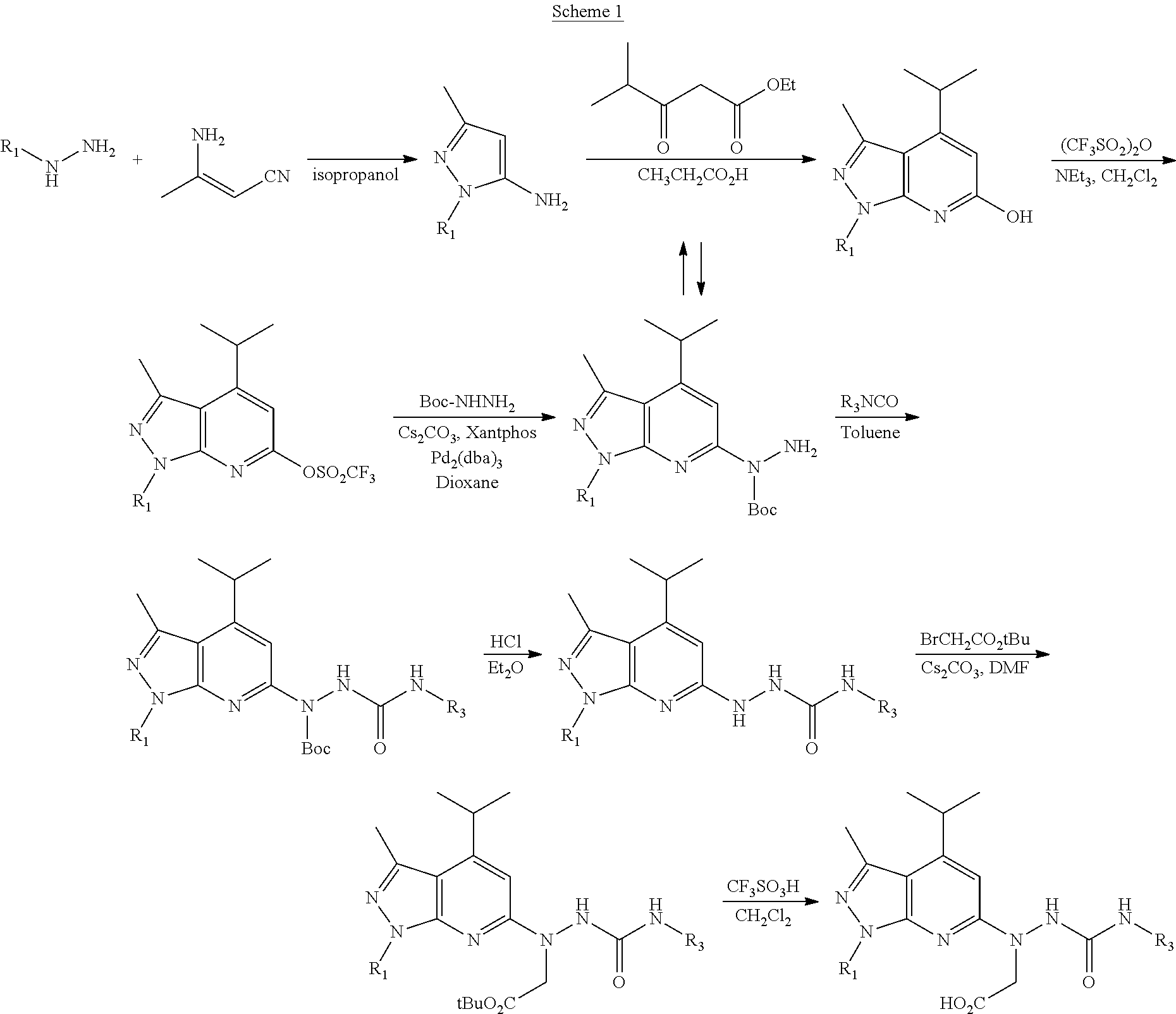
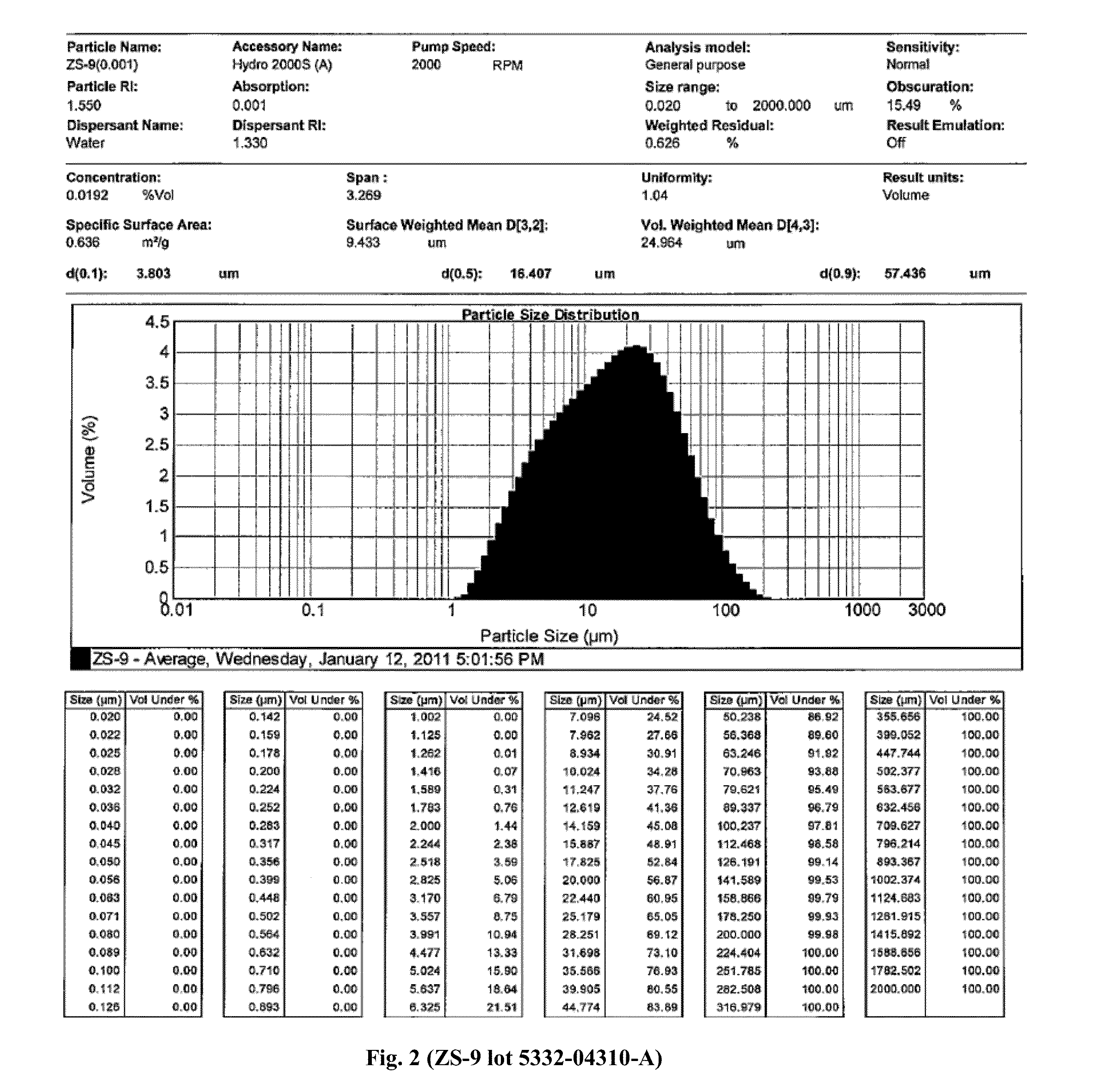
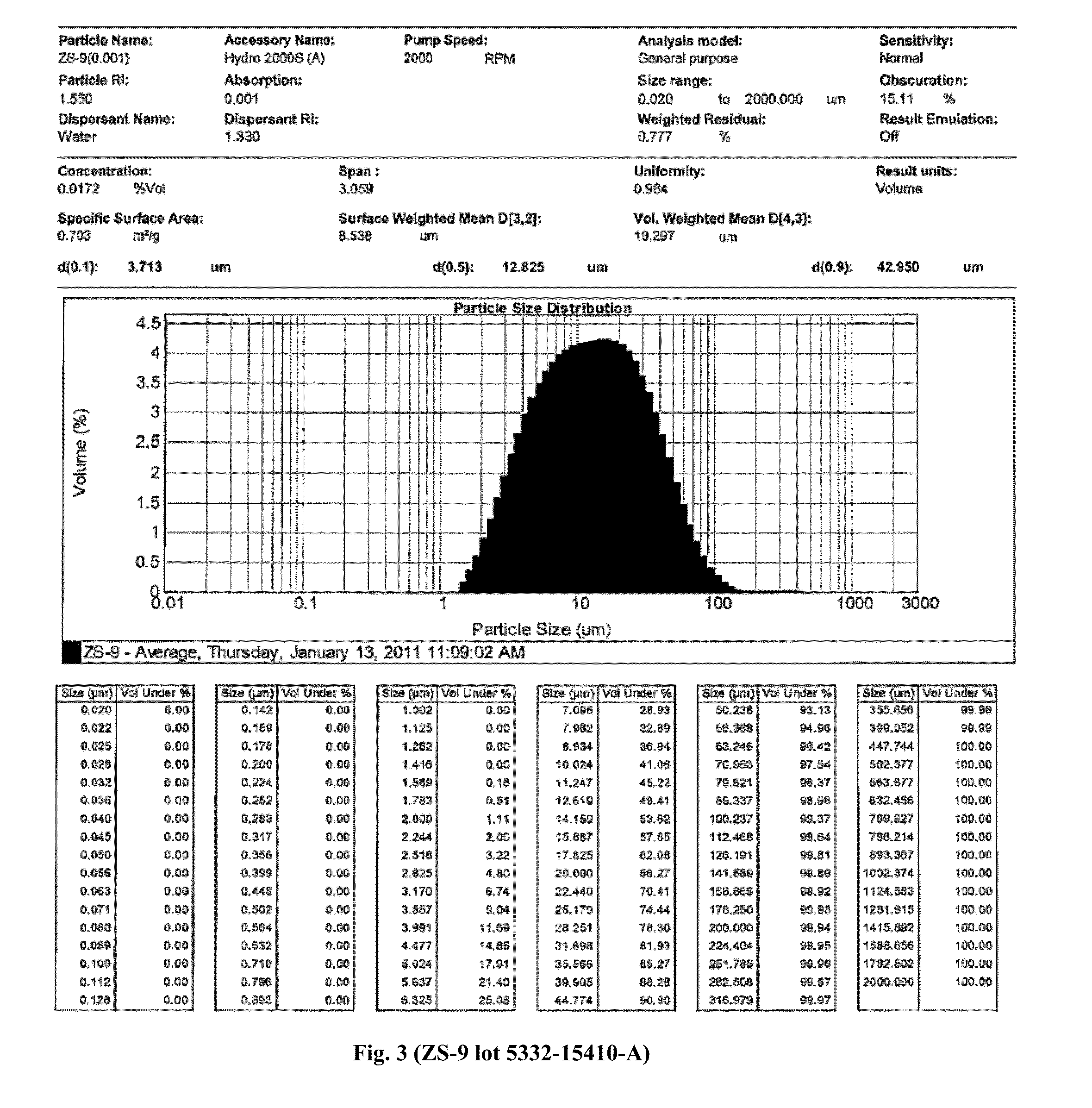
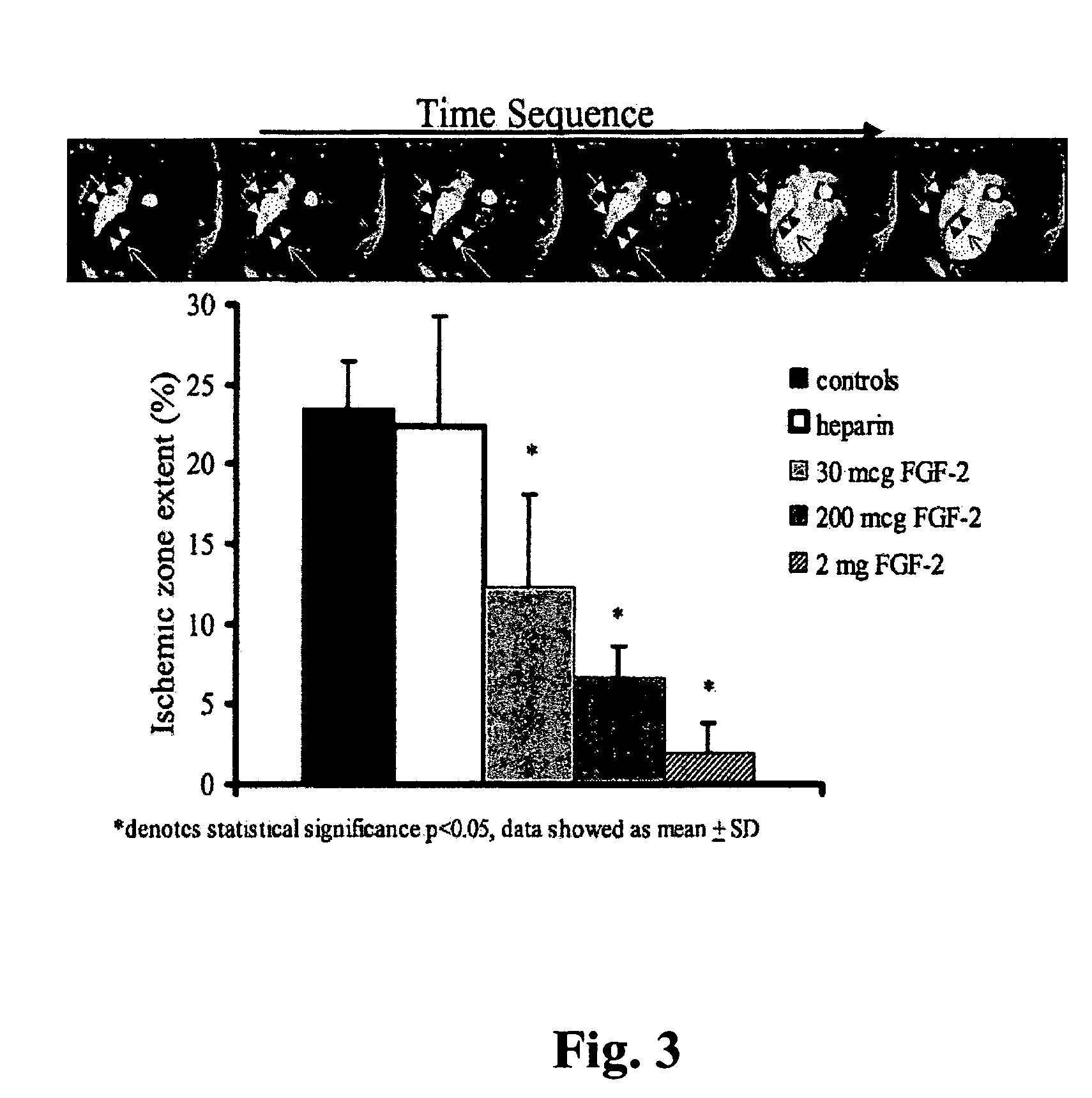
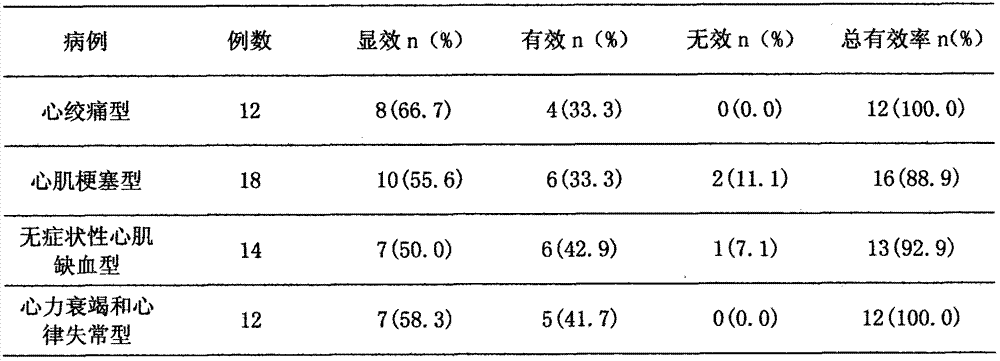
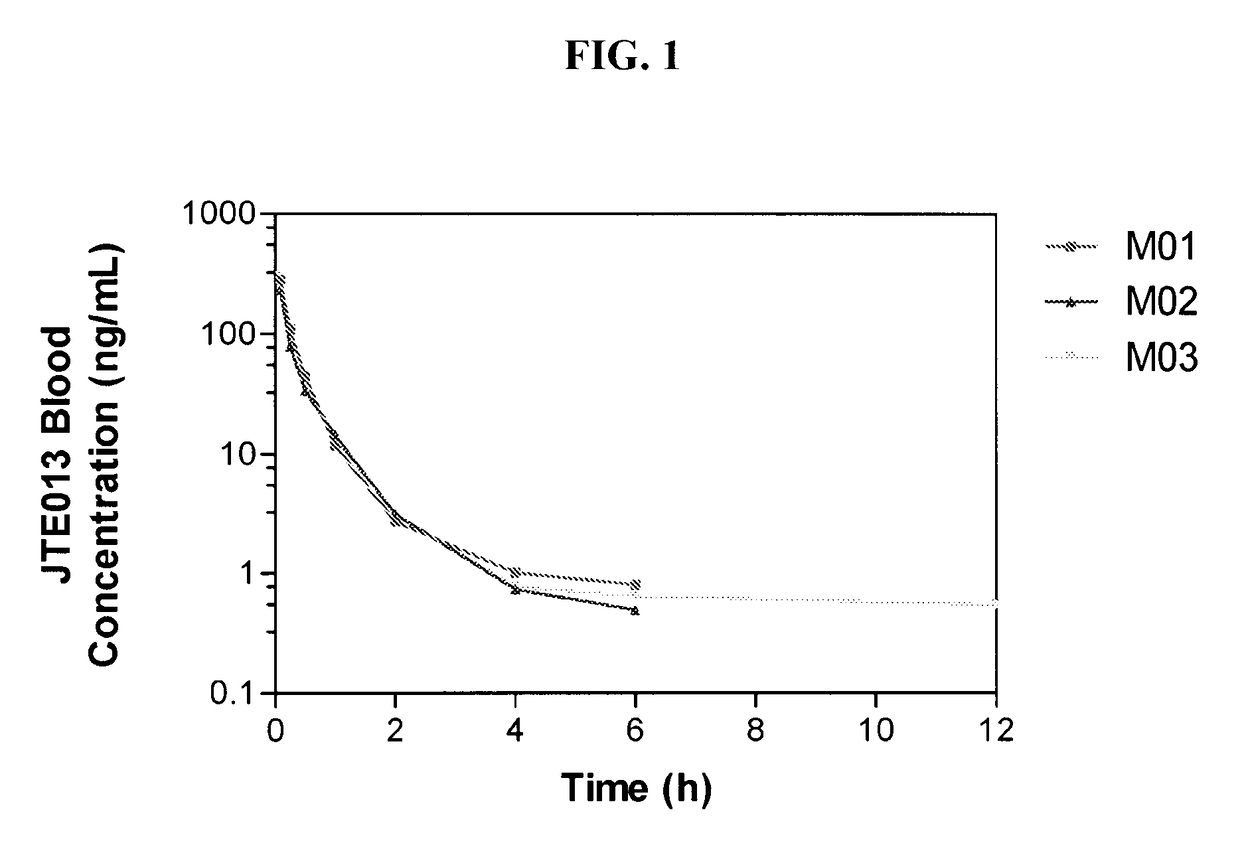
