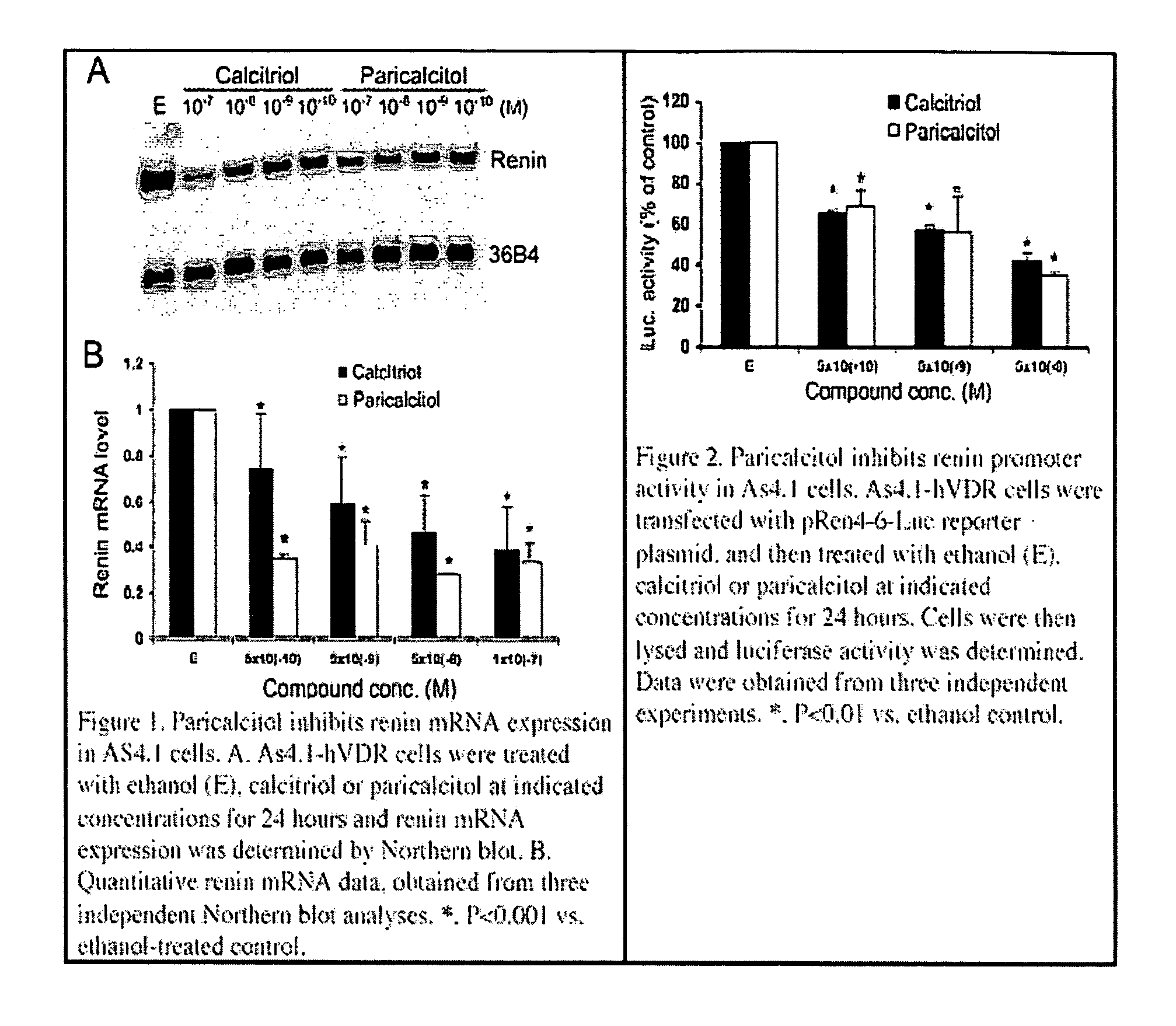
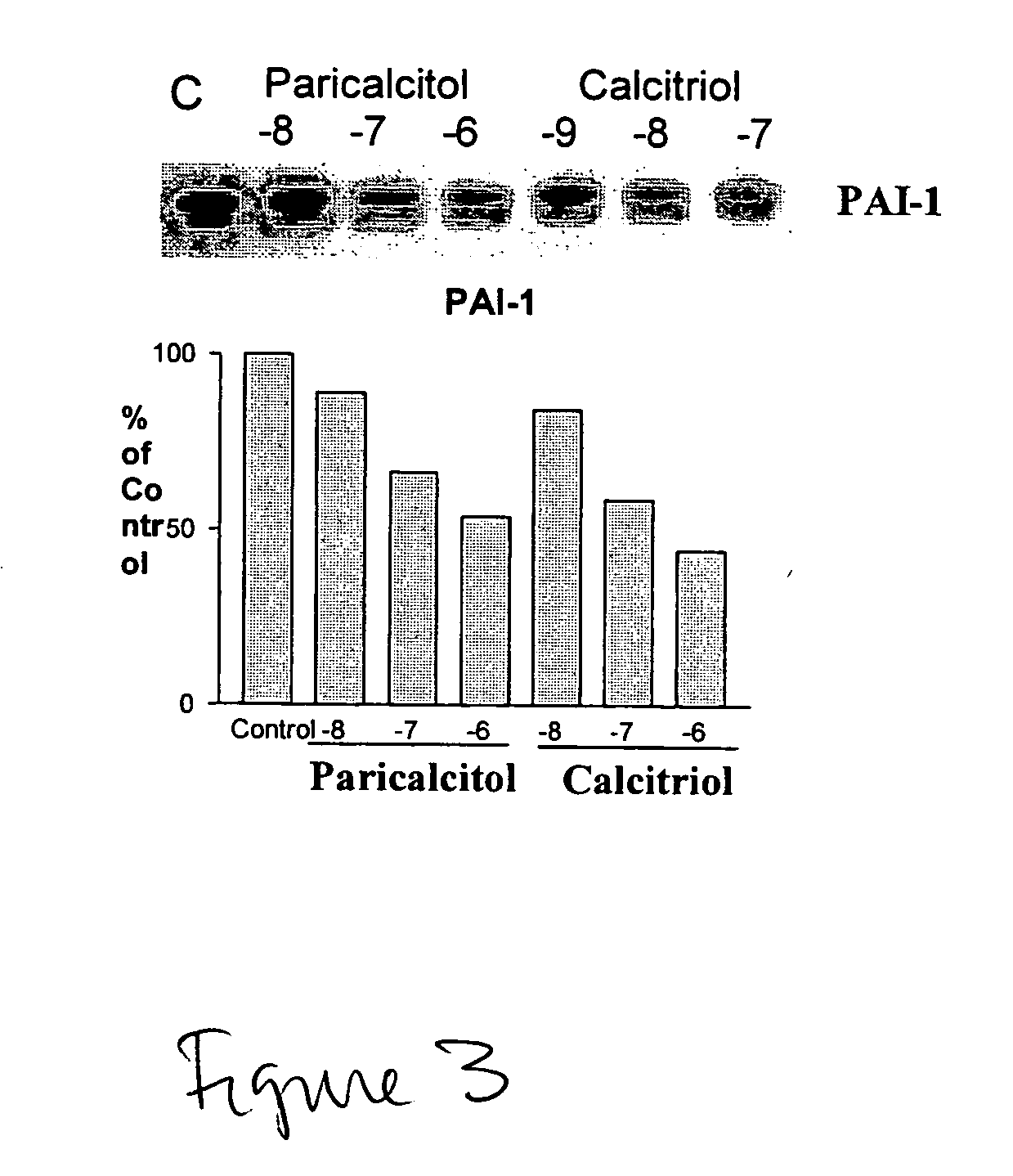
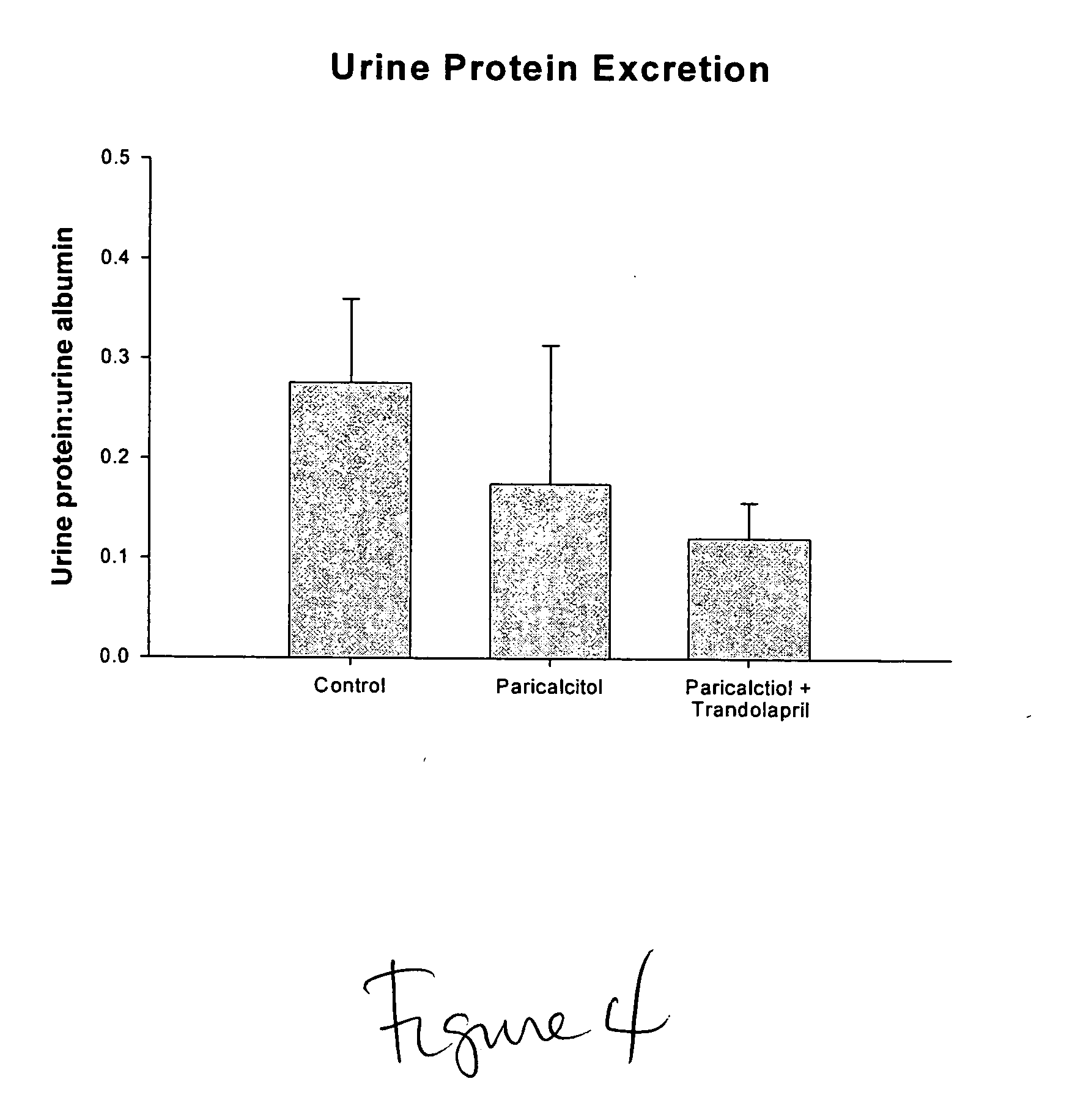
Use of vitamin Ds to treat kidney disease
InactiveUS20050124591A1Reduce inflammationSuppression problemBiocideUrinary disorderDiseaseAngiotensin Receptor Blockers
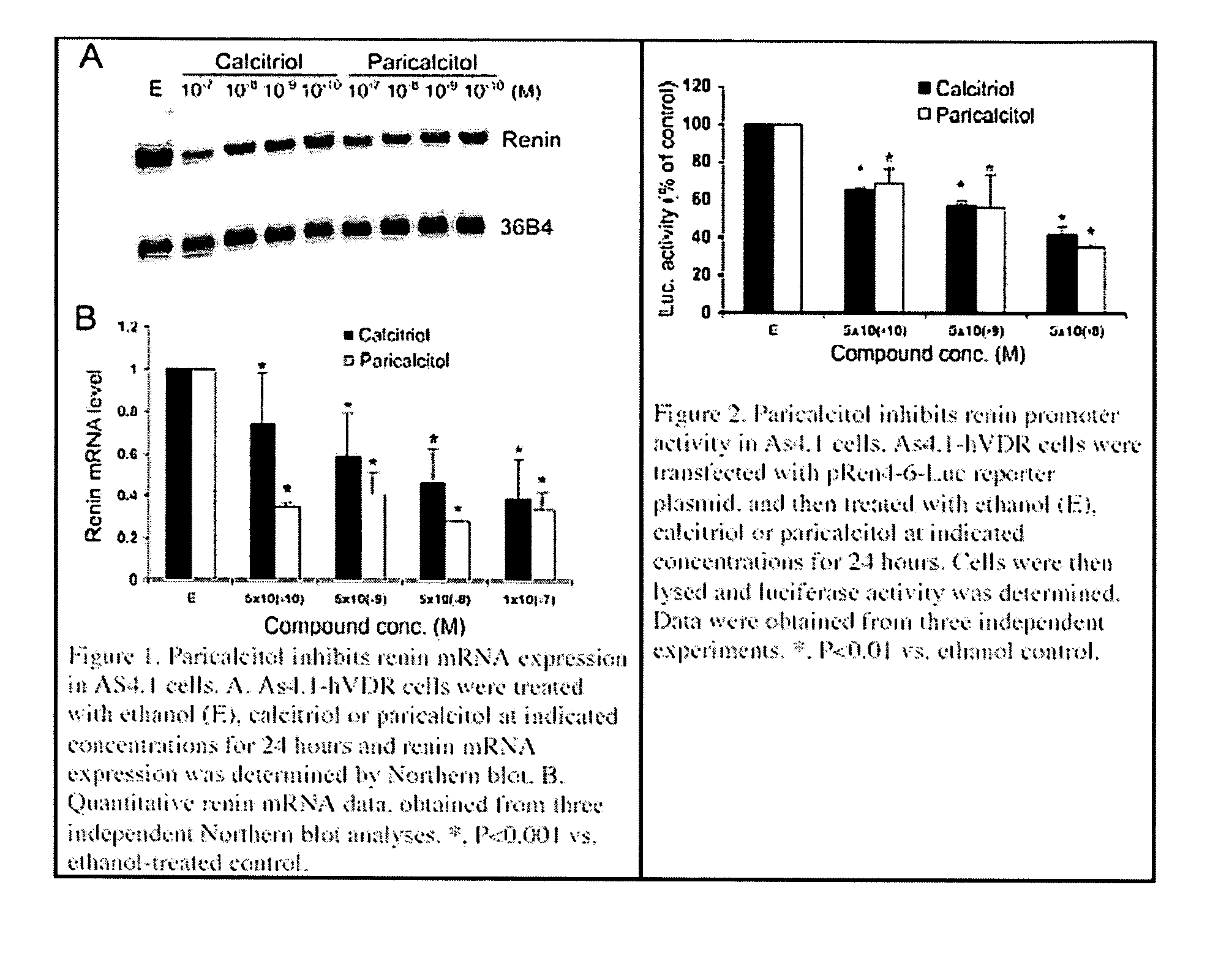
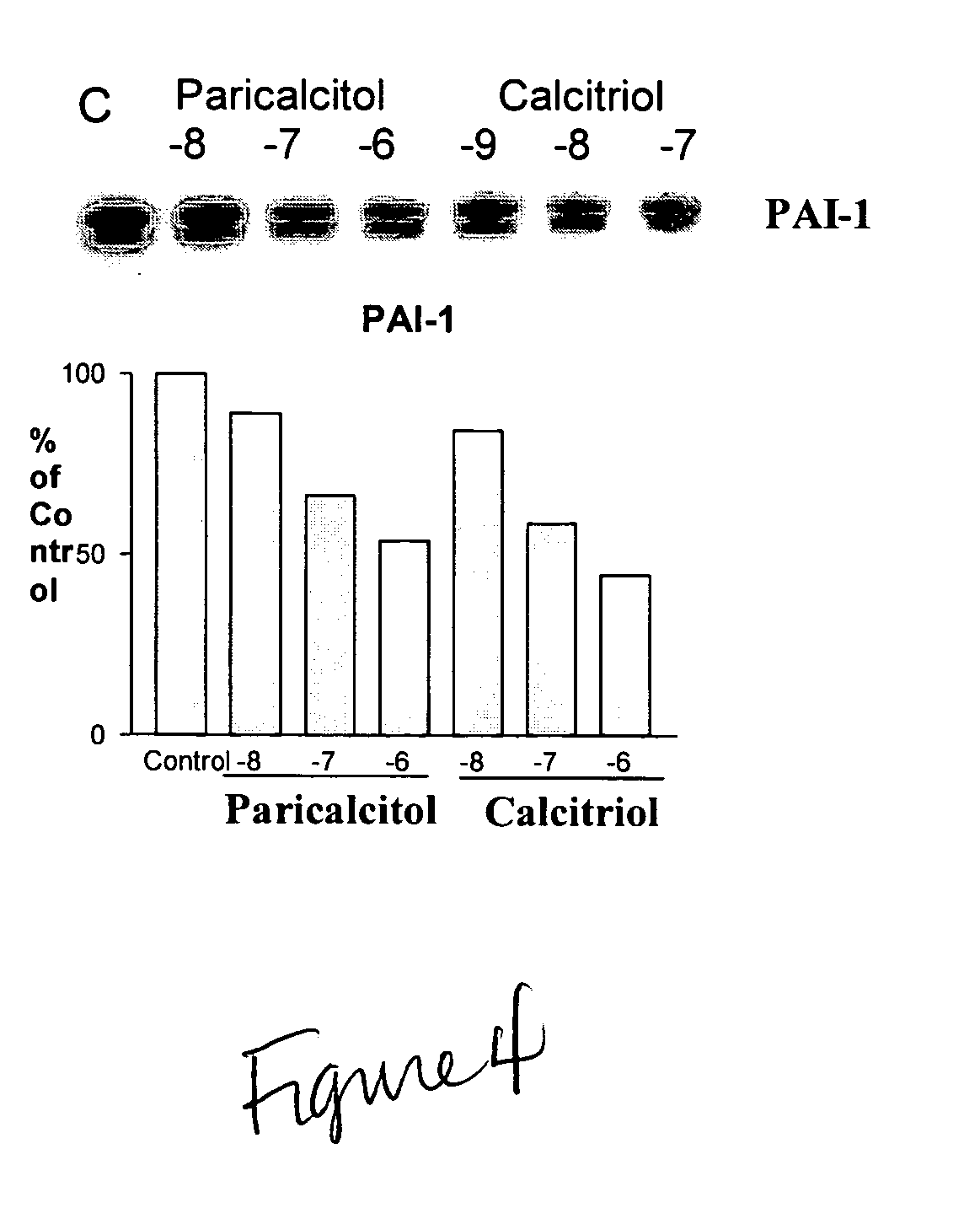
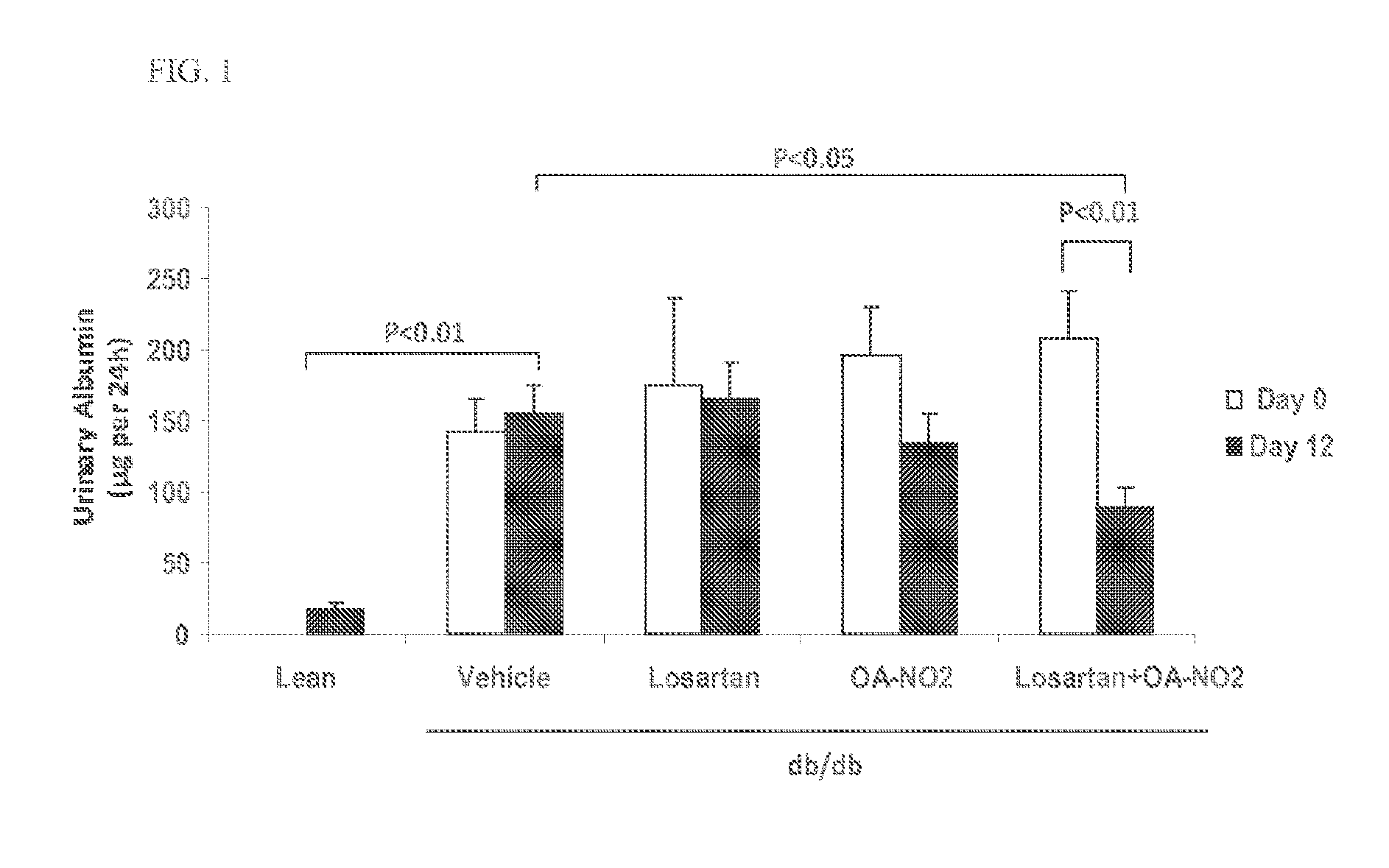
Disclosed are compositions containing a VDRA / Vitamin D analog to treat or prevent kidney disease, including chronic kidney disease. The present invention also relates to methods of treating kidney disease by administering to a patient a pharmaceutical composition containing a therapeutically effective amount of a VDRA / Vitamin D analog. Compositions according to the invention include a VDRA / Vitamin D analog and at least one of the following agents: an ACE inhibitor, an angiotensin (II) receptor blocker (ARB) and aldosterone blocker in therapeutically effective amounts to inhibit renin production or inhibit activation of the renin-angiotensin-aldosterone system. Preferred compositions contain paricalcitol with at least one of these other agents. Such combinations can avoid ACE inhibition escape and aldosterone escape with subsequent increase in angiotensin (II) and aldosterone generation.
Owner:ABBOTT LAB INC
Combination of an aldosterone receptor antagonist and an anti-diabetic agent
A combination therapy comprising a therapeutically-effective amount of an aldosterone receptor antagonist and a therapeutically-effective amount of an anti-diabetic agent is described for treatment of circulatory disorders, including cardiovascular disorders such as hypertension, congestive heart failure, cirrhosis and ascites. Preferred antidiabetic agents are those compounds having high potency and oral or parenteral bioavailability. Preferred aldosterone receptor antagonists are 20-spiroxane steroidal compounds characterized by the presence of a 9α,11α-substituted epoxy moiety.
Owner:PHARMACIA CORP
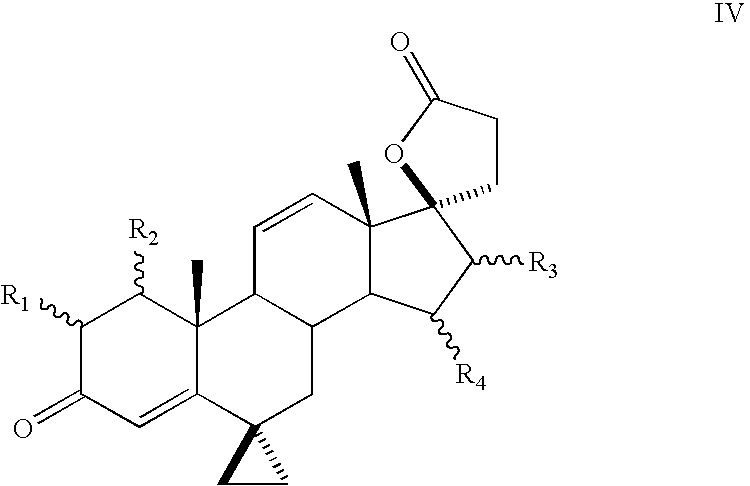
Progestational 3-(6,6-ethylene-17B-hydroxy-3-oxo-17A-pregna-4-ene-17A-yl)propionic acid G-lactones
Owner:EVESTRA
Use of Vitamin Ds to treat kidney disease
InactiveUS20050148557A1Reduce inflammationSuppression problemBiocideUrinary disorderDiseaseAldosterone escape
Disclosed are compositions containing a VDRA / Vitamin D analog to treat or prevent kidney disease, including chronic kidney disease. The present invention also relates to methods of treating kidney disease by administering to a patient a pharmaceutical composition containing a therapeutically effective amount of a VDRA / Vitamin D analog. Compositions according to the invention include a VDRA / Vitamin D analog and at least one of the following agents: an ACE inhibitor, an angiotensin (II) receptor blocker (ARB) and aldosterone blocker in therapeutically effective amounts to inhibit renin production or inhibit activation of the renin-angiotensin-aldosterone system. Preferred compositions contain paricalcitol with at least one of these other agents. Such combinations can avoid ACE inhibition escape and aldosterone escape with subsequent increase in angiotensin (II) and aldosterone generation.
Owner:UNIVERSITY OF CHICAGO
Potassium binding polymers for treating hypertension and hyperkalemia
ActiveUS20140105848A1Increase and stabilize kidney functionImprove survivalPowder deliveryHeavy metal active ingredientsNephrosisNephropathy
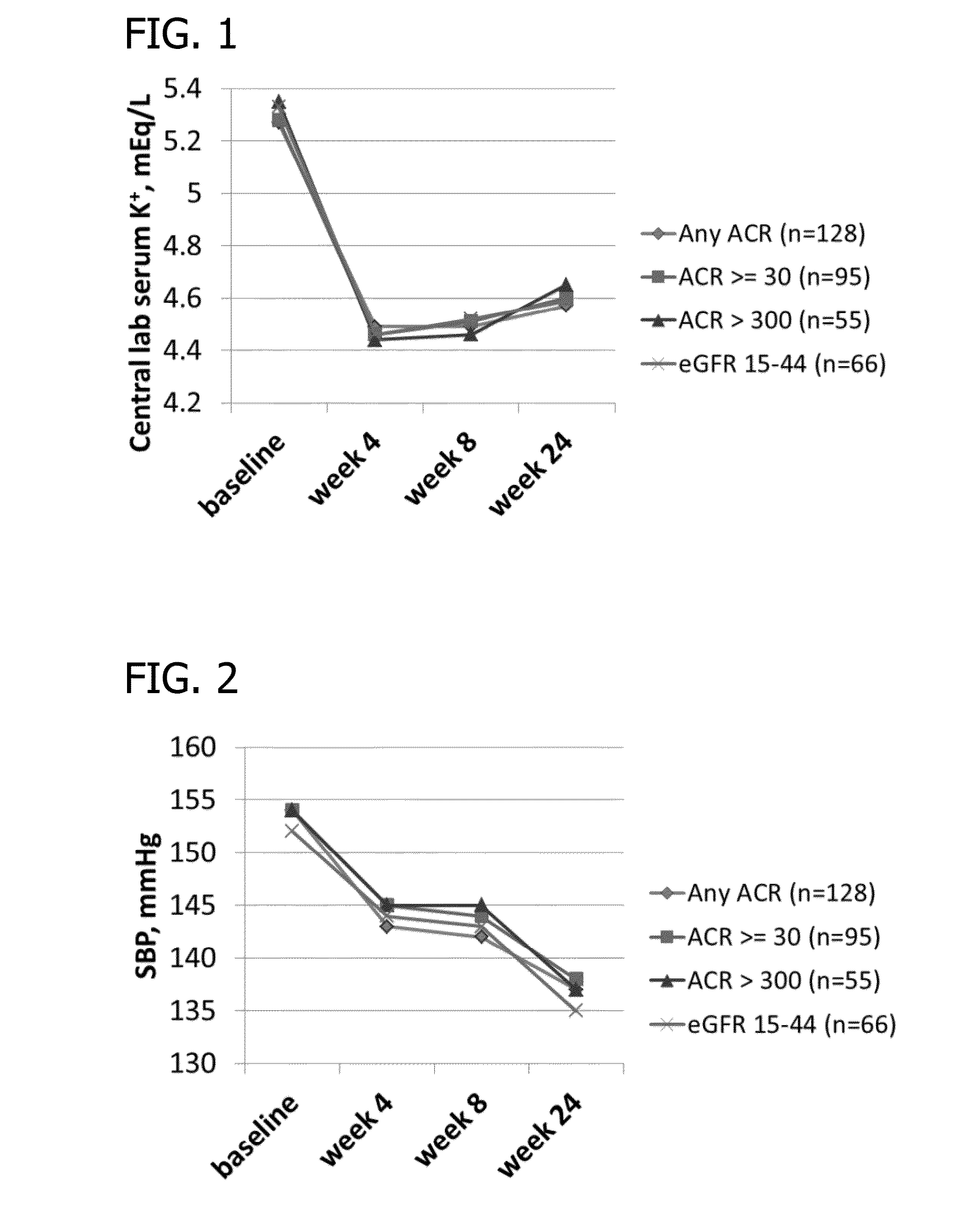
The present invention generally relates to methods of treating hypertension (HTN) in patients in need thereof wherein the patient optionally further suffers from chronic kidney disease (CKD) or Type II diabetes mellitus (T2DM). The invention also relates to methods of treating hyperkalemia in a patient in need thereof, wherein the patient suffers from CKD, T2DM or HTN and are optionally being treated with an effective amount of a renin-angiotensin-aldosterone system (RAAS) agent. The invention also relates to methods of treating kidney disease in a patient in need thereof, wherein the patient is optionally being treated with an effective amount of a renin-angiotensin-aldosterone system (RAAS) agent. The methods can comprise administering an effective amount of a potassium-binding polymer to the patient to lower the patient's blood pressure and / or increase or stabilize the patient's kidney function.
Owner:VIFOR INT AG
Tgf-beta antagonists combined with renin-angiotensin-aldosteron-system antagonists for treating renal insufficiency
InactiveUS20060286105A1Loss of renal functionPrevent and reduce riskBiocidePeptide/protein ingredientsRenal disorderFibrosis
The disclosure provides methods for treating, preventing, and reducing risk of occurrence of renal insufficiency in mammals. The disclosed methods include administering to a subject susceptible to, or afflicted with, renal disorder therapeutically effective amounts of a TGF-β antagonist and a renin-angiotensin-aldosterone system (RAAS) antagonist so as to maintain desirable levels of renal function. The populations treated by the methods of the invention include but are not limited to patients suffering or at risk for the development of renal insufficiency, such as those afflicted with type I or type II diabetes, hypertension, (auto)immune disease, systemic fibrosis, etc.
Owner:LEDBETTER STEVEN +2
Implantable medical device for drug delivery and method of use
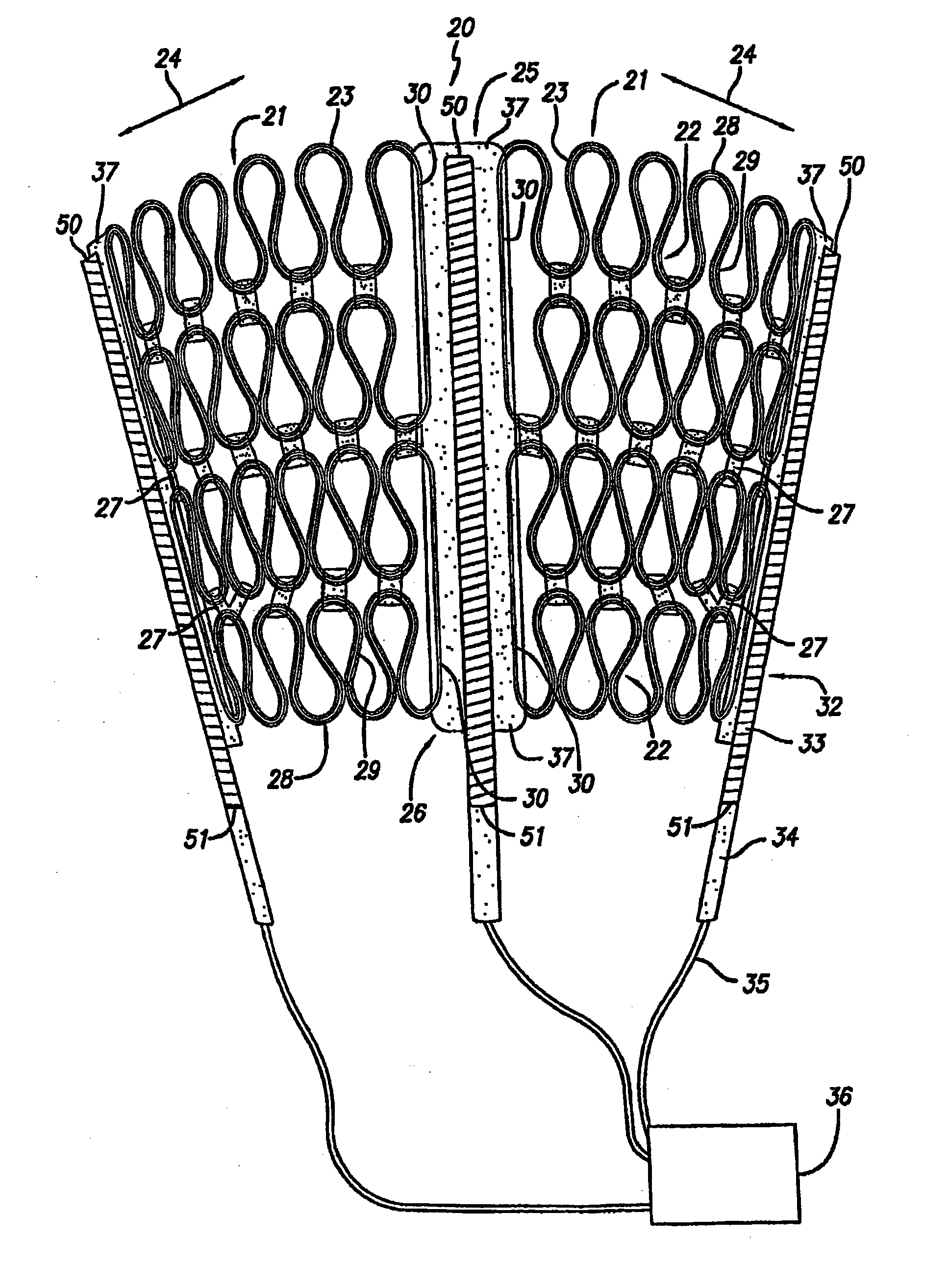
An apparatus and method of preparing a cardiac harness for use in controllably delivering a medicament such as an mTOR inhibitor or aldosterone blockade from the surface of the cardiac harness to the epicardium of a patient's heart for the site-specific treatment of cardiac and non-cardiac maladies. The delivery of the medicament from the cardiac harness is achieved by: coating the medicament on the surface of the cardiac harness; coating the cardiac harness with a polymer material or dielectric material and impregnating the coating with the medicament; or by loading the medicament into an implant attached to the cardiac harness.