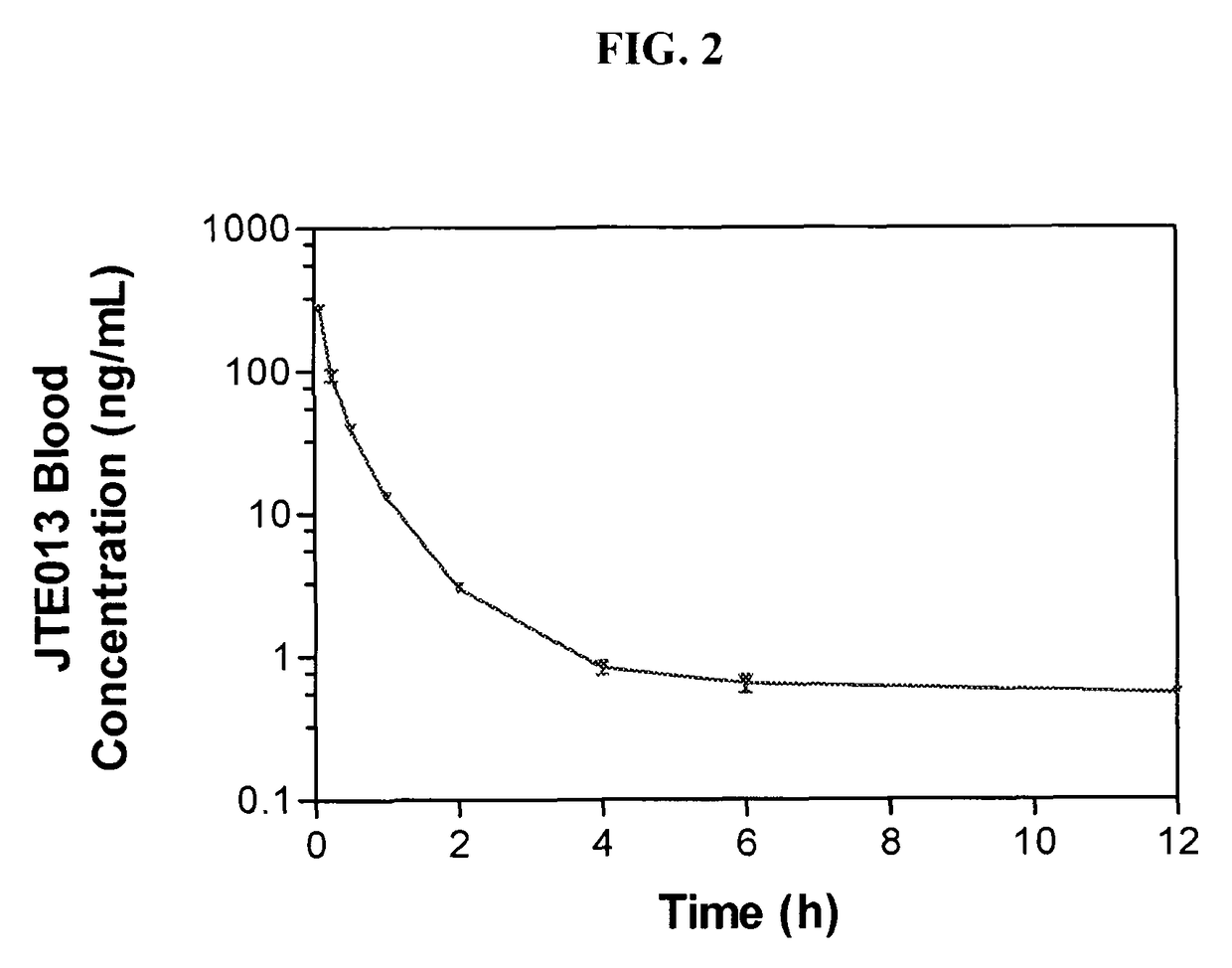
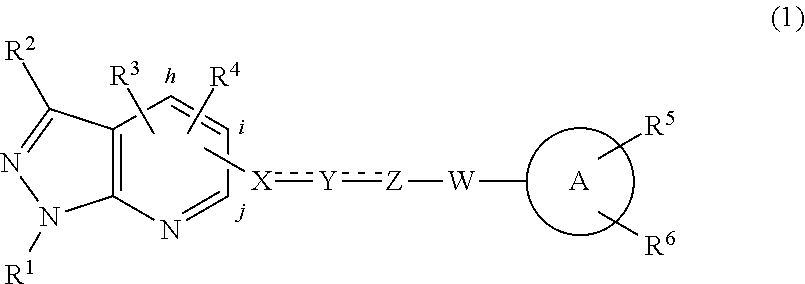Patents
Literature
44 results about "Chronic heart disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
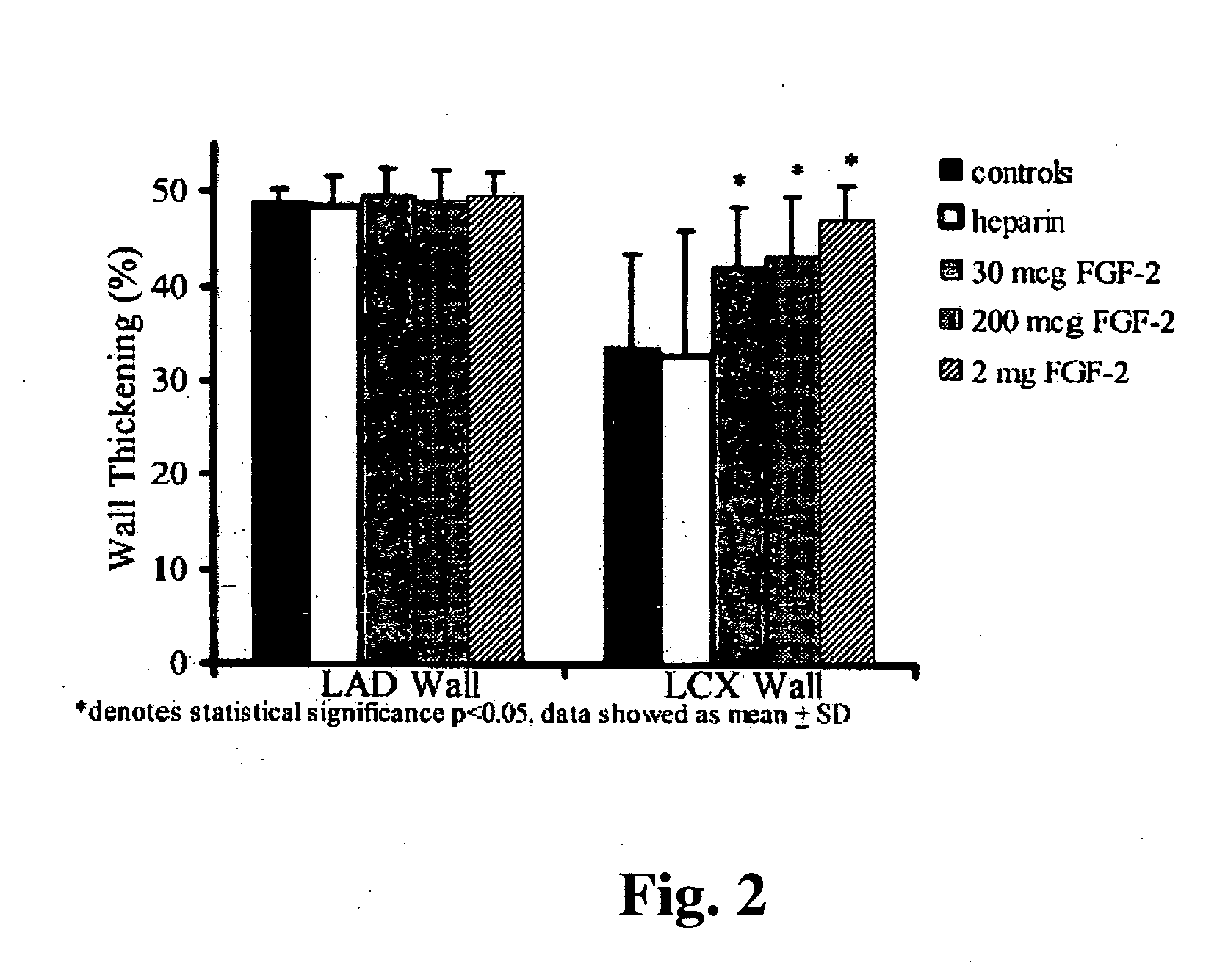
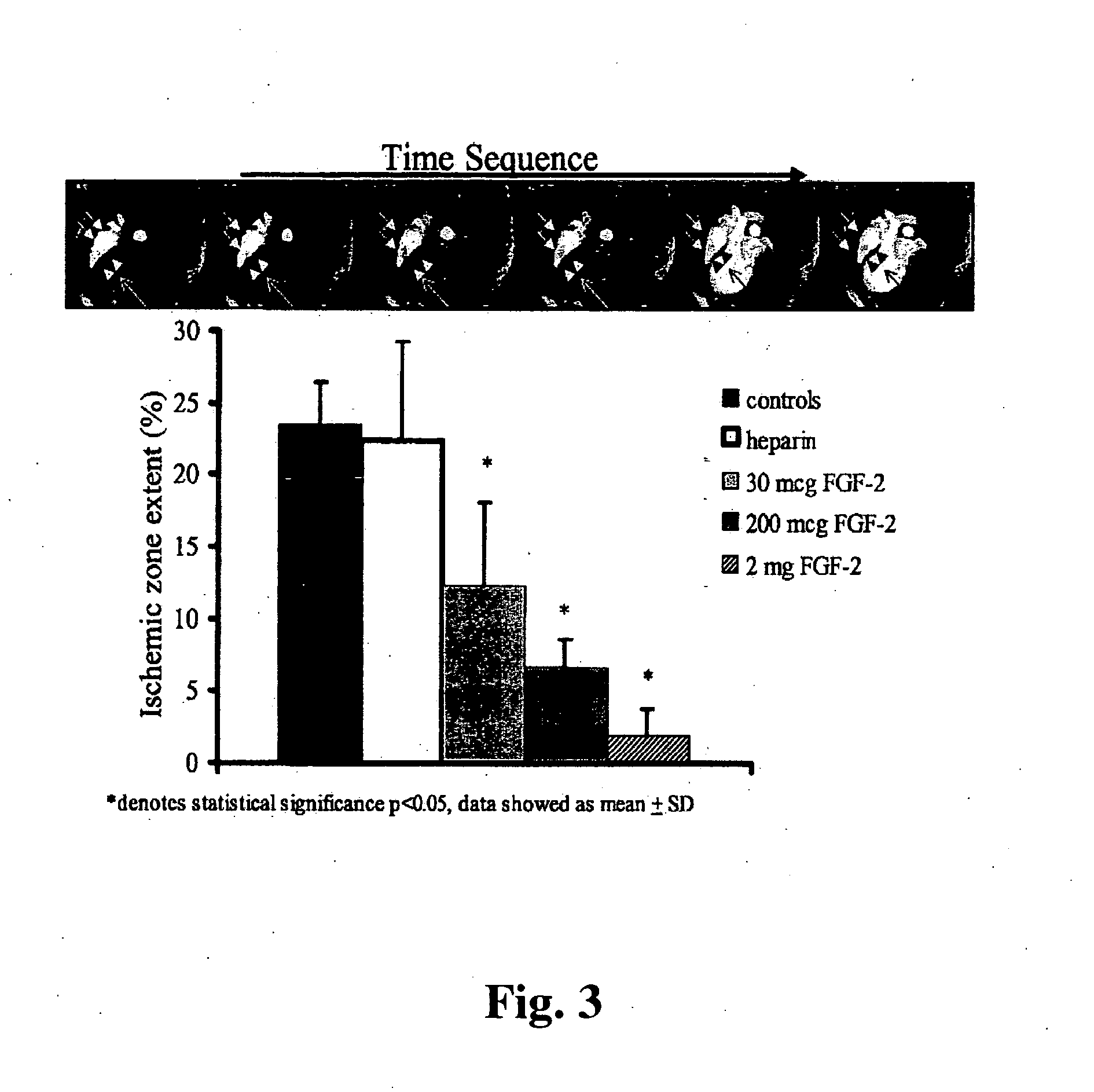
Combination growth factor therapy and cell therapy for treatment of acute and chronic heart disease
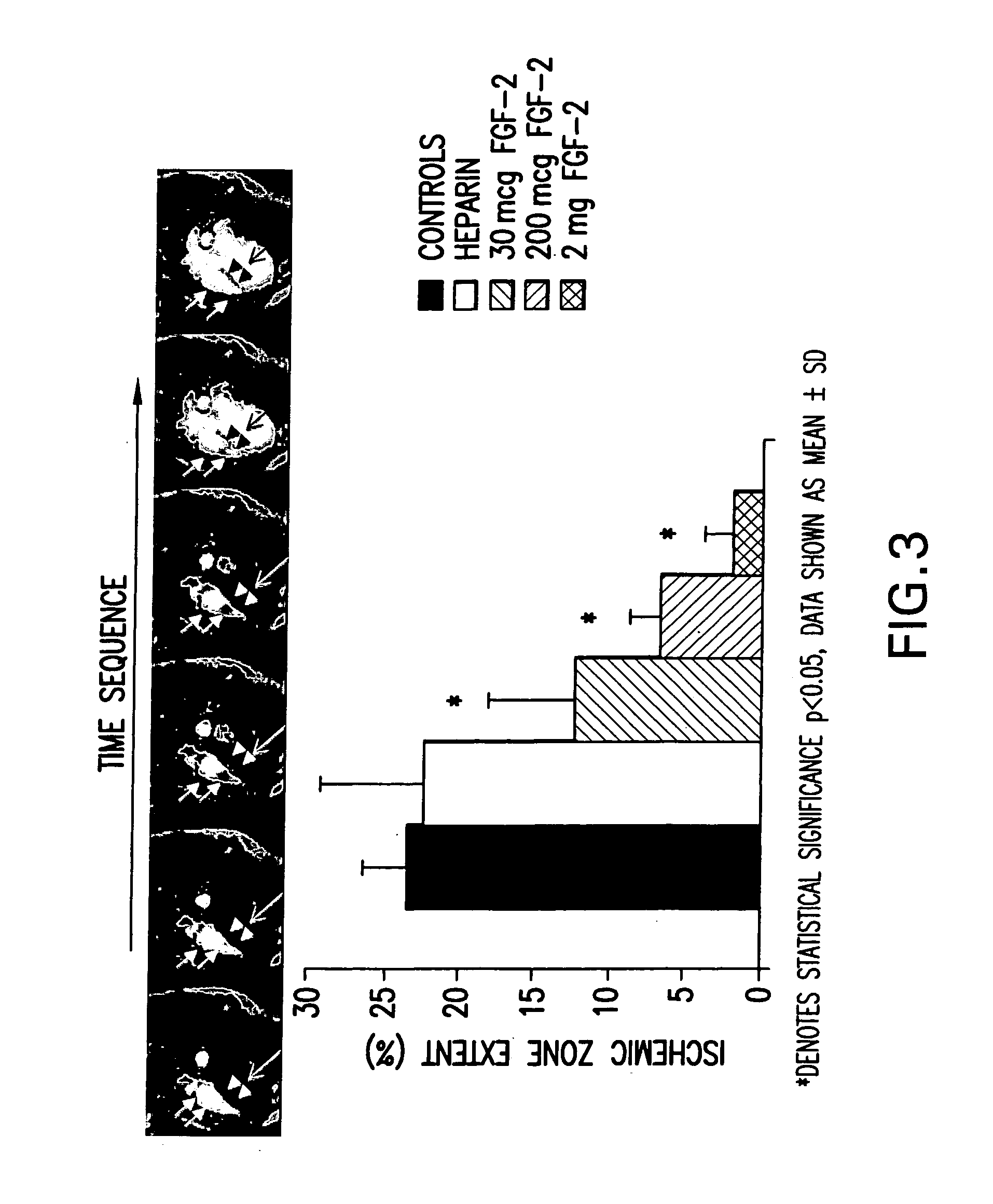
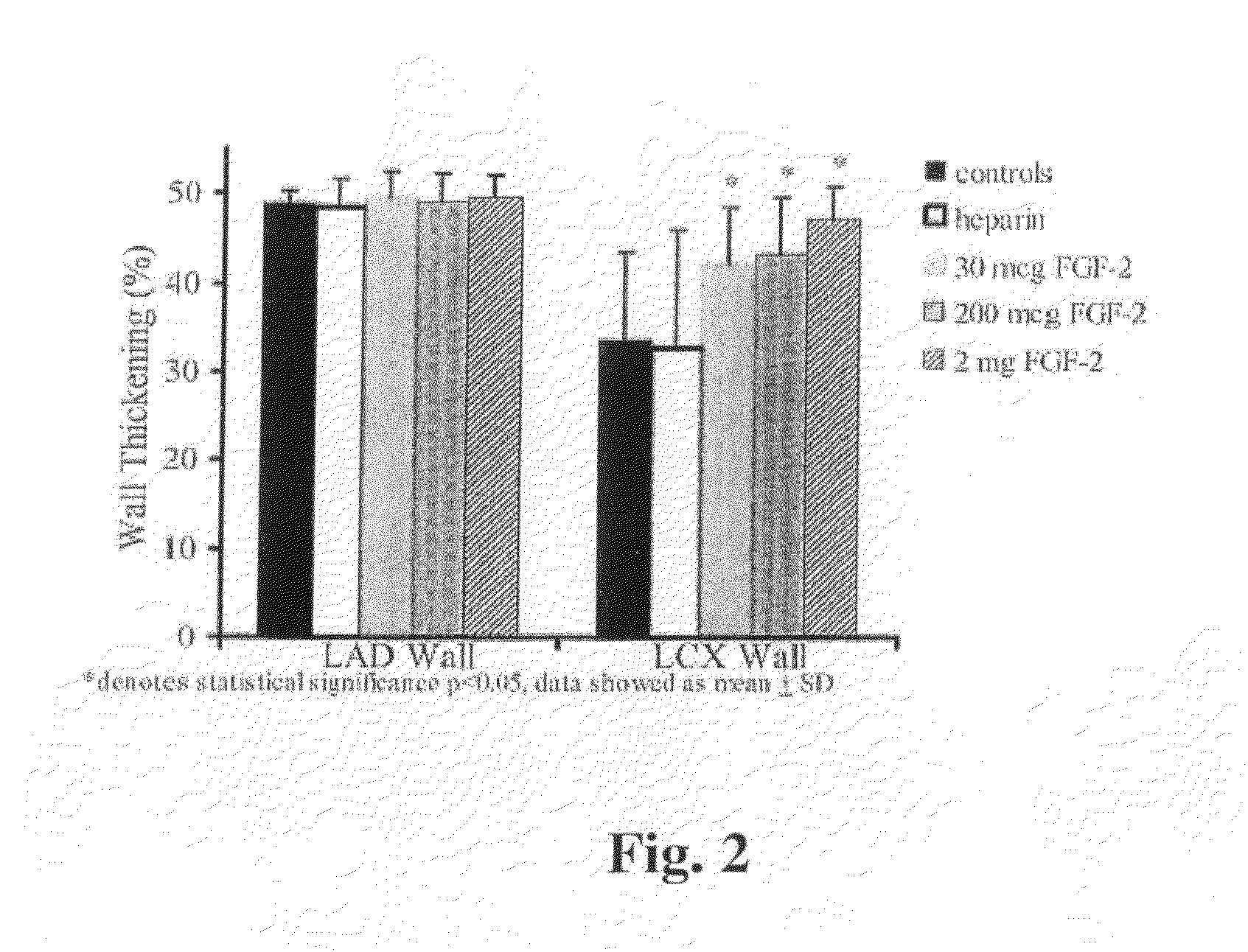