Owner:PARACOR MEDICAL
Compositions and methods related to heart failure
InactiveUS20060014828A1Reduce in quantityShorten the construction periodBiocideNervous disorderDiseaseType B Natriuretic Peptide
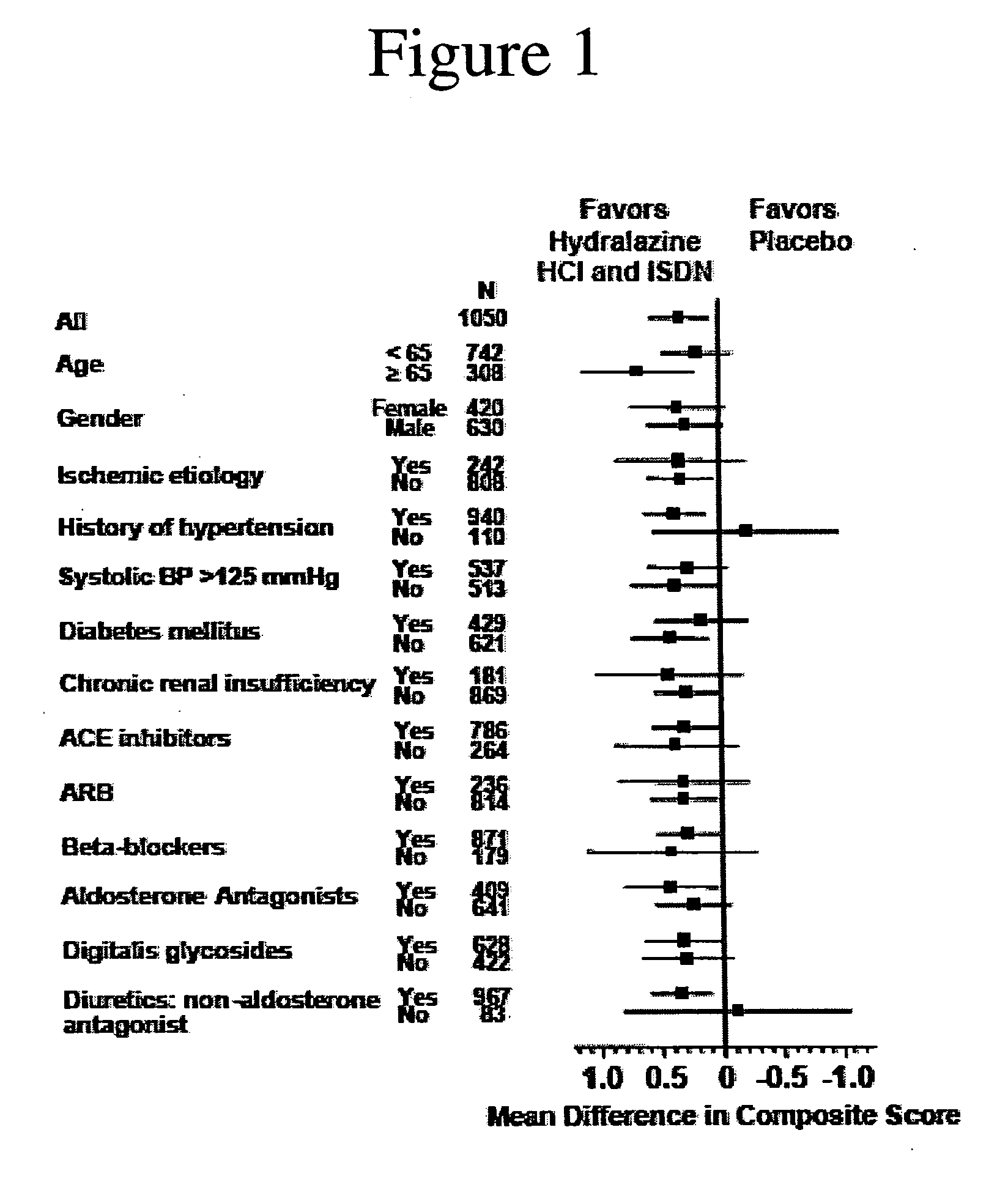
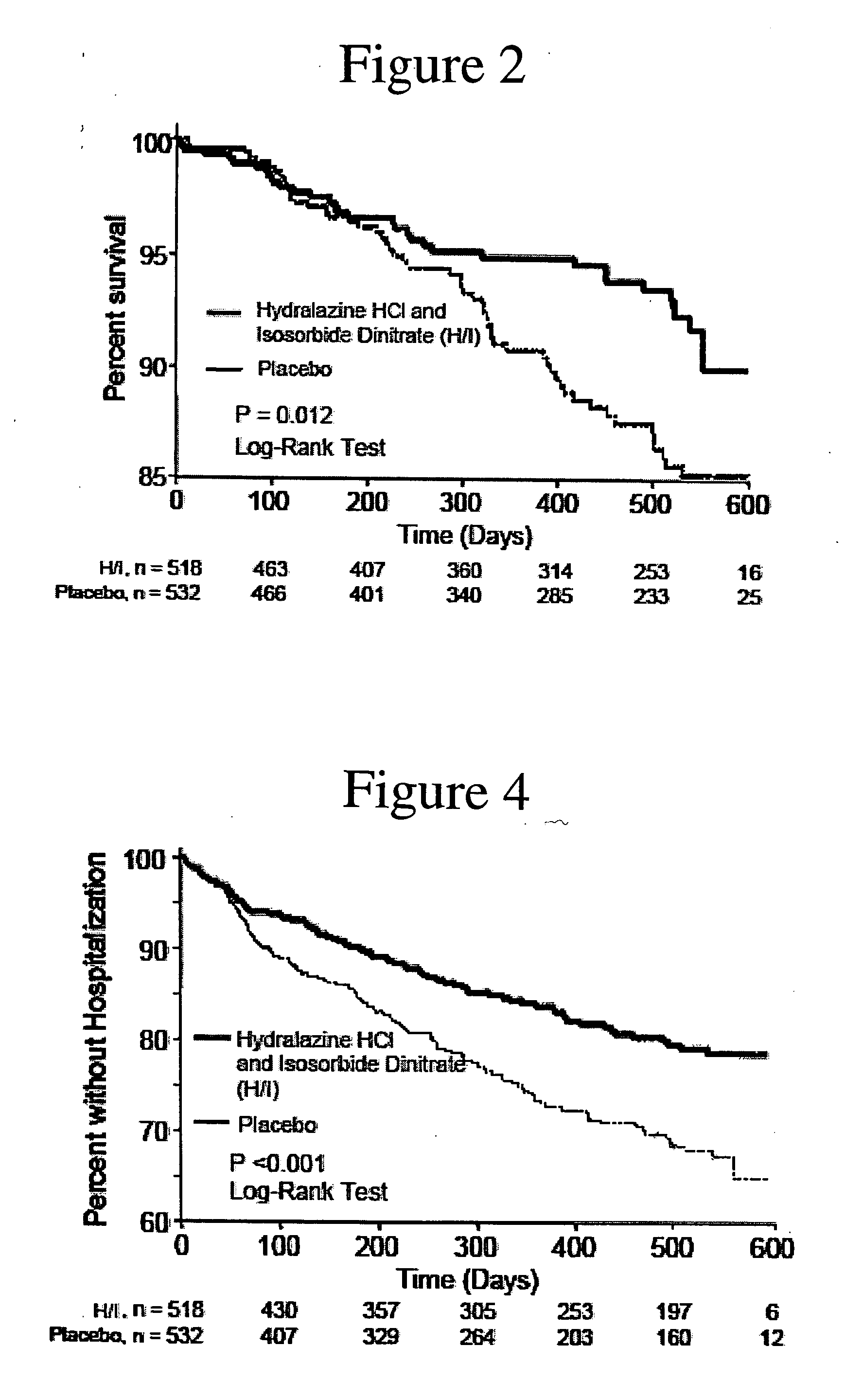
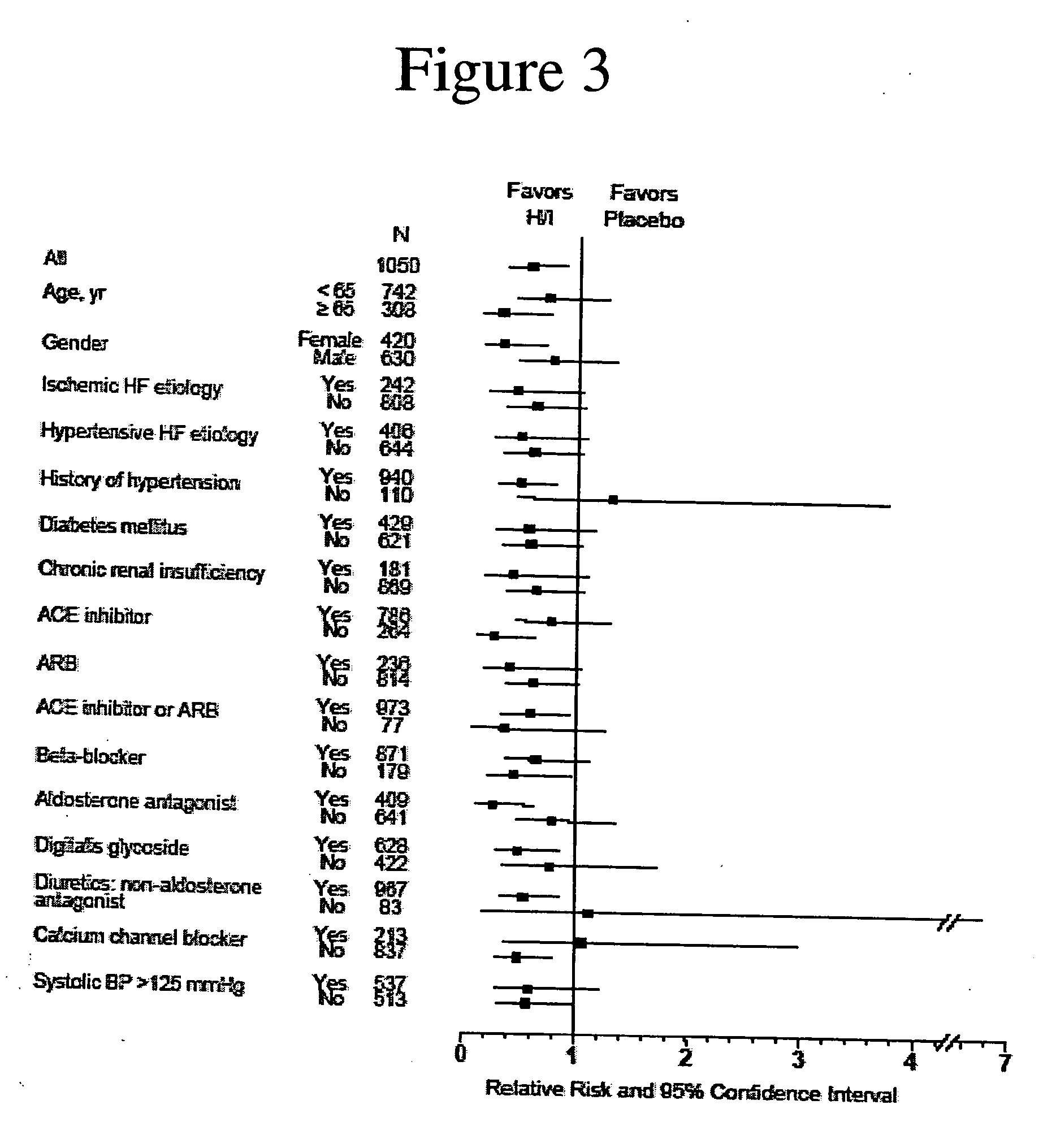
The invention provides methods for (a) prolonging time to hospitalization for heart failure; (b) prolonging time to first hospitalization for heart failure; (c) reducing the total number of days a patient with heart failure spends in the hospital for heart failure for a single hospital stay (i.e., reducing the duration of a single hospital stay for a patient with heart failure); (d) reducing the total number of days a patient spends in the hospital for heart failure for multiple hospital stays (i.e., two or more hospital stays); (e) reducing the number of hospital admissions for heart failure; (f) reducing mortality and reducing hospitalizations for heart failure (e.g., the total number of days in the hospital and / or the number of hospital visits); (g) increasing the left ventricular ejection fraction in a heart failure patient; (h) treating a sexual dysfunction (e.g., erectile dysfunction and female sexual dysfunction) (j) treating a headache in a heart failure patient by administering a non-steroidal antiinflammatory compound (i.e., NSAIDs); (k) treating a heart failure patient who has a history of hypertension (but who is not currently diagnosed with hypertension); (l) improving the quality of life in a heart failure patient based on the Minnesota Living with heart failure questionnaire; (m) decreasing the levels of B-type natriuretic peptide; (n) treating hypertension in a heart failure patient; (o) lowering blood pressure in a heart failure patient; (p) treating labile hypertension; (q) treating idiopathic hypertension; (r) increasing patient compliance with medication dosing in a heart failure patient; (s) treating hypertension in a patient with a dilated heart; (t) treating ischemic disease and / or coronary artery disease; and (u) reducing cardiomegaly in a patient in need thereof comprising administering to the patient a therapeutically effective amount of (i) a hydralazine compound or pharmaceutically acceptable salt thereof, (ii) isosorbide dinitrate and / or isosorbide mononitrate, and (iii) optionally at least one compound selected from the group consisting of angiotensin converting enzyme inhibitors, β-adrenergic antagonists, angiotensin II antagonists, aldosterone antagonists, cardiac glucosides (digitalis), and diuretic compounds.
Owner:NITROMED
Combination therapy with nitrated lipids and inhibitors of the renin-angiotensin-aldosterone system
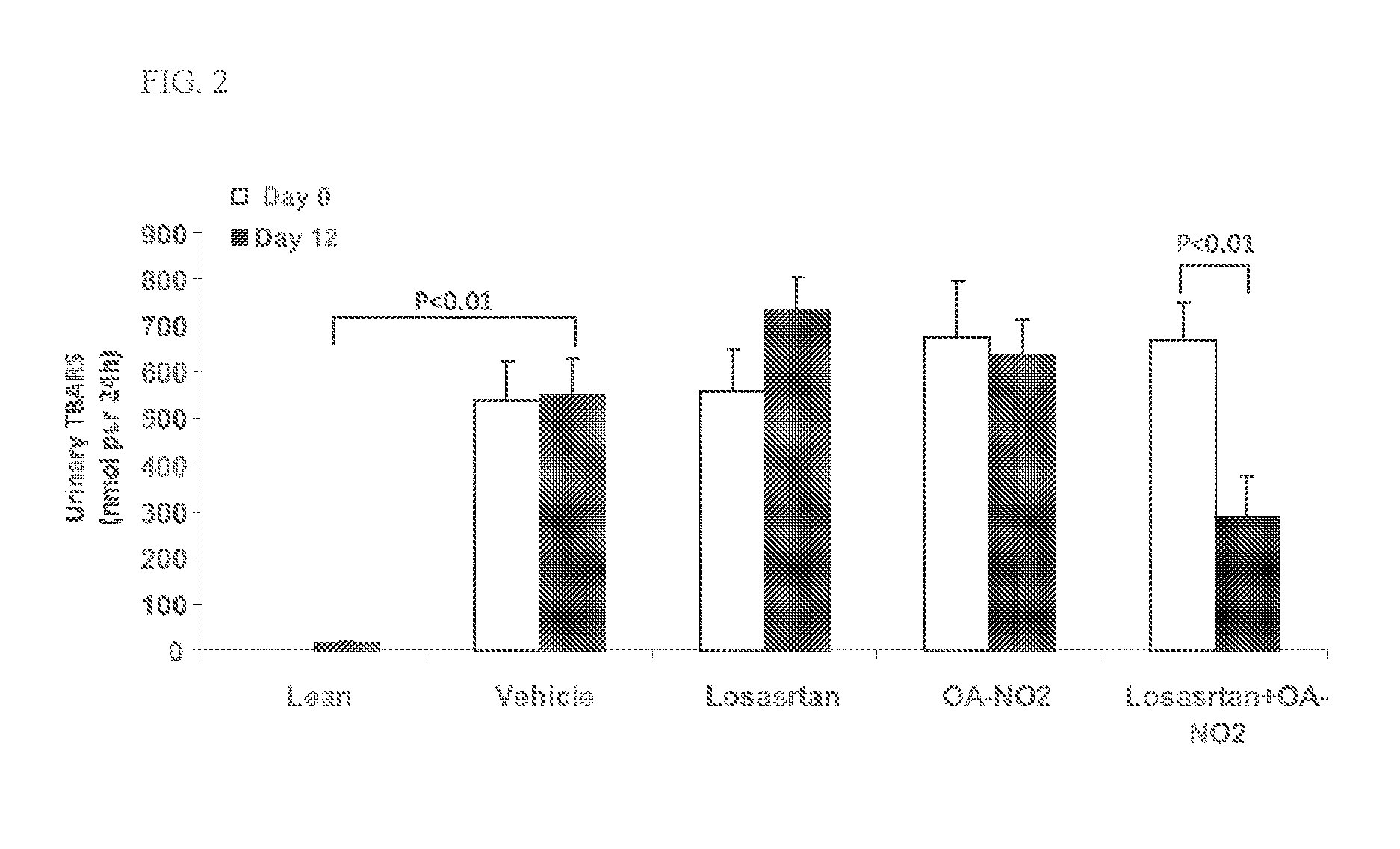
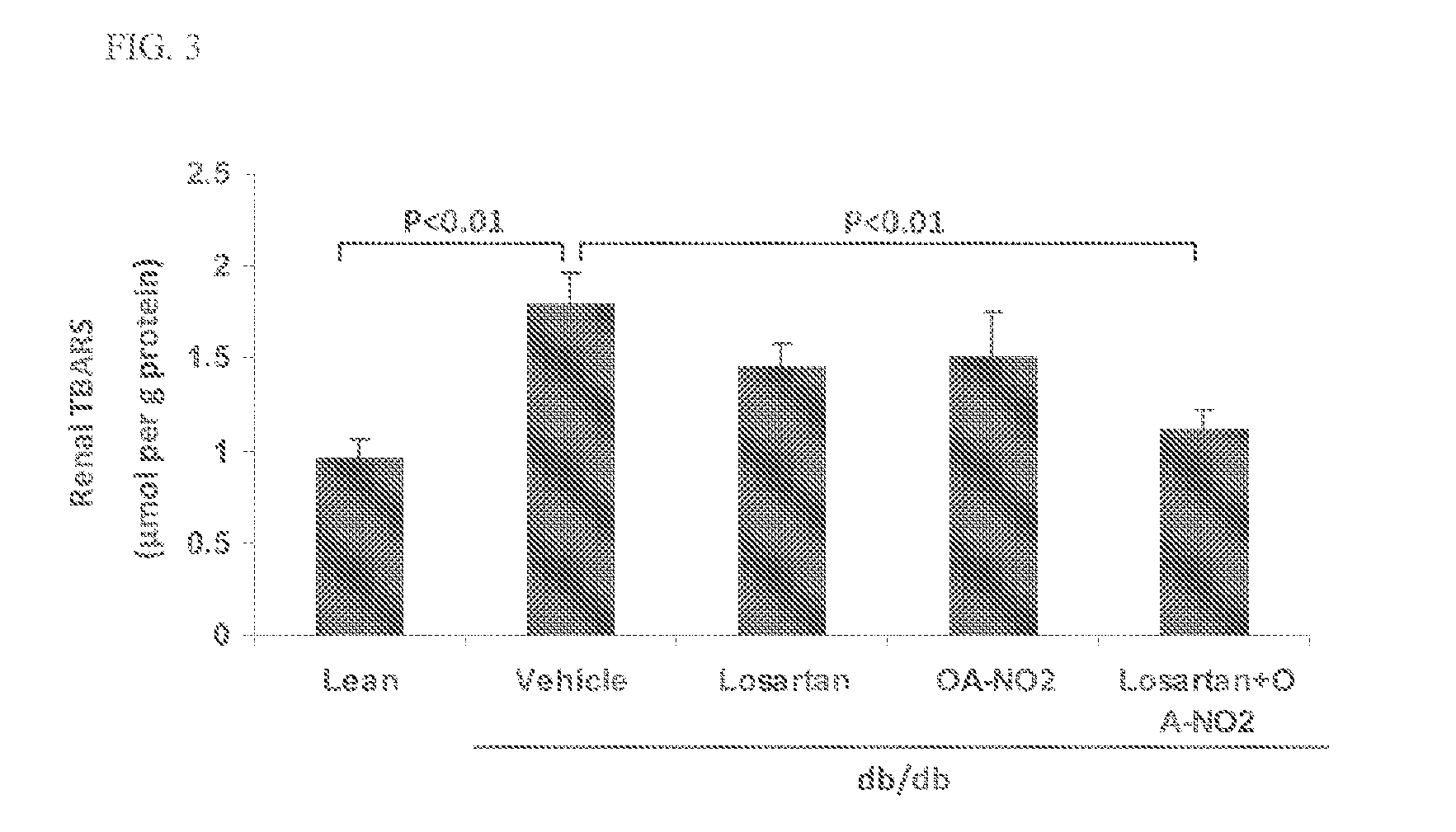
ActiveUS20140243380A1Reducing mRNA expressionHigh expressionBiocideUrinary disorderNephrosisEnd stage renal disease
The present technology provides compositions and methods for treating chronic kidney disease, end-stage renal disease, or diabetic nephropathy. The compositions comprise a nitrated lipid and an inhibitor of the renin-angiotensin-aldosterone system. The methods comprise administering a nitrated lipid in combination with an inhibitor of the renin-angiotensin-aldosterone system to a subject in need thereof, in an amount effective to treat diabetic nephropathy, chronic kidney disease, and / or end-stage renal disease. The use of a nitrated lipid with an inhibitor of the renin-angiotensin-aldosterone system exhibits a synergistic effect in treating chronic kidney disease and diabetic nephropathy.
Owner:UNIV OF UTAH RES FOUND
Microporous zirconium silicate and diuretics for the reduction of potassium and treatment of chronic kidney and/or chronic heart disease
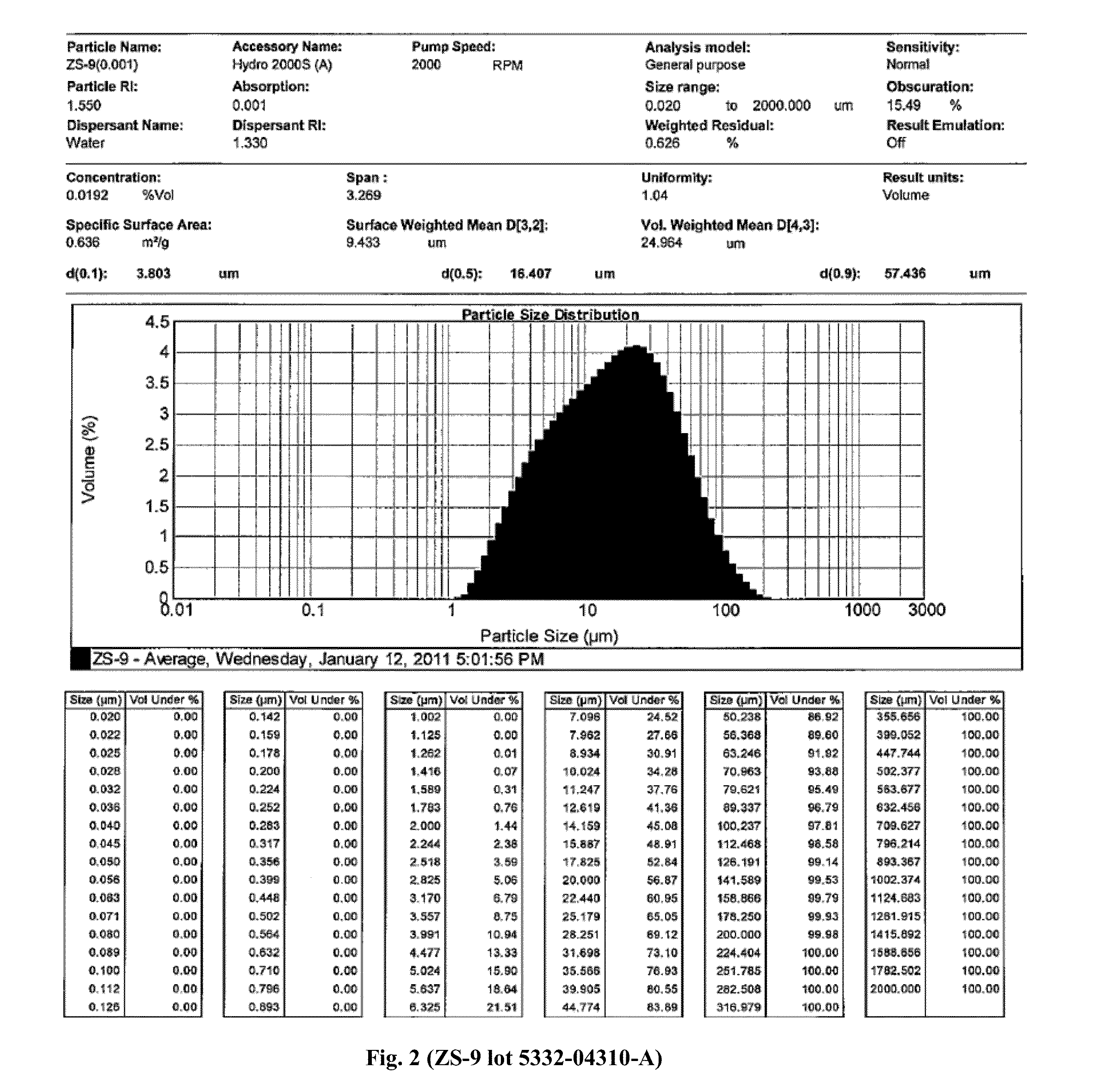
The present invention relates to novel methods of using microporous zirconium silicate to reduce the risk of hyperkalemia and to lower aldosterone levels in the treatment of chronic kidney disease and / or chronic heart disease with therapies comprising diuretics. The invention provides a safe way to reduce the risk of hyperkalemia and to lower aldosterone. The invention also relates to treatment of other conditions that can occur either alone or in connection with hyperkalemia, chronic kidney disease, and / or chronic heart disease.
Owner:ZS PHARMA
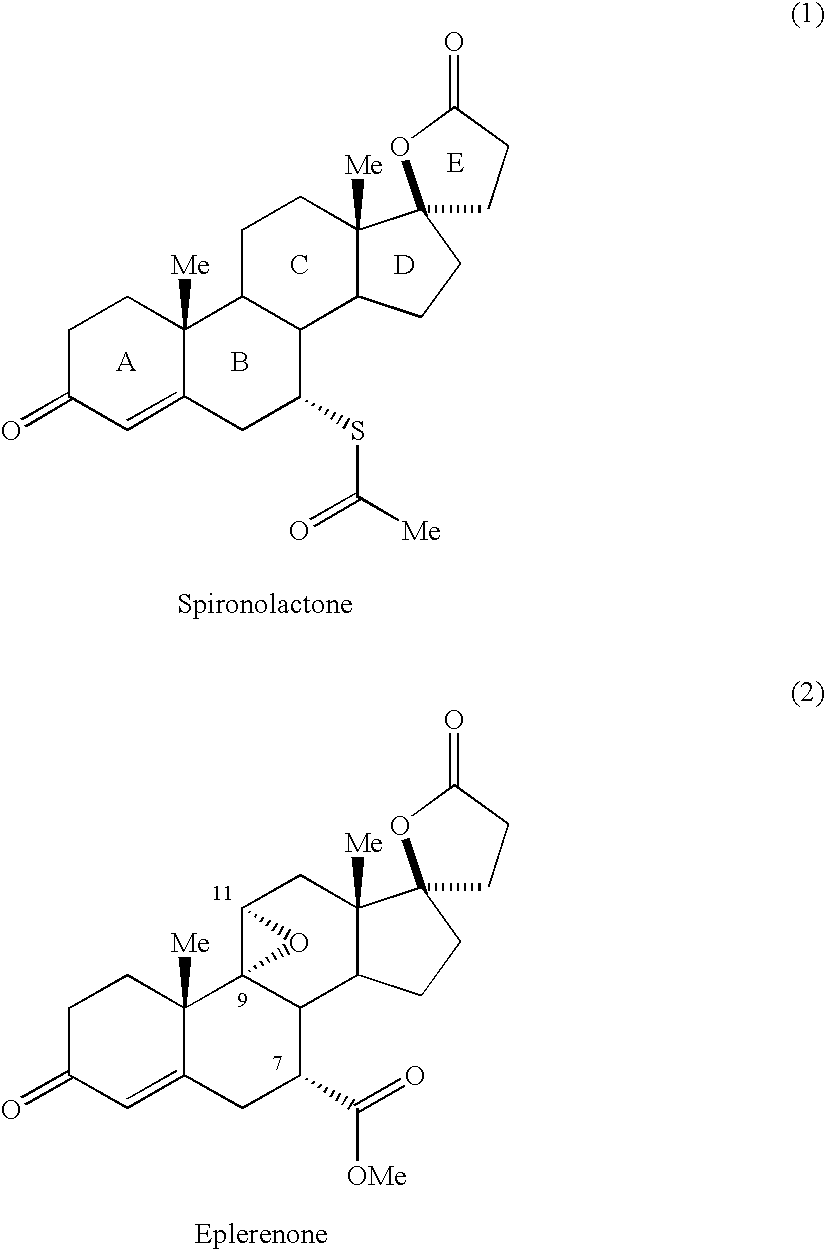
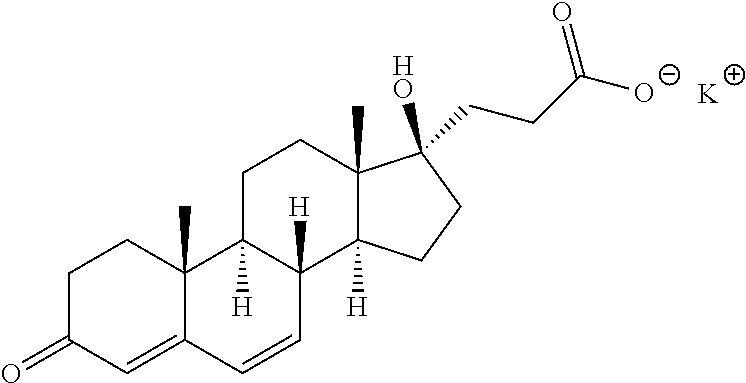
Eplerenone crystalline form exhibiting enhanced dissolution rate
A novel crystalline form (Form H) of the aldosterone receptor antagonist drug eplerenone is provided having a relatively rapid dissolution rate in aqueous media. Also provided are novel solvated crystalline forms of eplerenone that, when desolvated, can yield Form H eplerenone. Also provided is amorphous eplerenone. Pharmaceutical compositions are provided comprising Form H eplerenone, optionally accompanied by one or more other solid state forms of eplerenone, in a total unit dosage amount of eplerenone of about 10 to about 1000 mg, and further comprising one or more pharmaceutically acceptable excipients. Processes are provided for preparing Form H eplerenone and for preparing compositions comprising Form H eplerenone. A method for prophylaxis and / or treatment of an aldosterone-mediated condition or disorder is also provided, comprising administering to a subject a therapeutically effective amount of eplerenone, wherein at least a fraction of the eplerenone present is Form H eplerenone.
Owner:GD SEARLE & CO
Cardiovascular Compounds Comprising Nitric Oxide Enhancing Groups, Compositions and Methods of Use
The invention describes compositions and kits comprising at least one cardiovascular compound comprising at least one nitric oxide enhancing group, or pharmaceutically acceptable salts thereof, and, optionally, at least one nitric oxide enhancing compound and / or at least one therapeutic agent. The invention also provides methods for (a) treating cardiovascular diseases; (b) treating renovascular diseases; (c) treating diabetes; (d) treating diseases resulting from oxidative stress; (e) treating