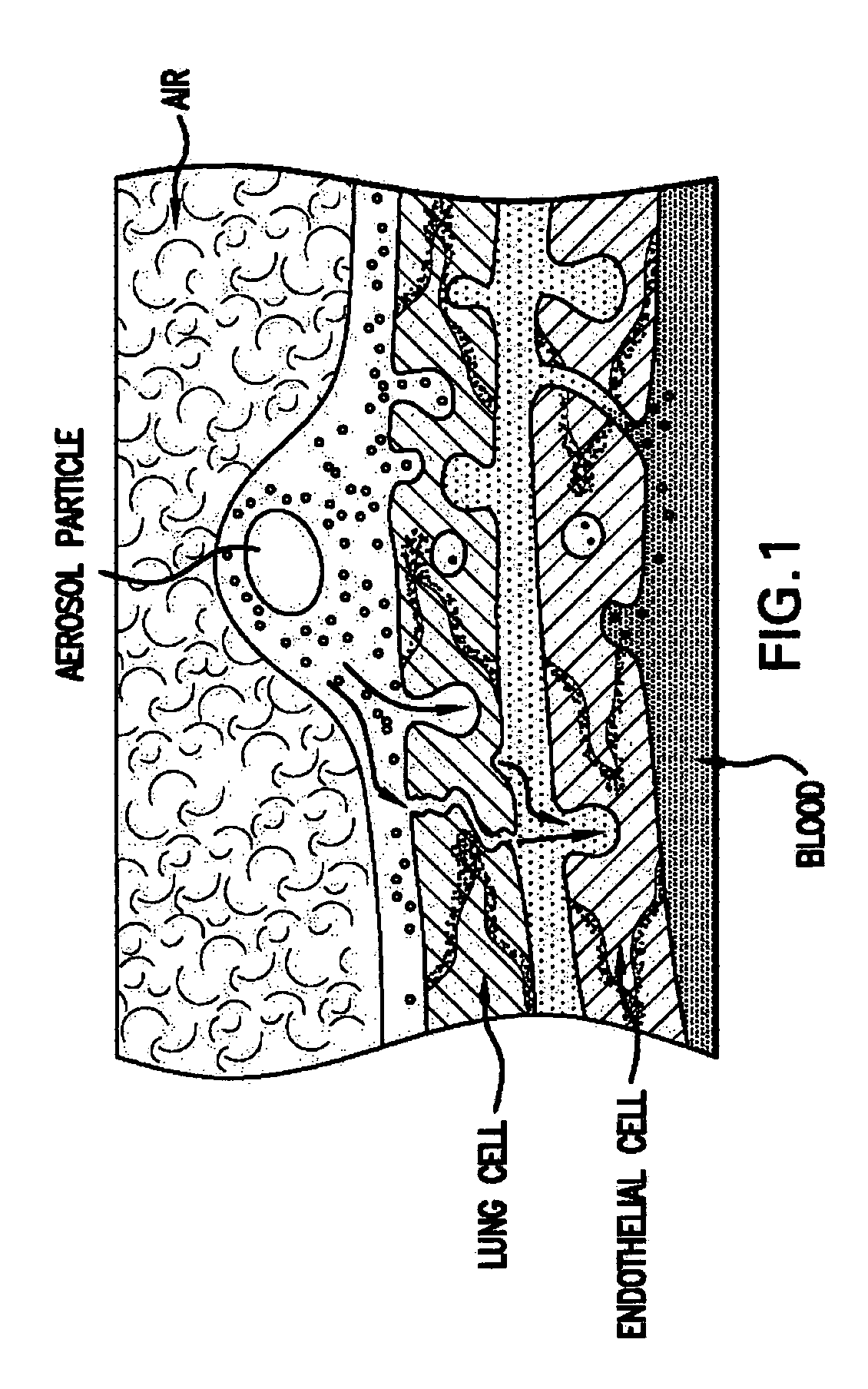
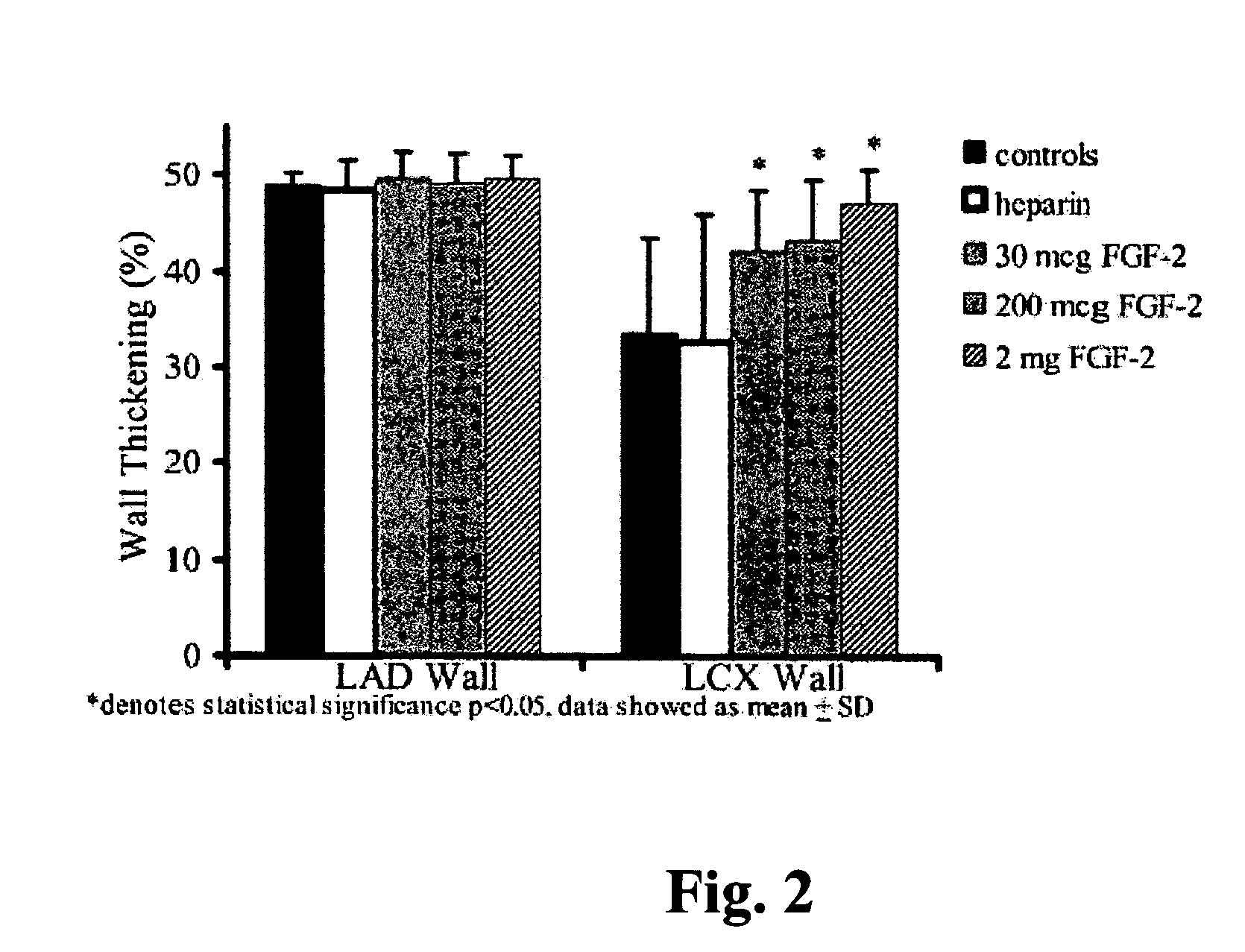
Acute and chronic heart disease is treated using a rational, multi-tier approach. A patient is pretreated with growth factor proteins or gene therapy, followed by the administration of adult stem cells. The progress of treatment is continuously monitored by echo-cardiogram with growth factor treatment and / or stem cell administration adjusted according to the results of the echo-cardiogram or clinical status of the patient. Heart disease is also treated by a method that comprises administration of a therapeutically effective amount of a growth factor protein by oral inhalation therapy.