endothelial dysfunctions; (f) treating diseases caused by endothelial dysfunctions; (g) treating cirrhosis; (h) treating pre-eclampsia; Q) treating osteoporosis; (k) treating nephropathy; (l) treating peripheral vascular diseases; (m) treating portal hypertension; (n) treating ophthalmic disorders; (o) treating metabolic syndrome; and (p) treating hyperlipidemia. The cardiovascular compounds are angiotensin II antagonists, aldosterone antagonists, endothelin antagonists, hydralazine compounds, neutral endopeptidase inhibitors and renin inhibitors. The nitric oxide enhancing groups are nitroxides and / or heterocyclic nitric oxide donors.
Owner:NICOX SA
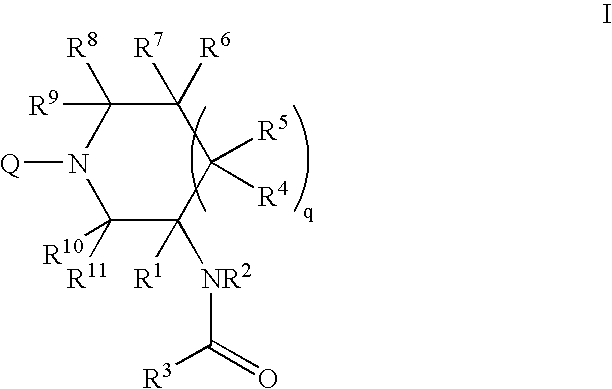
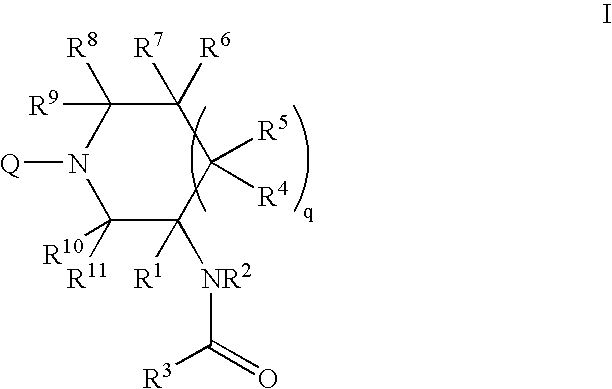
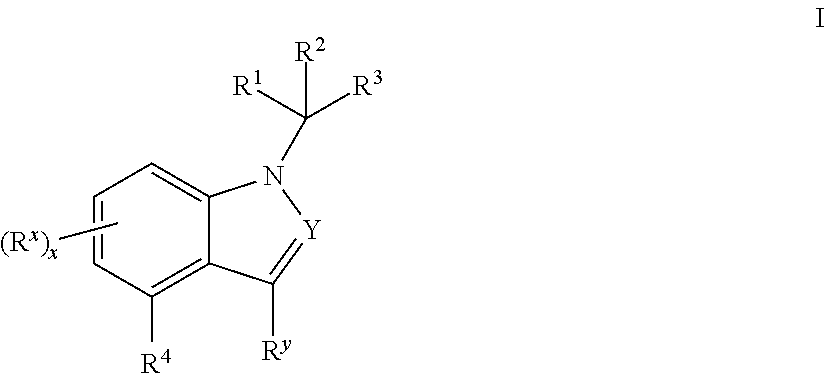
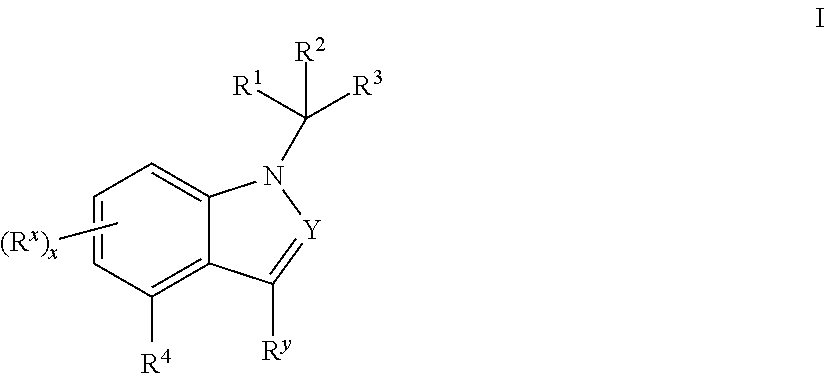
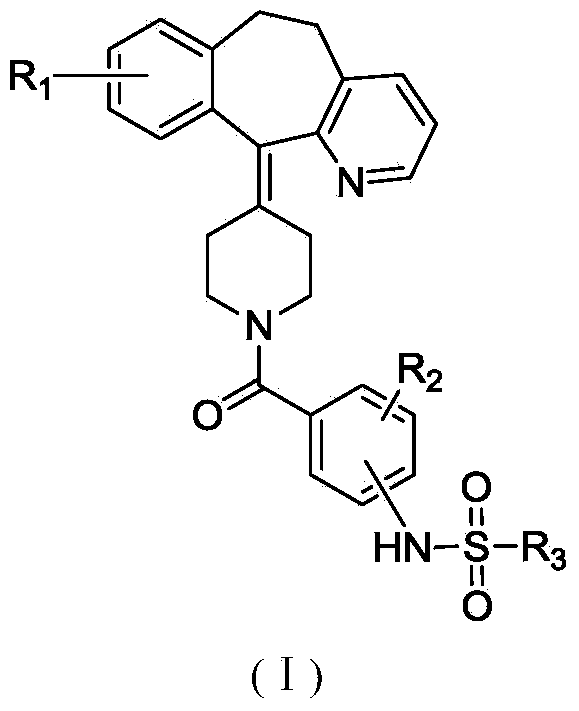
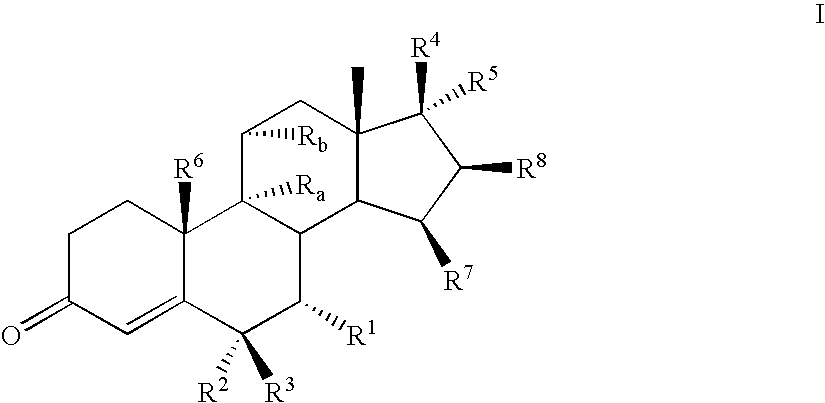
Mineralocorticoid receptor antagonists
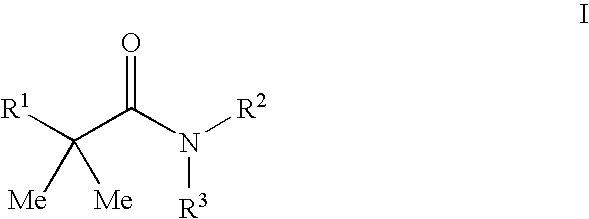
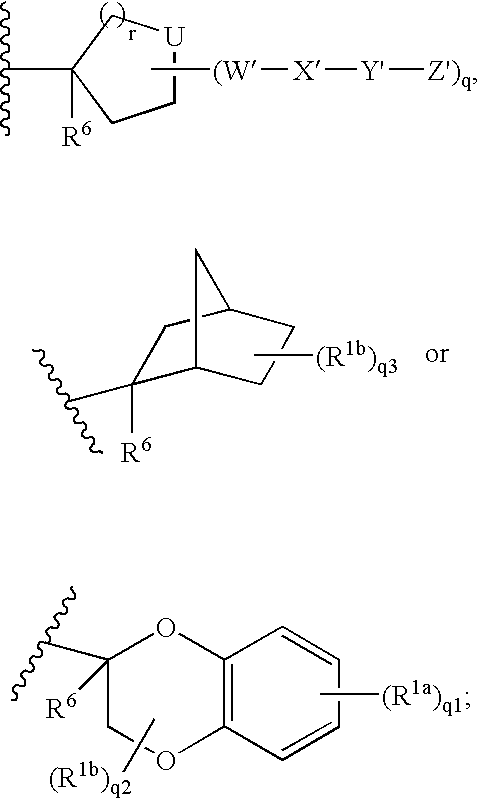
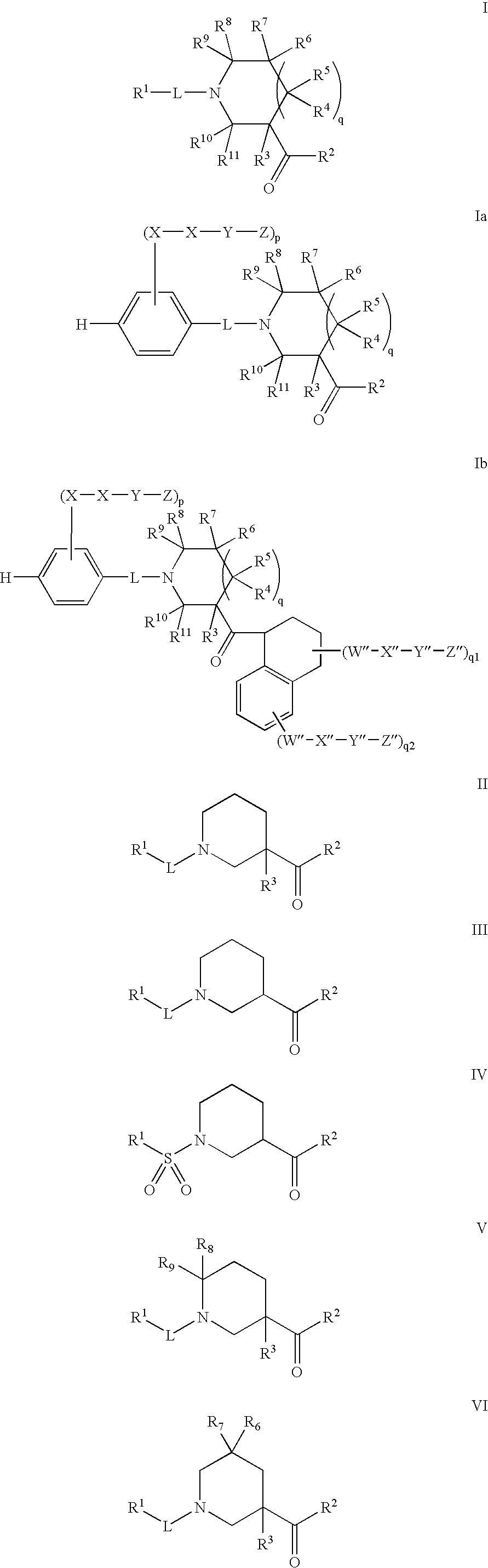
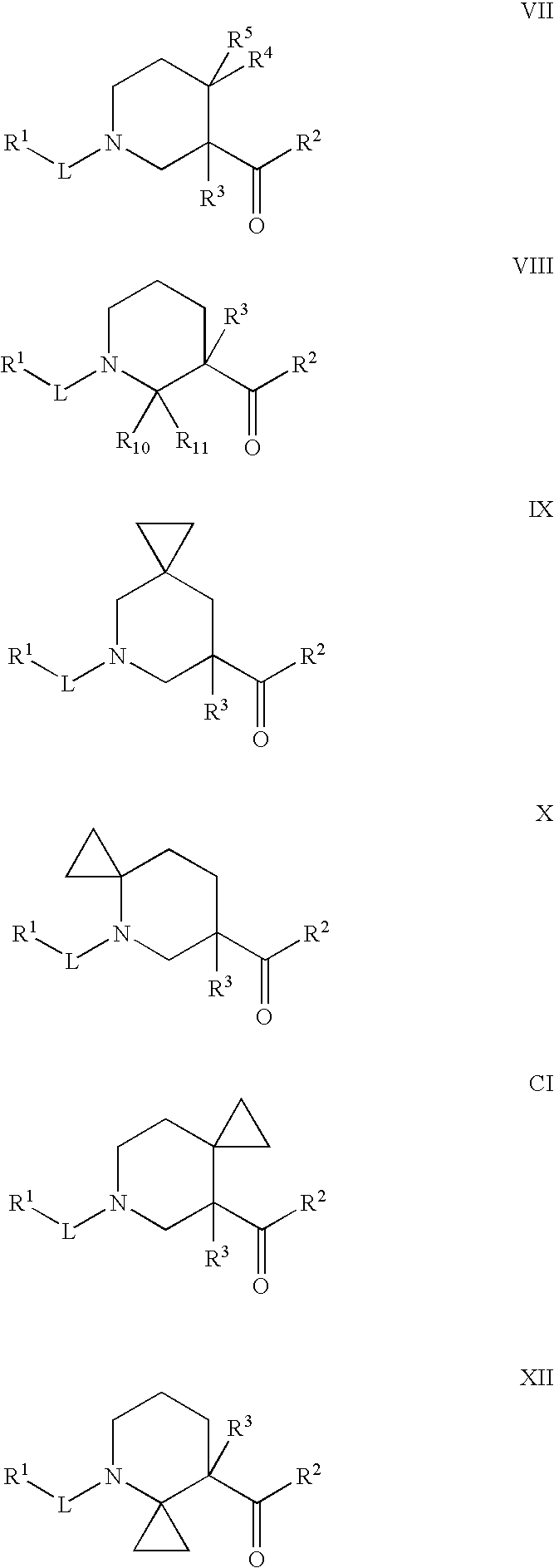
Disclosed are the compounds of the Formula (I) as well as pharmaceutically acceptable salts thereof, which are useful for treating aldosterone-mediated diseases. The processes for preparing compounds of the Formula (I), the use for the therapy and prophylaxis of the abovementioned diseases and for preparing pharmaceuticals for this purpose, and the pharmaceutical compositions which comprise compounds of the formula (I) are disclosed too.
Owner:MERCK SHARP & DOHME LLC
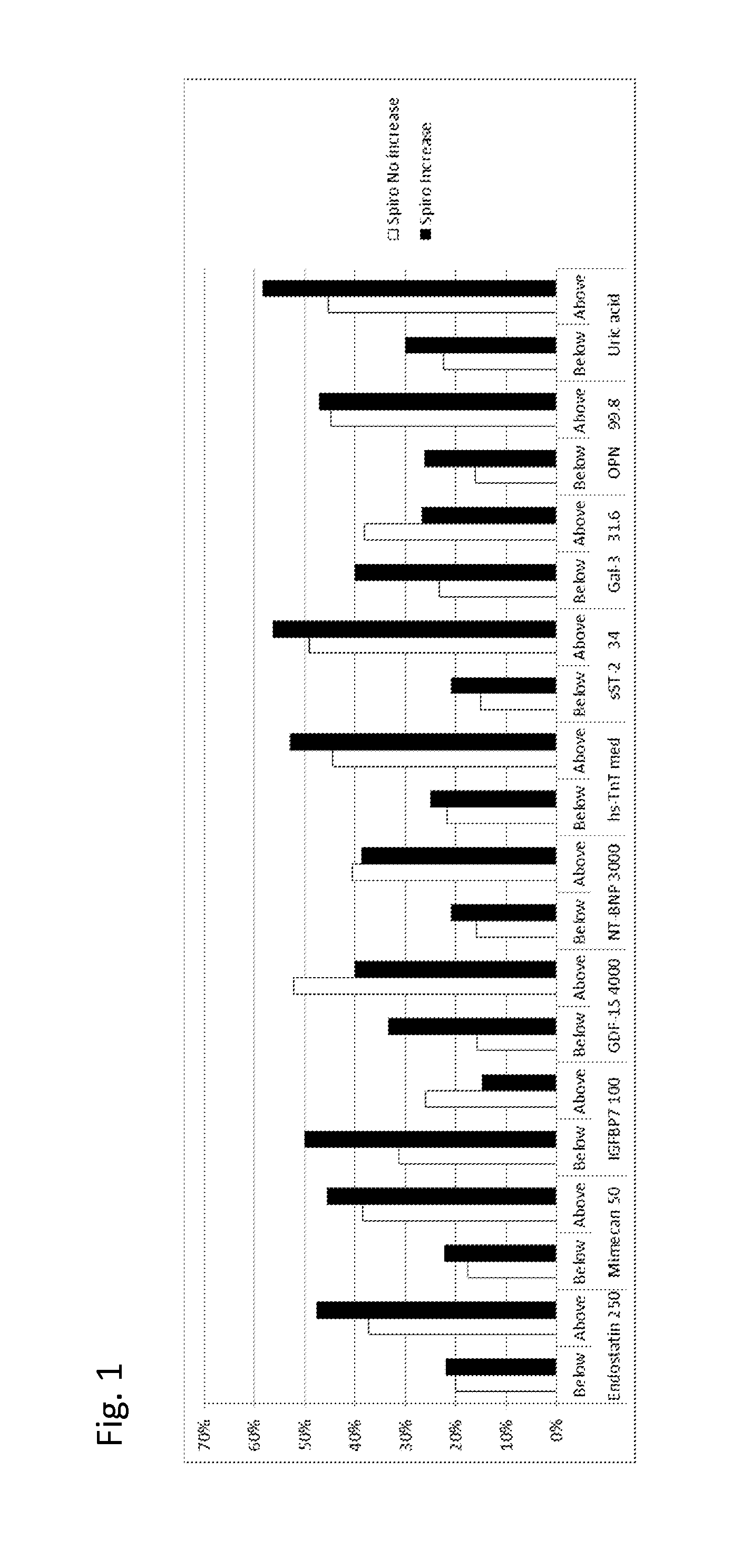
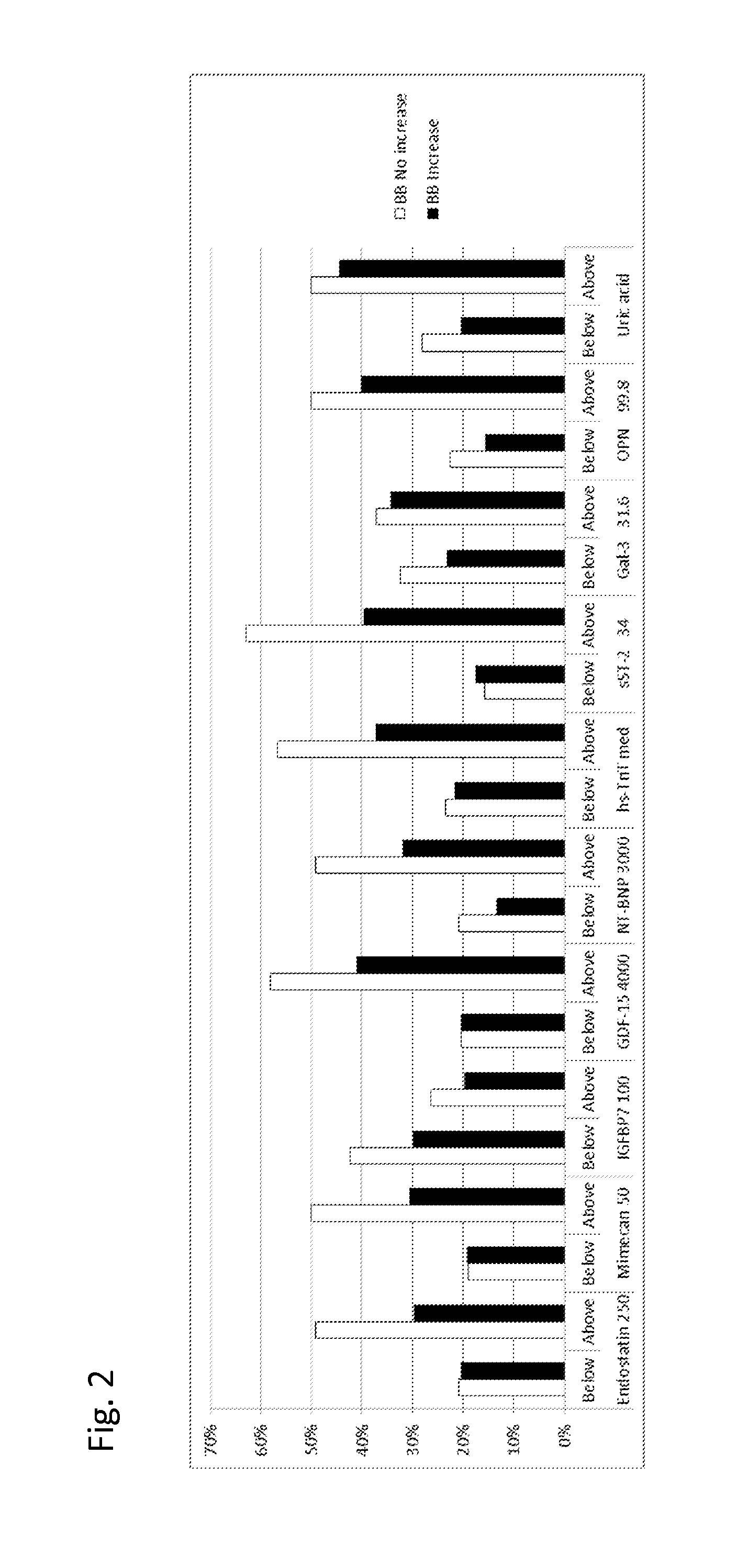
Biomarkers in the selection of therapy of heart failure
InactiveUS20150268251A1Bioreactor/fermenter combinationsBiological substance pretreatmentsBeta blockerBiomarker (petroleum)
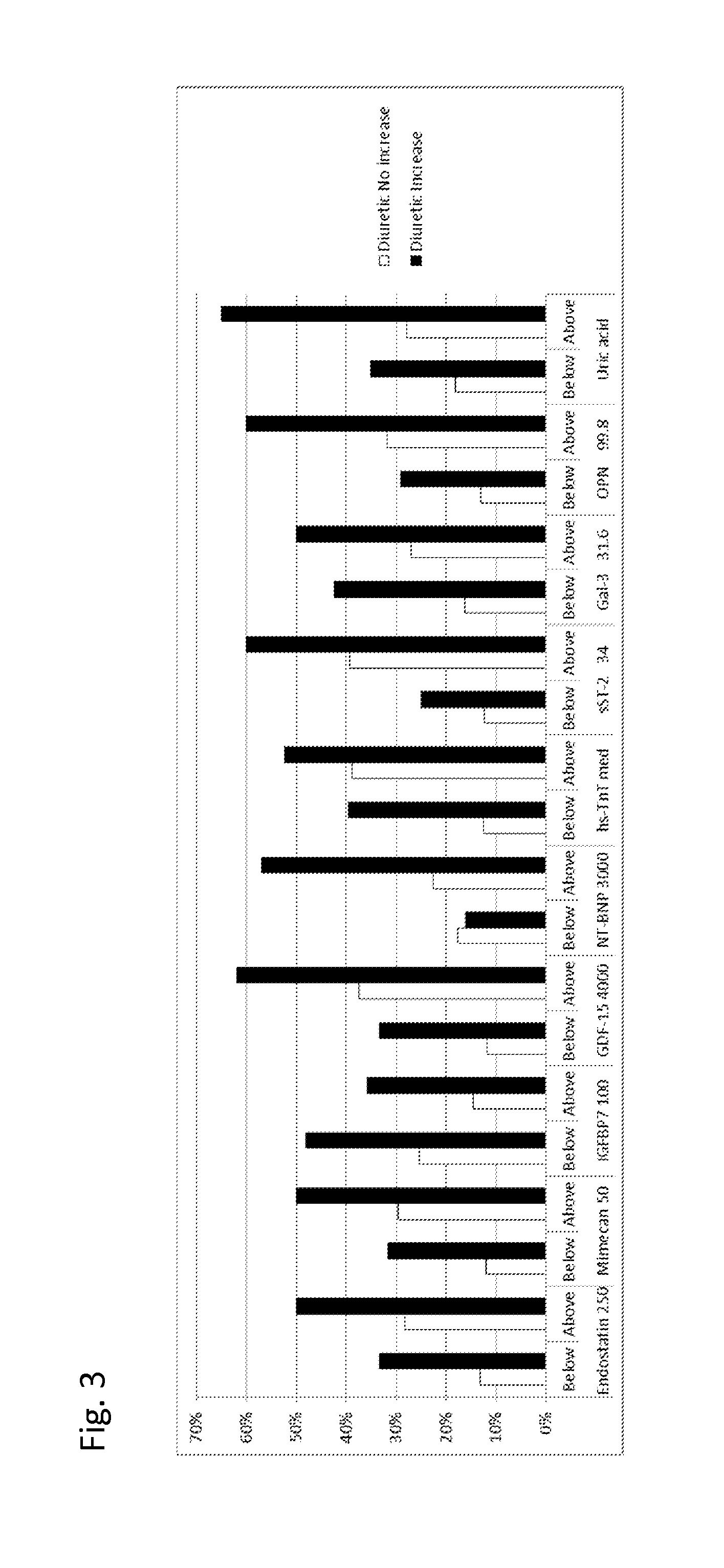
The present invention relates to a method for identifying a subject being eligible to the administration of at least one medicament selected from the group consisting of a beta blocker, an aldosterone antagonist, a diuretic, and an inhibitor of the renin-angiotensin system. The method is based on the determination of the amount of at least one biomarker selected from the group consisting of GDF-15 (Growth Differentiation Factor 15), endostatin, mimecan, IGFBP7 (IGF binding protein 7), a cardiac Troponin, a BNP-type peptide, uric acid, Gal3 (Galectin-3), osteopontin, sST2 (soluble ST2), PlGF, sFlt-1, P1NP, Cystatin C, Prealbumin, and Transferrin in a sample from a subject suffering from heart failure. Further, the method comprises the step of comparing the, thus, determined amount with a reference amount. Further envisaged by the present invention are kits and devices adapted to carry out the method of the present invention. The present invention also relates to a system for identifying a subject being eligible to the administration of at least one medicament as disclosed herein and to reagents and kits used in performing the methods as disclosed herein.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
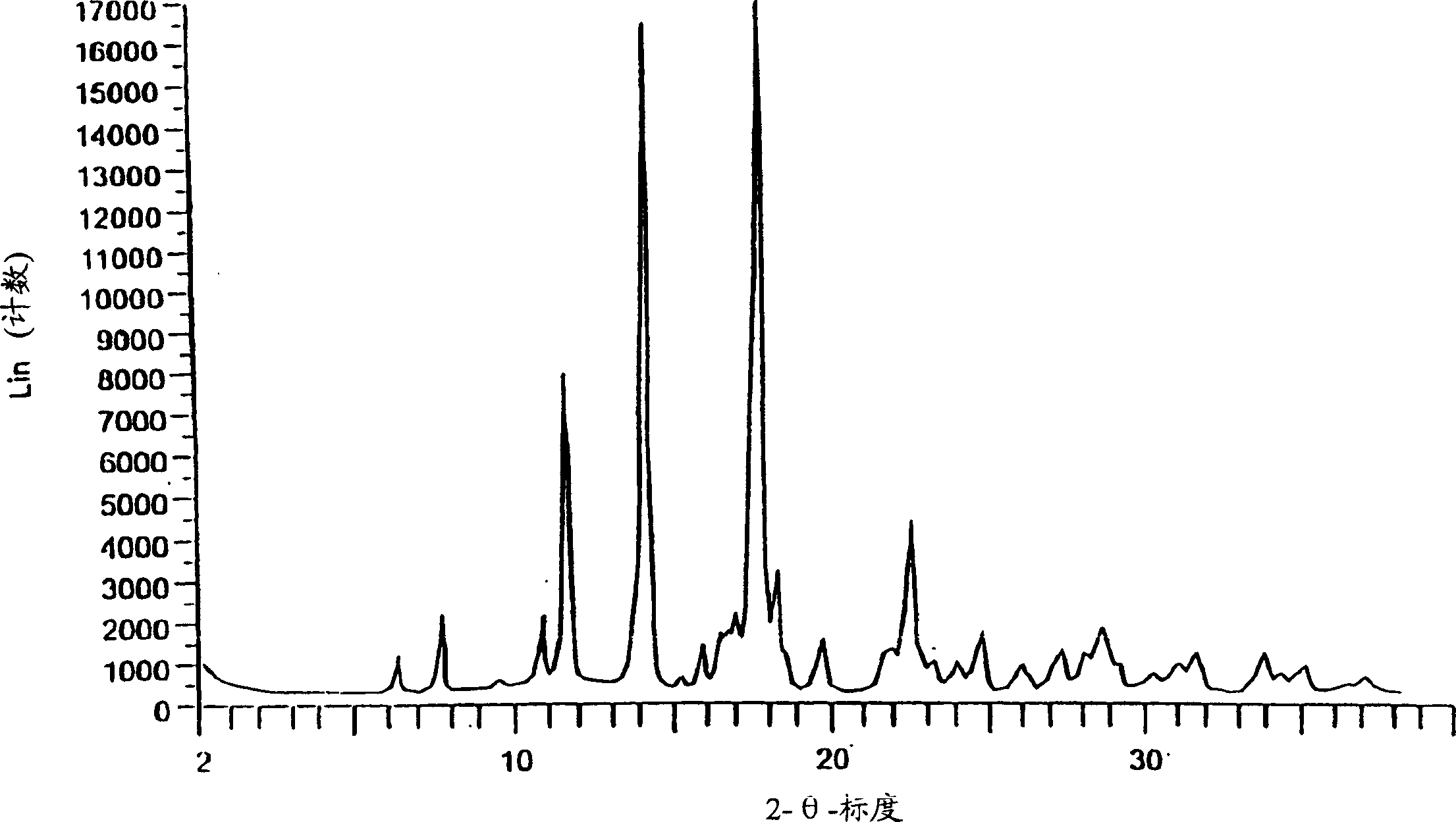
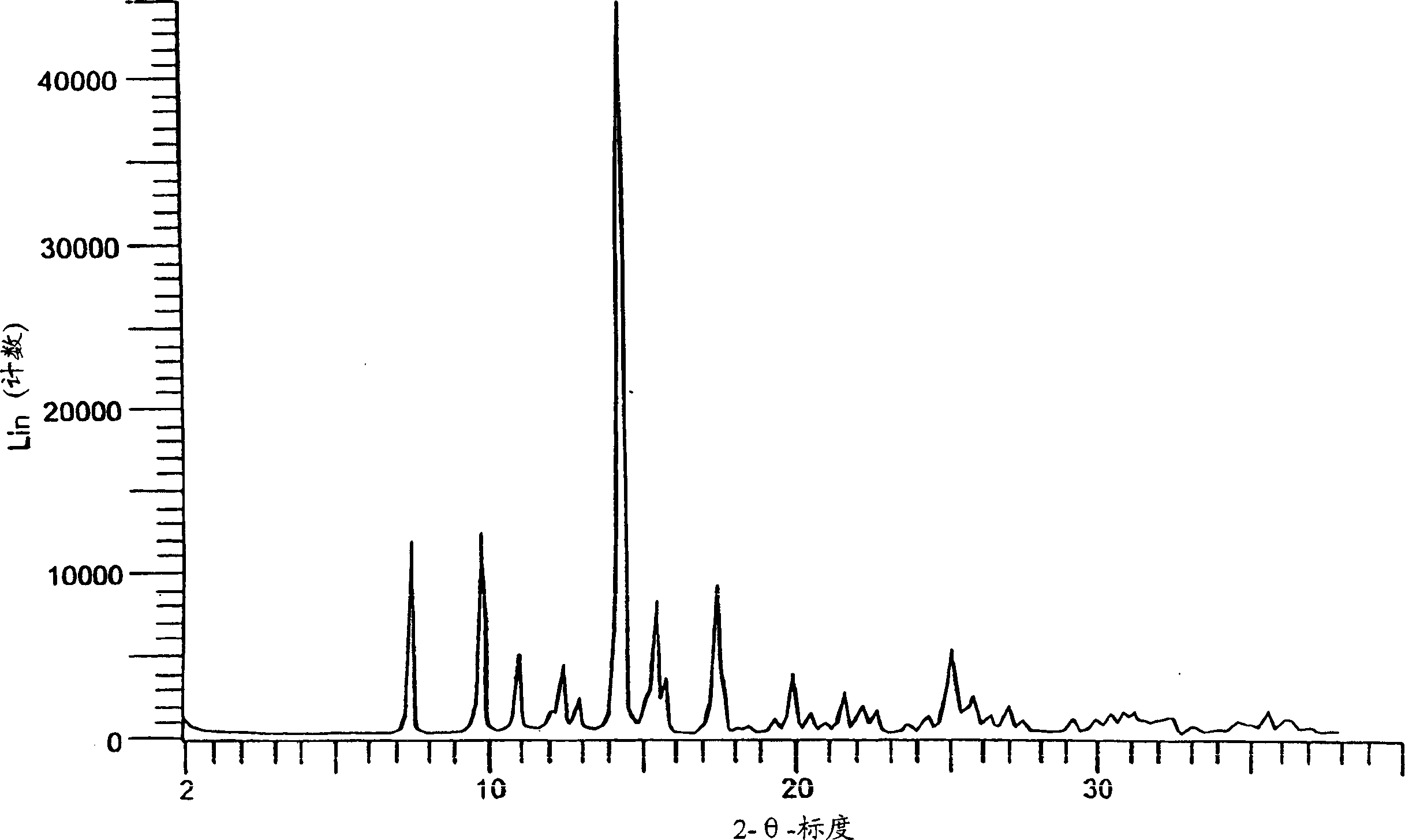
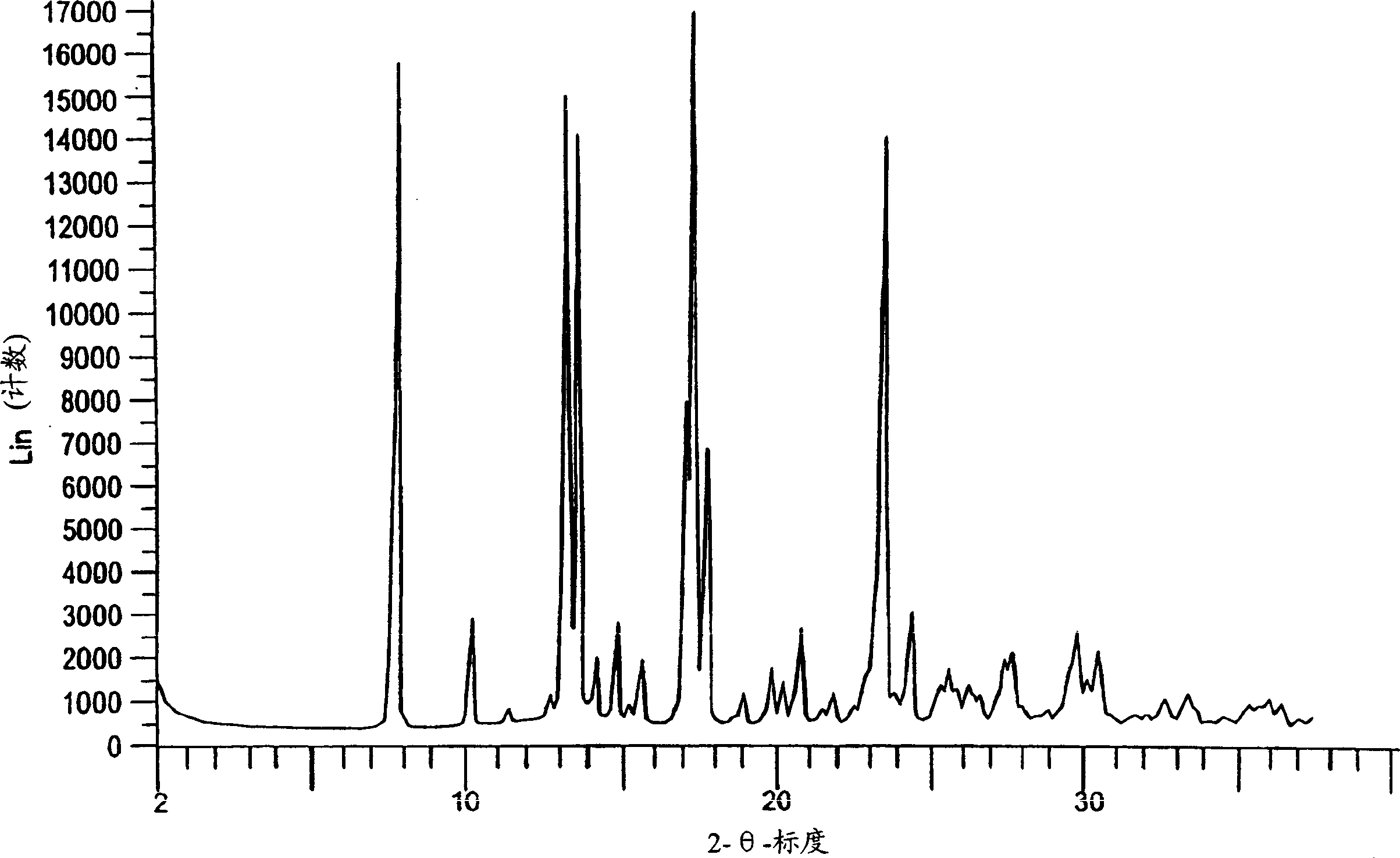
Eplerenone crystalline form
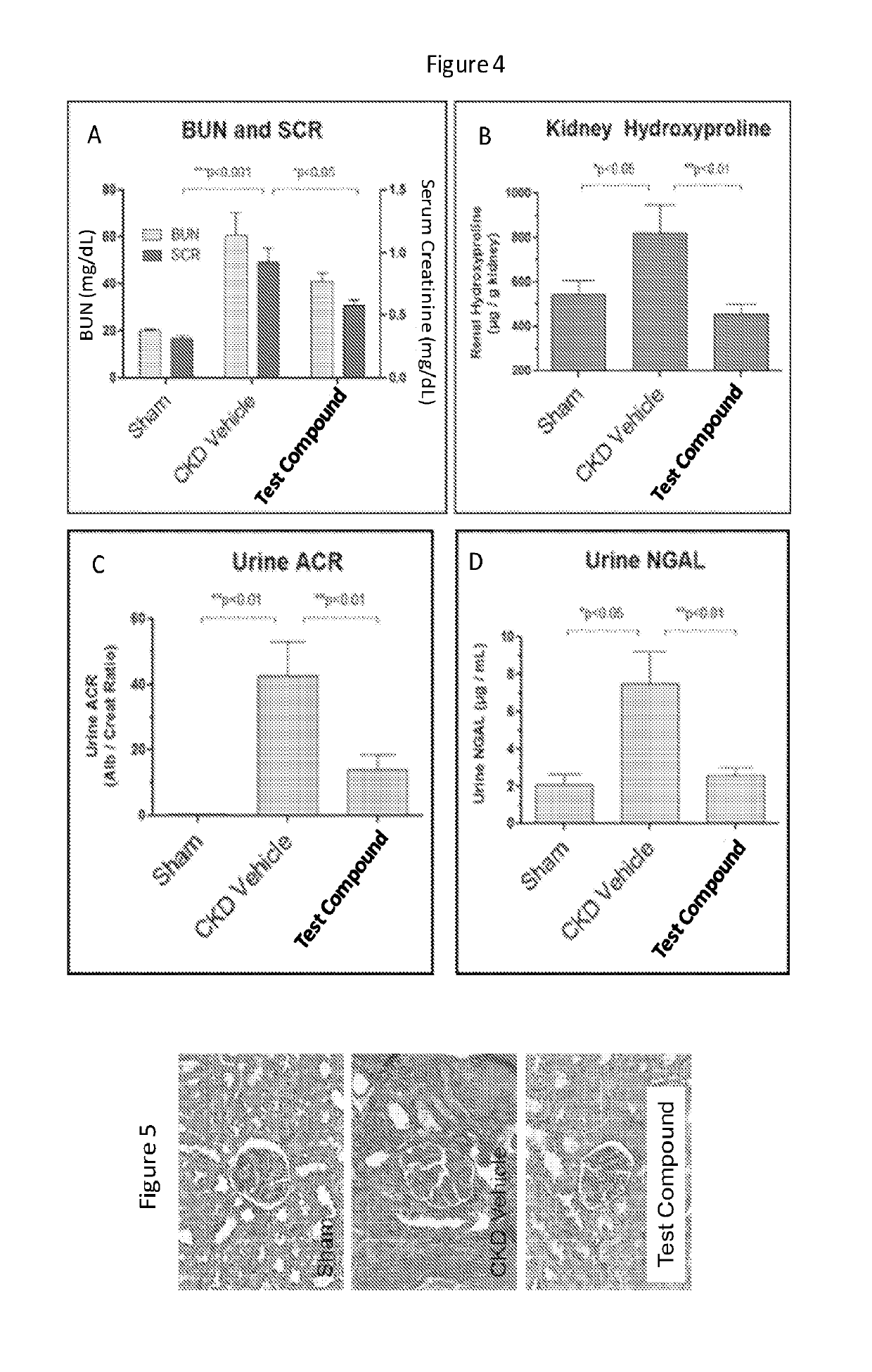
InactiveCN1433427AHigh chemical purityOrganic active ingredientsNervous disorderDiseaseIncreased aldosterone
Owner:PHARMACIA CORP
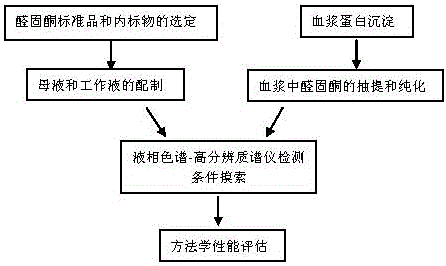
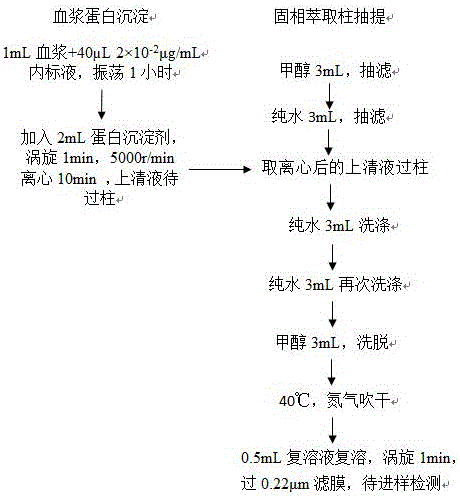
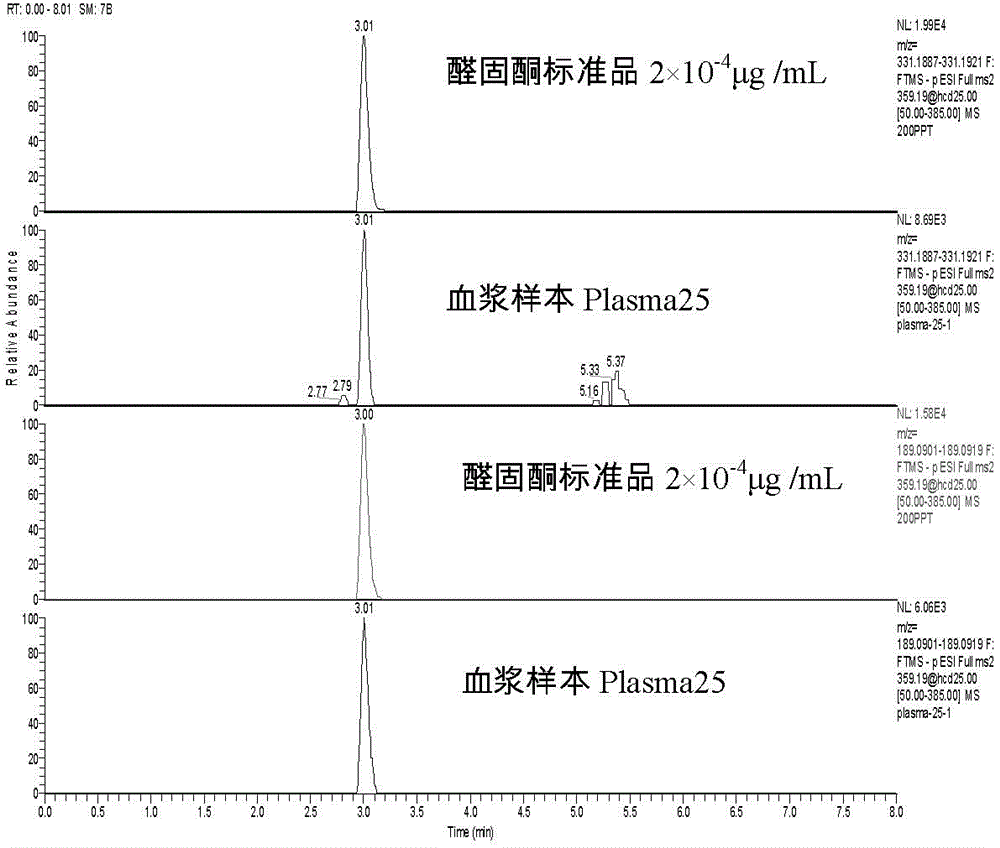
Method for detecting trace amount of aldosterone in plasma by using high-resolution mass spectrometer
The invention relates to a method for detecting a trace amount of aldosterone in plasma by using a high-resolution mass spectrometer. The problem that the trace amount of aldosterone in the plasma cannot be detected by a mass spectrometer can be solved effectively. The method comprises the following steps: preparing a standard working solution and an internal standard working solution; adding a plasma sample in the internal standard working solution, mixing evenly, then adding a protein precipitation solution, carrying out eddying and centrifuging, enabling supernate to flow through an activated HLB solid-phase extracting column, carrying elution with methanol, collecting elution liquid, drying to obtain an extract, redissolving the extract with a methanol aqueous solution, filtering to obtain filtrate, and detecting the filtrate by using a liquid chromatography-mass spectrometer; setting detecting conditions of the liquid chromatography-mass spectrometer, comparing the result, which is measured by a ThermoFisher QE mass spectrometer, of the plasma sample with the standard curve of an aldosterone standard working solution of six working points with known concentration so as to detect the trace amount of aldosterone in plasma. The method is simple, is easy to operate, accurate in detection and good in effect, and has quite high actual application value and remarkable economic benefit and social benefit.