Owner:FRANCO WAYNE P
Combination growth factor therapy and cell therapy for treatment of acute and chronic heart disease
InactiveUS20050214260A1Increase the number ofBiocidePeptide/protein ingredientsChronic heart diseaseInhalation
Acute and chronic heart disease is treated using a rational, multi-tier approach. A patient is pretreated with growth factor proteins or gene therapy, followed by the administration of adult stem cells. The progress of treatment is continuously monitored by echo-cardiogram with growth factor treatment and / or stem cell administration adjusted according to the results of the echo-cardiogram or clinical status of the patient. Heart disease is also treated by a method that comprises administration of a therapeutically effective amount of a growth factor protein by oral inhalation therapy.
Owner:FRANCO WAYNE P
Methods of use of fibroblast growth factor, vascular endothelial growth factor and related proteins in the treatment of acute and chronic heart disease
Disclosed herein is a rational, multi-tier approach to the administration of growth factor proteins in the treatment of heart disease. Also disclosed is a method to evaluate the effectiveness of the administration of growth factor proteins comprising the clinical assay of CPK-MB levels in a patient undergoing treatment with growth factor proteins. In addition, there is disclosed a method for treatment of heart disease comprising administration of a therapeutically effective amount of a growth factor protein by oral inhalation therapy.
Owner:FRANCO WAYNE P
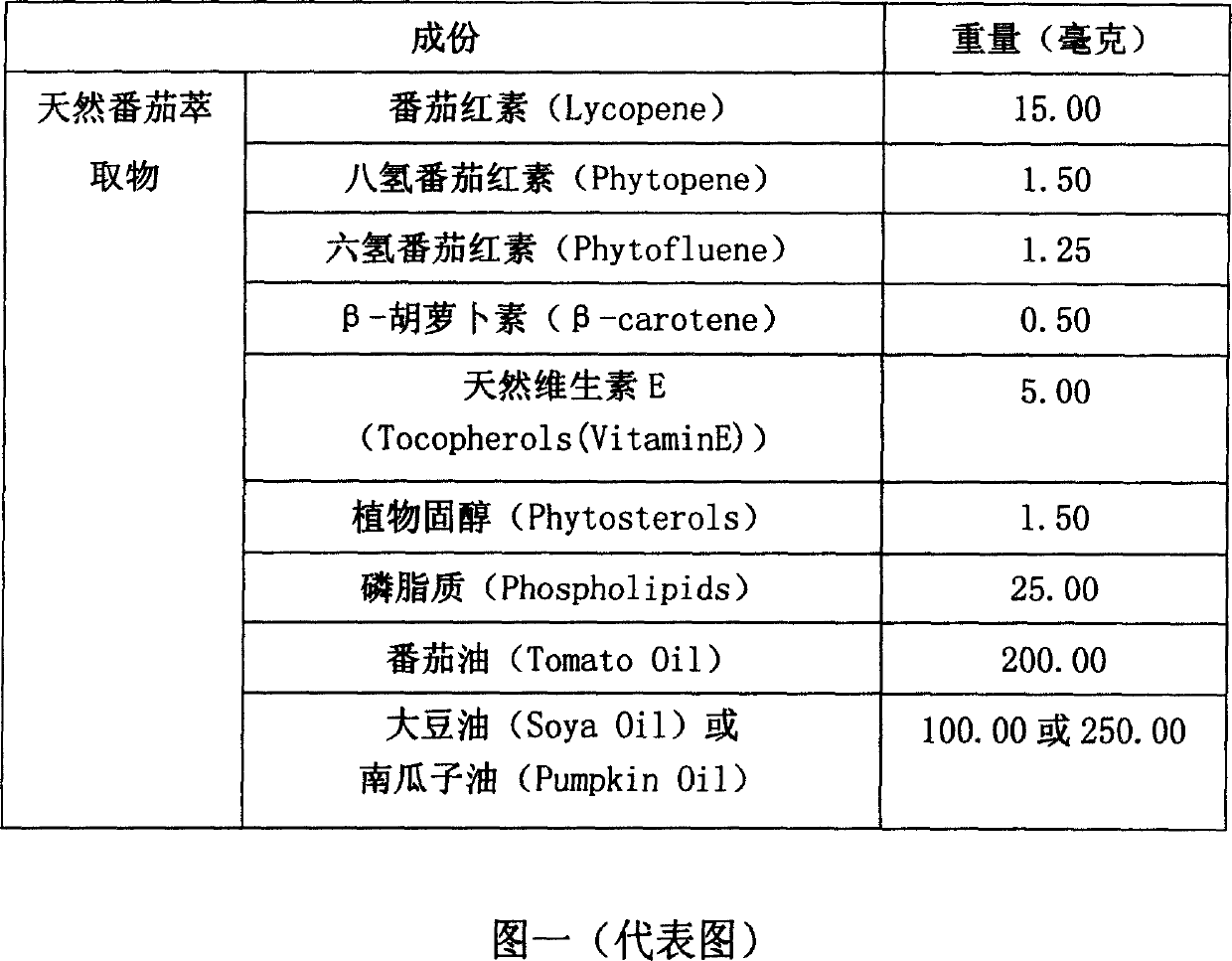
Lycophyll mixture
ActiveCN1650951APromote absorptionNo harmful side effectsMetabolism disorderUnknown materialsChronic heart diseasePlant sterol
A lycopene mixtuer for preventing arteriosclerosis, myocardial infarction and chronic heart disease is proportionally prepared from natural tomato extract and soya oil. Said natural tomato extract is composed of lycopene, phytopene, phytofluene, beta-carotene, VE, phytosterols, phospholipids, and tomato oil.
Owner:健永生物科技国际私人有限公司
Drug for treating coronary heart disease
ActiveCN101554466AImprove clinical efficacyDilated coronary vesselsCardiovascular disorderPlant ingredientsChronic heart diseaseCoronary artery disease
The invention discloses a drug for treating coronary heart disease, which comprises 230 of salvia, 70 of piper longum, 115 of aquilaria, 51 of rosewood, 76 of nutmeg, 38 of kaempferol, 76 of wide jujube, 25 of sandalwood, 76 of sea buckthorn, and 25 of red sandal according to the weight ratio. The salvia, the piper longum, the kaempferol, the wide jujube, the sea buckthorn, and the nutmeg are extracted for three times; for the first time, 14 times of 75 percent of ethanol are added and heated with 2 hours of reflux; for the second time, 12 times of 75 percent of ethanol are added and heated with 2 hours of reflux; for the third time, 10 times of 75 percent of ethanol are added and heated with 2 hours of reflux and the ethanol is recycled. After the coarse powder of aquilaria, rosewood, red sandal and sandalwood are immersed into 8 times of water for 5 hours, volatile oil is extracted for twice with 14 hours for each time; the water extract liquid of the volatile oil is filtered; the herb residue is added with 8 times of water and then heated and recycled for 2 hours, and then filtered; later the extract liquid is merged, concentrated, and dried for obtaining cream powder. By crushing the cream powder into fine powder and then mixing evenly the powder, later adding suitable amount of PEG-6000 for melting and then adding the volatile oil, dropping pills are then made.