Owner:INSPECTION & QUARANTINE TECH CENT HENAN ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
Pharmaceutical composition containing isosorbide mononitrate for treating high blood pressure
InactiveCN1634022AGood synergistic antihypertensive effectGood curative effectCardiovascular disorderHeterocyclic compound active ingredientsClinical efficacyPulse pressure
The expansion of pulse pressure (PP) is the individual dangerous cardiovascular (CV) factor, by integrating Isosorbide Mononitrate with other hypertension resisting medicaments, good clinic curative effect is obtained, and synergy effect is achieved in terms of reduction of contraction pressure, wherein the combination of calcium ion antagonist, rennin-angiotensins-aldosterone system antagon, blood vessel expanding agent, epinephrine acceptor retarding agent can make the most ideal effect. The invention realizes the functions of reducing contraction pressure, mitigating unwanted reaction caused by hypertension medicament, and effectively increasing survival rate of hypertension patients.
Owner:LUNAN PHARMA GROUP CORPORATION
Sulfonamide compound, and preparation method and application thereof
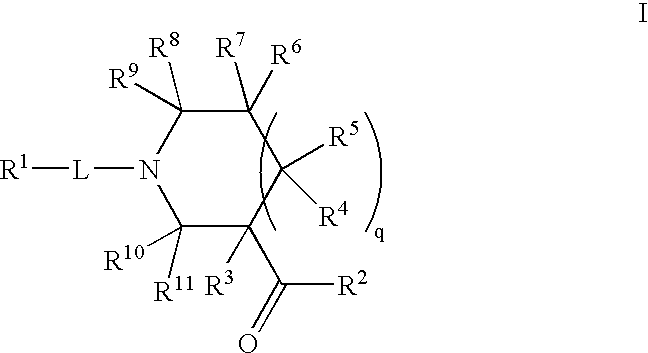
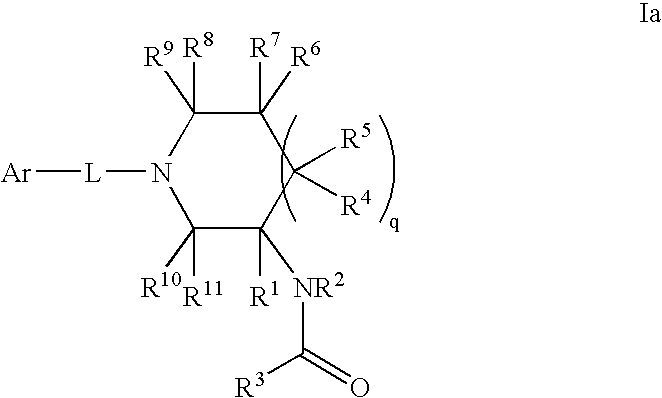
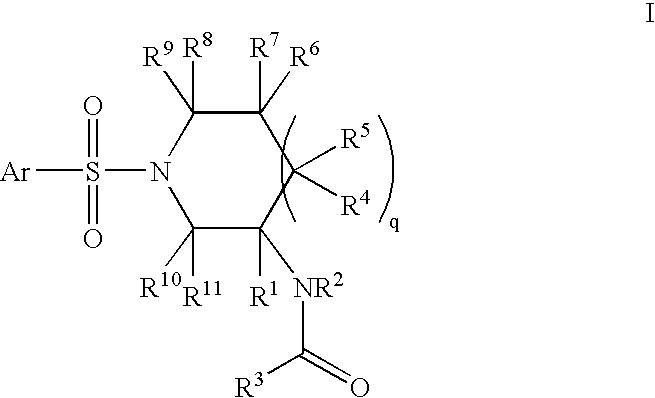
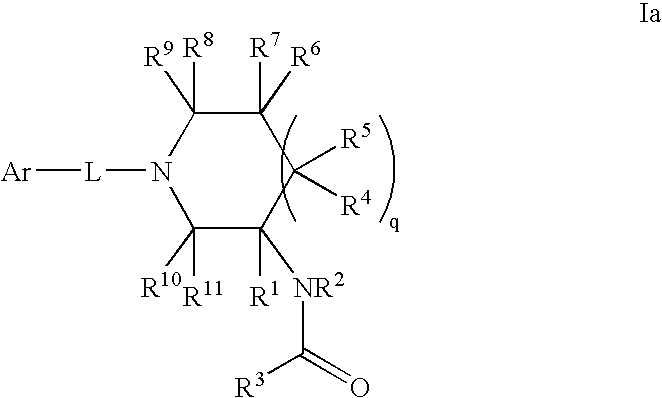
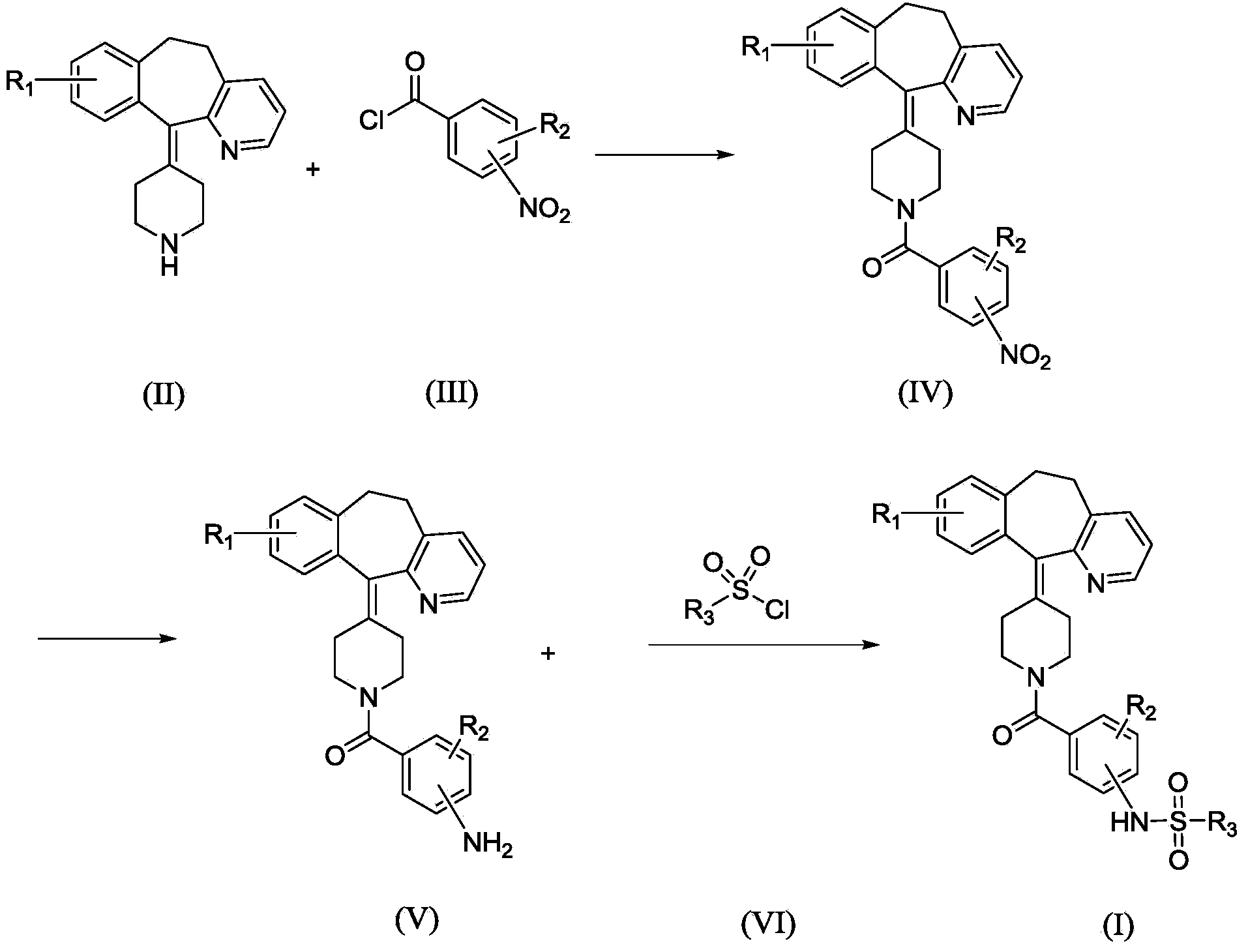
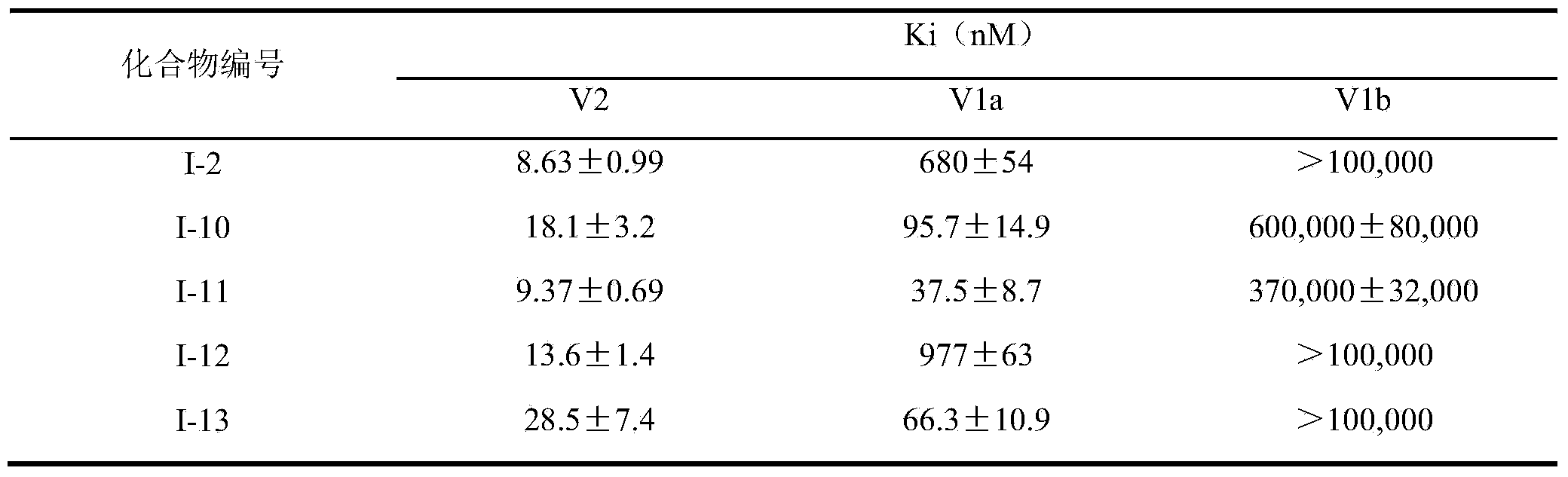
InactiveCN103965162AHas receptor antagonistic effectReceptor antagonismOrganic active ingredientsNervous disorderArginineCombinatorial chemistry
The invention relates to a sulfonamide compound, and a preparation method and application thereof. Particularly, the invention relates to the sulfonamide compound with a structure shown in the formula I, an acceptable salt of the sulfonamide compound on the pharmacy, and a preparation method of the sulfonamide compound. A medicine composition uses the sulfonamide compound with the structure shown in the formula I, and the acceptable salt of the sulfonamide compound on the pharmacy as active ingredients to be used for preventing or treating diseases related to arginine vasopressin V1a receptors, arginine vasopressin V1b receptors, arginine vasopressin V2 receptors, a sympathetic nervous system or a renin-angiotensin-aldosterone system.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Novel aldosterone antagonists and uses thereof
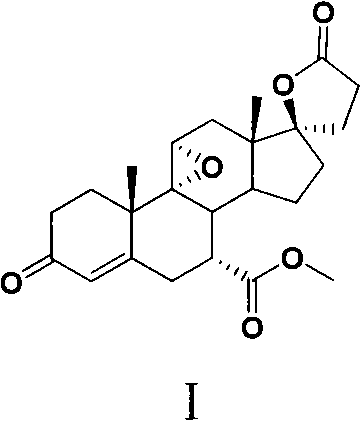
InactiveUS20070066580A1Inhibiting aldosterone receptor activityOrganic active ingredientsLactone steroidsIncreased aldosteroneTreatment use
Owner:RECORDATIE IRELAND LTD
(-)-Hydroxycitric acid for the modulation of angiotensin-converting enzyme
InactiveUS20060025482A1Preventing and treating and ameliorating conditionStabilize and improve healthBiocideAnimal repellantsDiagnostic Radiology ModalityAdjuvant
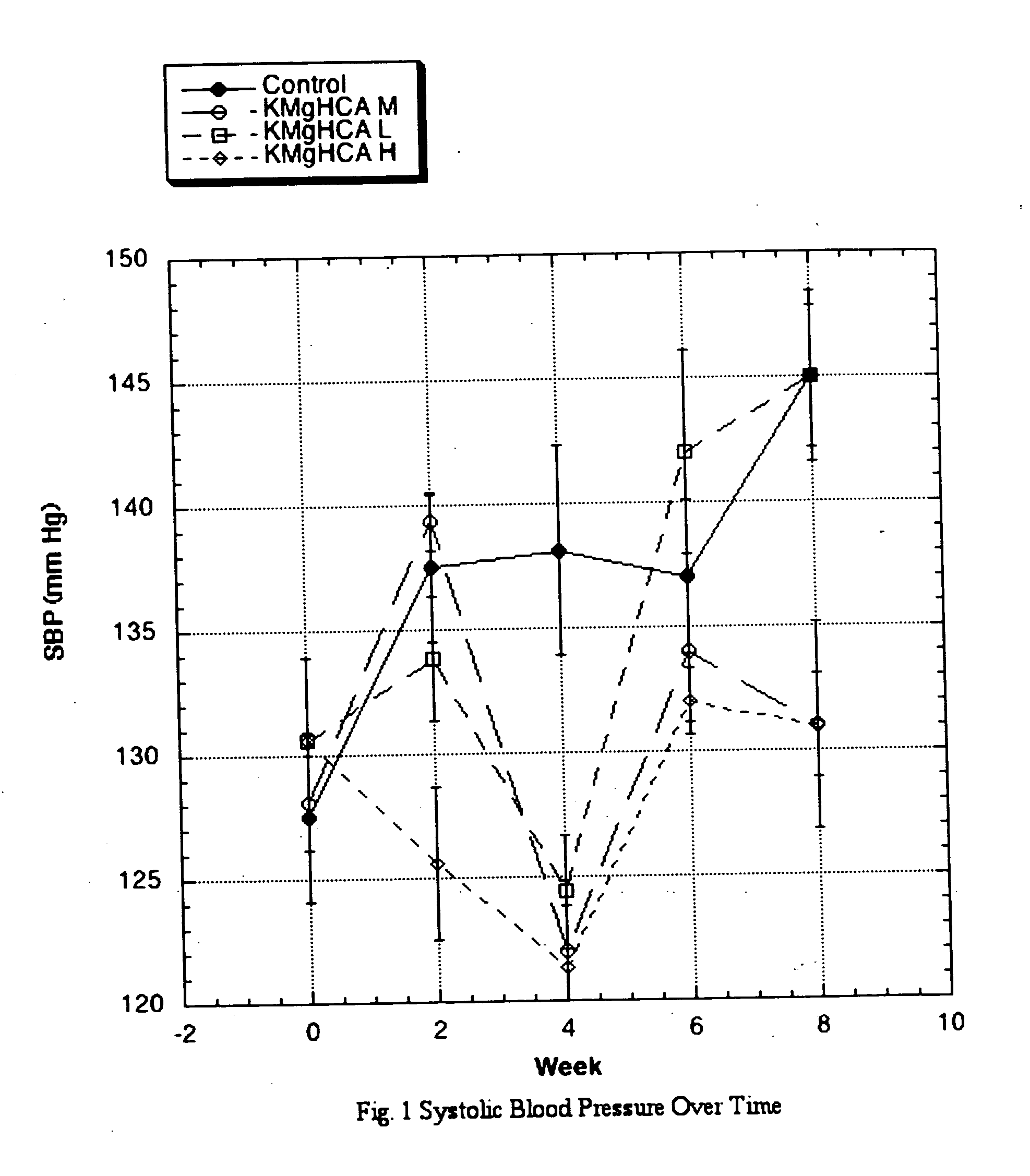
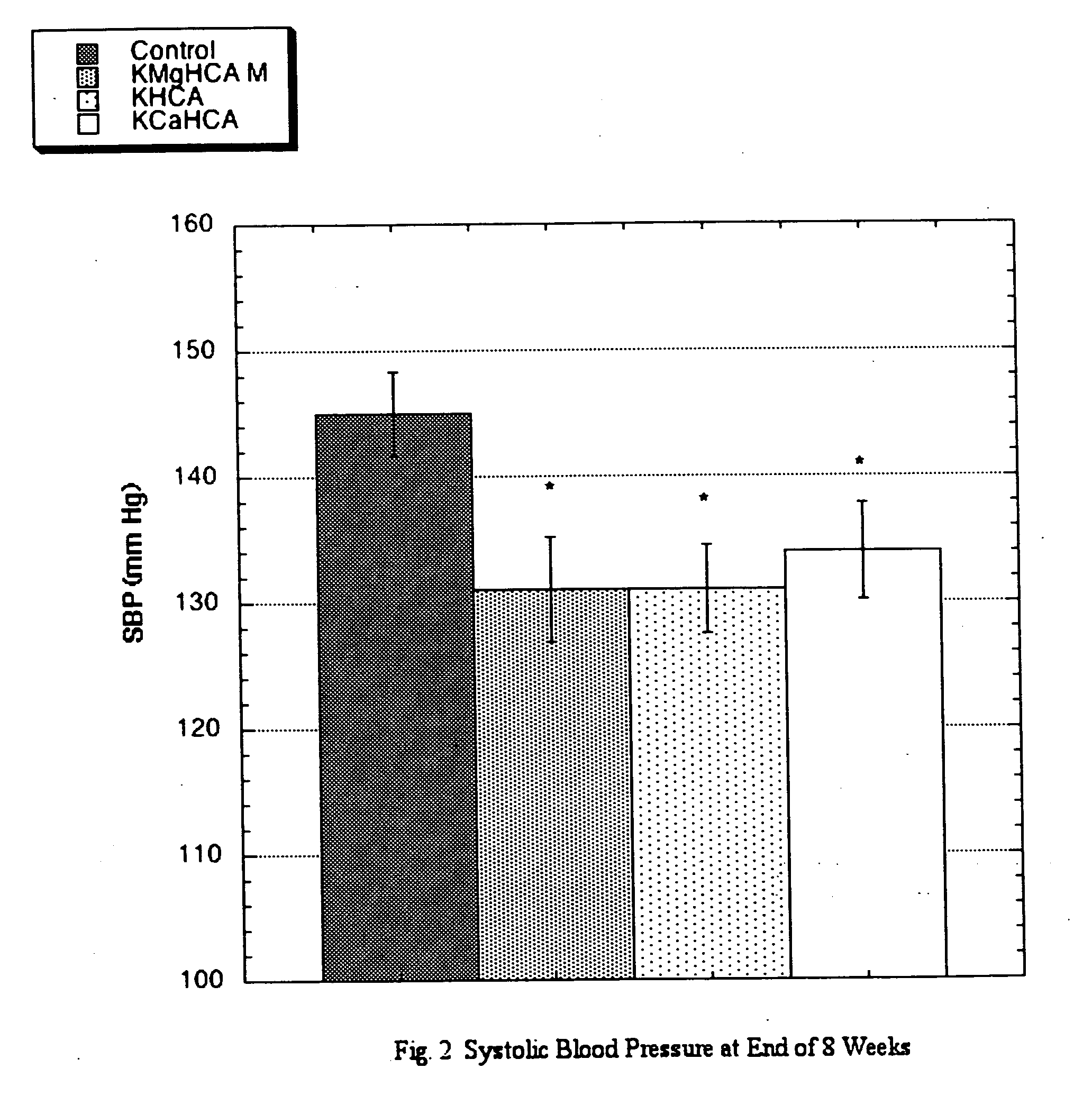
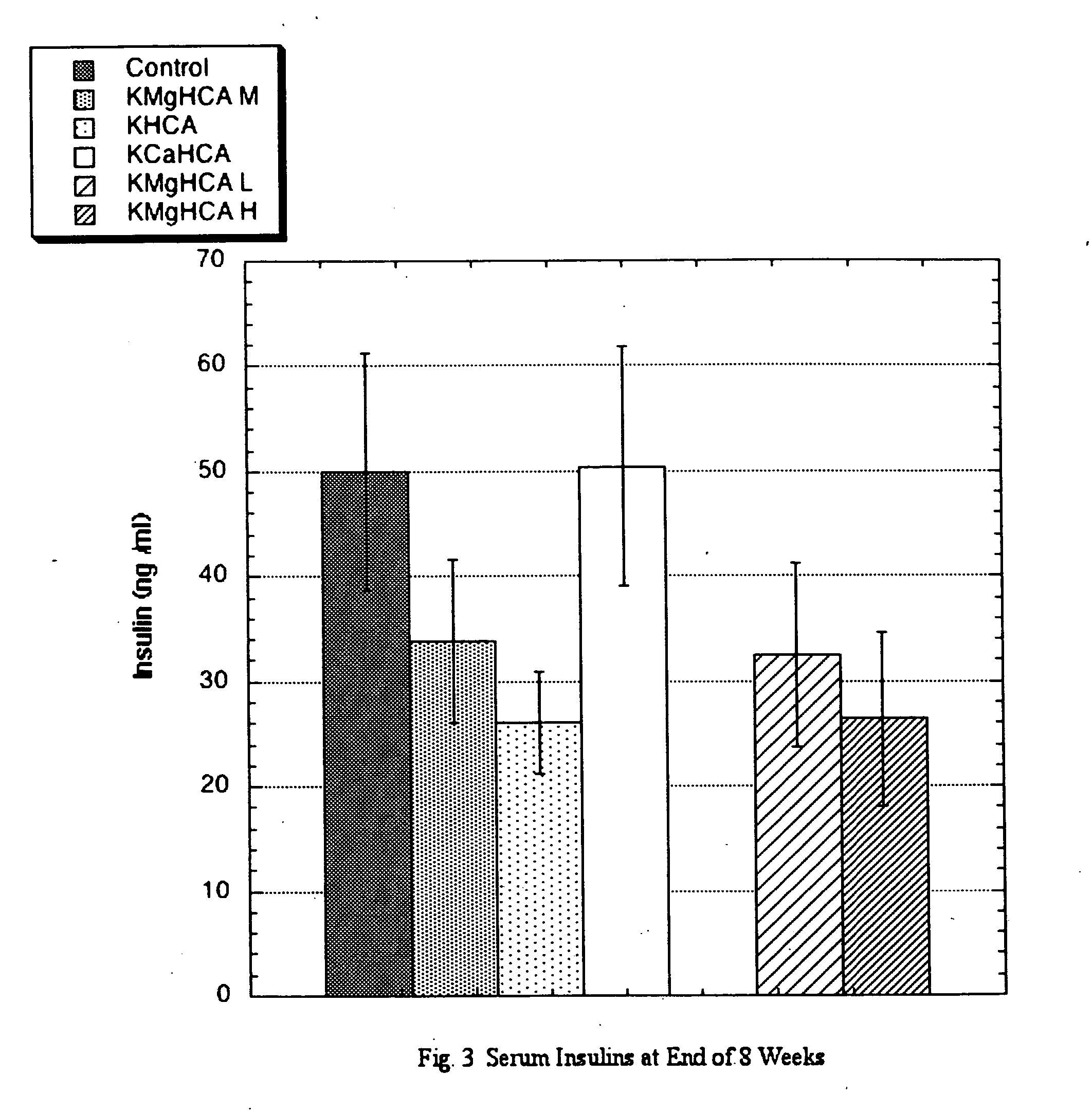
The invention teaches that supplementation with (−)-hydroxycitrate constitutes a novel means of modulating the angiotensin-converting enzyme (ACE) / renin-angiotensin-aldosterone system and is useful for preventing, treating and ameliorating conditions involving the angiotensin-converting enzyme (ACE) / renin-angiotensin-aldosterone system. The discovery that HCA has angiotensin-converting enzyme (ACE) / renin-angiotensin-aldosterone system-moderating effects allows for the creation of novel and more efficacious approaches to preventing and ameliorating conditions that arise from excessive ACE activity. These include cardiovascular diseases in general, heart failure, ventricular remodeling, ejection fraction issues, atrial fibrillation, and a wide variety of renal conditions. Other health conditions discovered to be influenced by the angiotensin-converting enzyme (ACE) / renin-angiotensin-aldosterone system would similarly be expected to be influenced. It is yet a further advantage of the present invention to provide a means—one that is accompanied by few or no side effects—of maintaining such improved status without resort to special diets. Furthermore, this discovery makes possible the development of adjuvant modalities that can be used to improve the results realized with other treatment compounds while at the same time reducing the side effects normally found with such drugs. HCA delivered in the form of its potassium salt is efficacious at a daily dosage (bid or tid) of between 750 mg and 10 grams, preferably at a dosage of between 3 and 6 grams for most individuals. A daily dosage above 10 grams might prove desirable under some circumstances, such as with extremely large or resistant individuals, but this level of intake is not deemed necessary under normal conditions.
Owner:CLOUATRE DALLAS L
Combination therapy for treating heart disease
InactiveUS20060135497A1BiocideOrganic active ingredientsCombined Modality TherapyCompound (substance)
A combination therapy and co-therapy method for administering therapeutic doses of an aldosterone antagonist agent and a metolazone-related compound to a subject in need of treatment for hypertension, congestive heart failure, and chronic kidney disease are provided. A pharmaceutical composition comprising these therapeutic agents is also provided.
Owner:GUPTA AJAY
New method for preparing eplerenone
InactiveCN102617697ASources are cheap and readily availableControllableSteroidsMicrobial transformationDouble bond
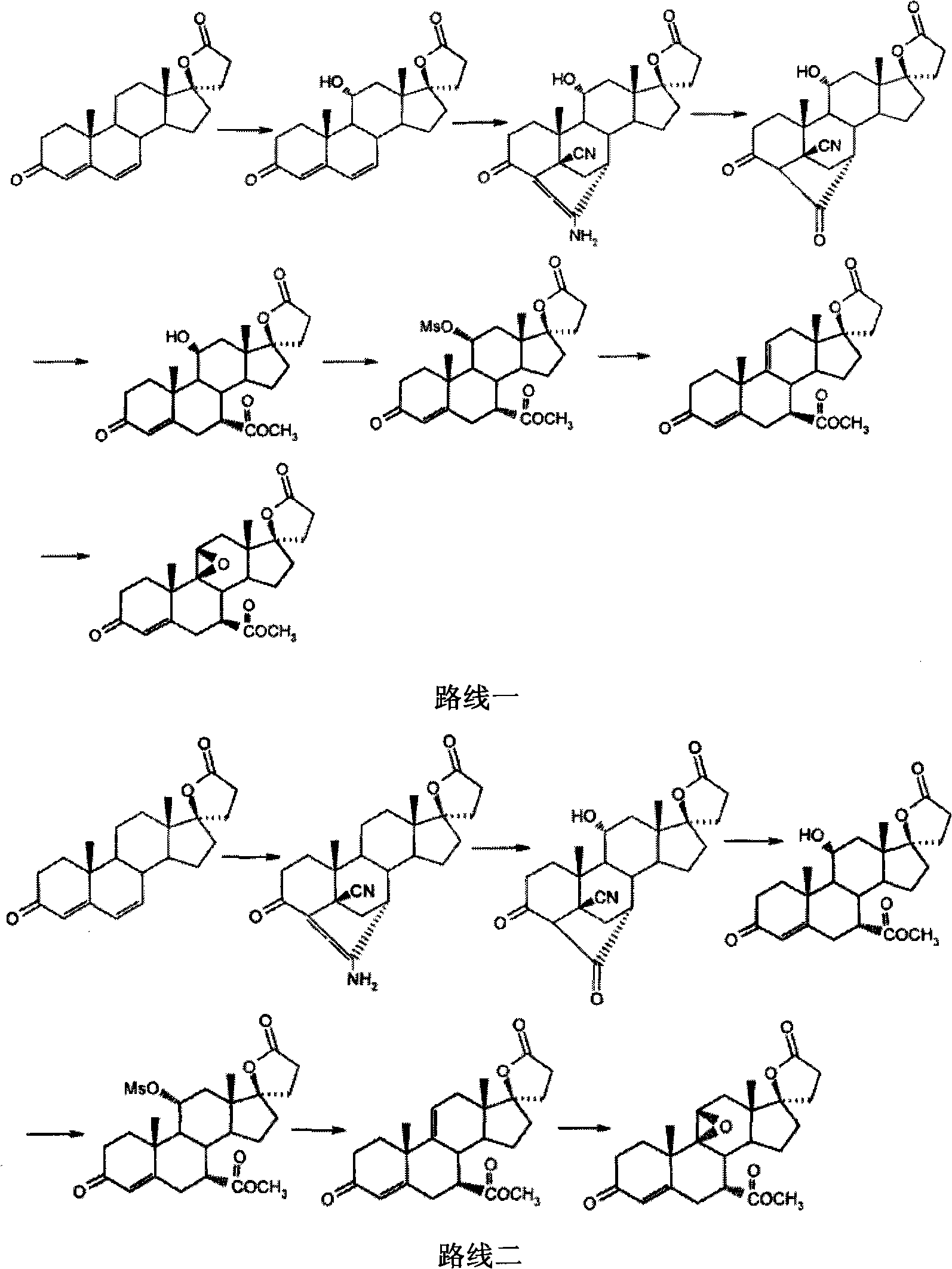
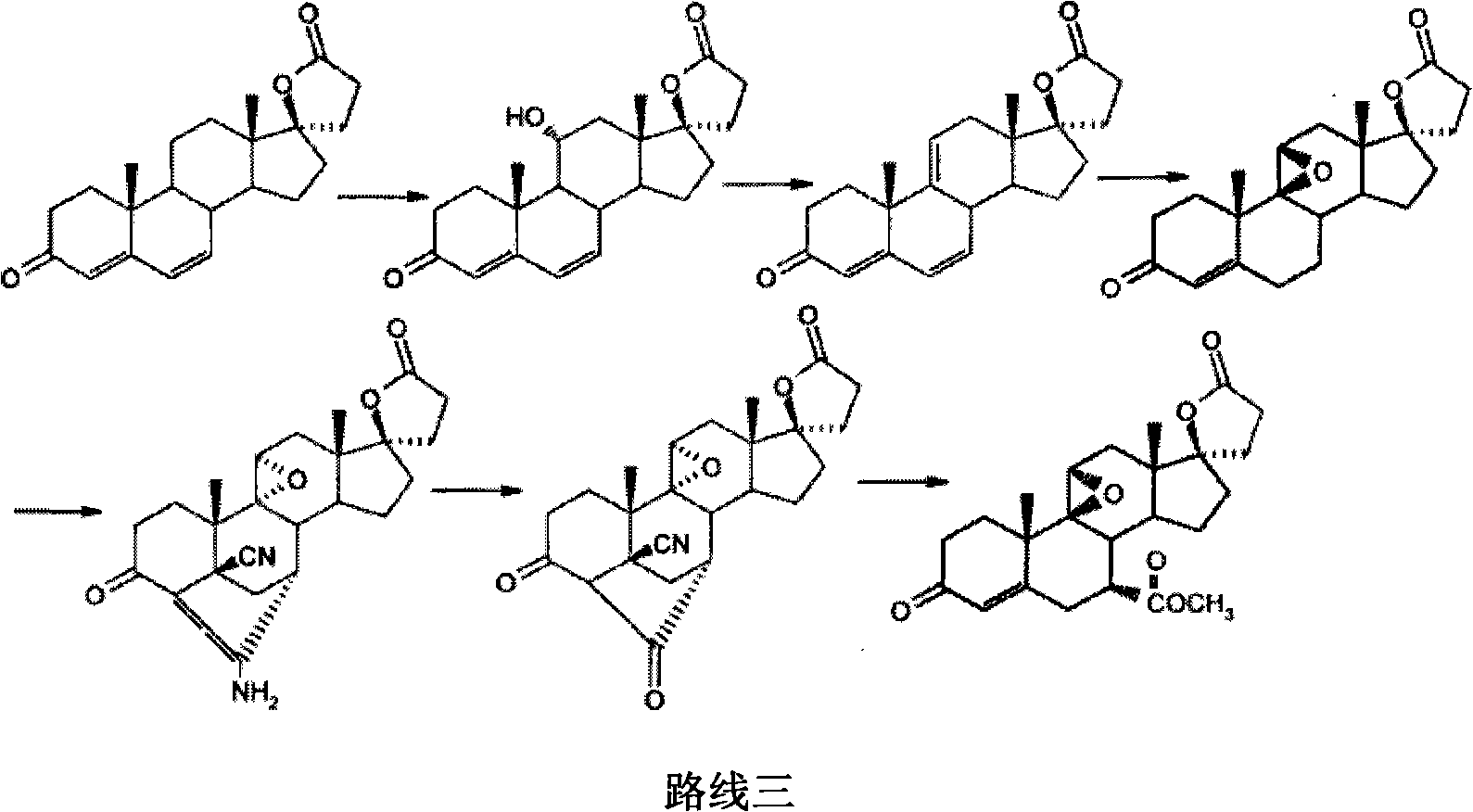
The invention provides a method for preparing a novel steroid aldosterone acceptor antagonist eplerenone. The method comprises the following steps: producing 9alpha-hydroxyandrostenedione (9alpha-OH-AD) through carrying out microbial conversion on a raw material androstenedione (4-AD), carrying out 17-keto epoxidation, condensation and carboxyl removal, introducing double bonds to 9(11) and 6(7), and carrying out 6(7) furanization, oxidation and esterification, and carrying out 9, 11 double bond epoxidation to generate eplerenone. The method which utilizes the cheap and easily available raw material and adopts a semisynthetic technology of microbial fermentation has the advantages of environmental protection, easy control of the operation, high yield, and low cost, and is suitable for industrialized production.
Owner:SHANGHAI SCIENPHARM CO LTD
Methods and agents for treating disease
Owner:ANGION BIOMEDICA CORP
Combination
ActiveUS10172914B2More efficaciousAddress bad outcomesOrganic active ingredientsCyclic peptide ingredientsCardioprotectionReperfusion injury
The present invention provides a combination comprising at least two of the following: (i) an insulin modulator, (ii) an immunosuppressive agent and (iii) an aldosterone antagonist, for instance a combination comprising at least two of (i) exenatide, or a functional derivative or analog thereof, or a pharmaceutically acceptable salt thereof; (ii) cyclosporine, or a functional derivative or analog thereof, or a pharmaceutically acceptable salt thereof; and (iii) potassium canrenoate, or a functional derivative or analog thereof. Said combinations are suitable for cardioprotection and for treating and / or preventing ischemia and / or reperfusion injury. Further aspects of the invention relate to pharmaceutical products and pharmaceutical compositions comprising said combinations according to the invention, and methods of treatment using the same.
Owner:GENESIS PHARMA SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com