Owner:INNER MONGOLIA TIANQI HAN&MONGOLIA PHARMA CO
Novel Sphingosine 1-Phosphate Receptor Antagonists
InactiveUS20150045332A1Improve featuresRaise the concentration levelBiocideSenses disorderChronic heart diseaseDiabetic retinopathy
The present invention relates to sphingosine-1-phosphate (S1P) receptors and compounds of the general formula:that are useful in the treatment and prevention of conditions associated with such receptors. More specifically, the present invention relates to the synthesis and use of sphingosine 1-phosphate receptor 2 (S1P2) antagonists that are useful in the treatment of cancer, atherosclerosis, diabetic retinopathy, and other inflammatory diseases. Among these inflammatory diseases that could be treated with these S1P2 antagonist are those characterized by fibrosis including chronic lung disease, chronic kidney and liver disease, chronic heart disease, and skin diseases such as sclerosis / scleroderma. The S1P2 antagonists can also be used in the treatment of glioblastoma multiforme (brain cancer), pediatric neuroblastoma, and other cancers.
Owner:ARROYO BIOSCI L L C
Microporous zirconium silicate and diuretics for the reduction of potassium and treatment of chronic kidney and/or chronic heart disease
The present invention relates to novel methods of using microporous zirconium silicate to reduce the risk of hyperkalemia and to lower aldosterone levels in the treatment of chronic kidney disease and / or chronic heart disease with therapies comprising diuretics. The invention provides a safe way to reduce the risk of hyperkalemia and to lower aldosterone. The invention also relates to treatment of other conditions that can occur either alone or in connection with hyperkalemia, chronic kidney disease, and / or chronic heart disease.
Owner:ZS PHARMA
Monascus compound preparation capable of regulating pressure and lipid and improving arteriosclerosis, coronary heart disease and cerebral embolism
ActiveCN102319278AGood curative effectNo pollution in the processFungiMetabolism disorderLipid formationCitrinin
The invention relates to a monascus compound preparation capable of regulating pressure and lipid and improving arteriosclerosis, coronary heart disease and cerebral embolism. The preparation is prepared through the following steps: fermenting ginseng and gingko leaves in a liquid state to obtain ginseng fermentation liquor and gingko fermentation liquor, culturing monascus 9901 bacterium or 9906strain in a liquid state, and adding water into the ginseng fermentation liquor, the gingko fermentation liquor and grains to prepare a solid-state culture medium; grafting and performing solid statefermentation to initially obtain a monascus strain; sterilizing malt juice or potato juice, sugar and agar to prepare a plate; grafting and culturing to prepare a pure monascus strain; preparing a strain enlarged culture fluid; grafting and performing strain enlarged culture; culturing in a shaker; performing sterilization grafting and culturing on the ginseng extract, the gingko extract and a solid fermentation nutrient solution; sterilizing, baking, crushing and uniformly mixing the fermented solid culture to obtain monascus fine powder; and preparing the preparation according to a corresponding preparation process of the preparation. The non-citrinin high-color value functional monascus produced by adopting a liquid-state fermentation technology in the invention has the advantages of low cost, edible safety, tiny toxic or side effect, stable and reliable curative effect and short course of treatment.
Owner:上海考门医药科技有限公司
Drug for treating hyperlipidemia, hyperviscosity, hypertension and coronary heart diseases
InactiveCN102018950AReduce dosageNo side effectsMetabolism disorderCardiovascular disorderChronic heart diseaseSide effect
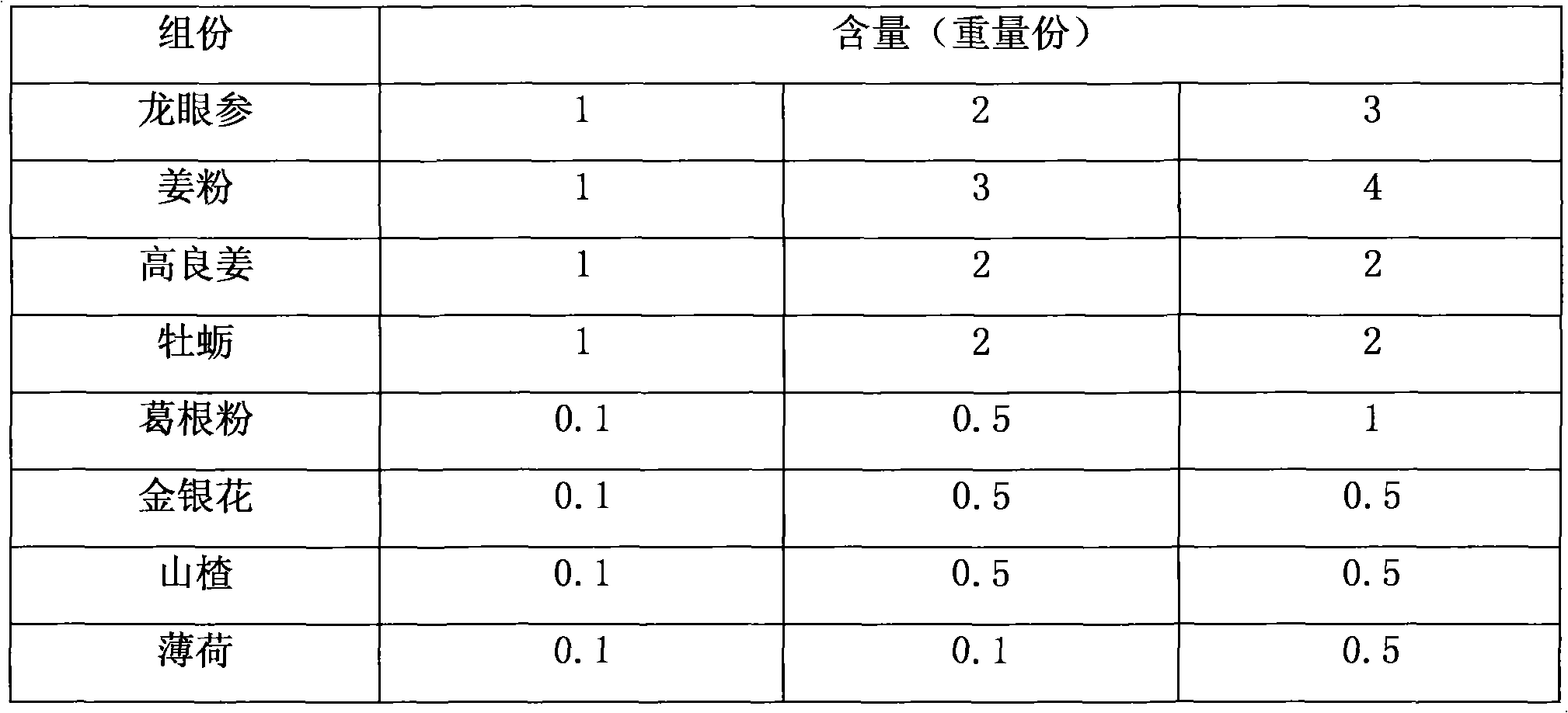
The invention relates to a drug for treating hyperlipidemia, hyperviscosity, hypertension and coronary heart diseases, which takes special medicinal materials in the Guangxi zhuang nationality autonomous region as raw materials, and comprises the following components by weight part: 1-3 parts of lysidice rhodostegia hance, 1-4 parts of ginger powder, 1-2 parts of galangal, 1-2 parts of oyster, 0.1-1 part of puerarin powder, 0.1-0.5 part of honeysuckle, 0.1-0.5 part of hawthorn and 0.1-0.5 part of mint. The drug has the characteristics of obvious curative effect, no toxic or side effect, long-term use and no dependence.
Owner:NANJING DUOLING BIOLOGICAL TECH
Traditional Chinese medicine for treating cardiovascular and cerebrovascular diseases and preparation method of traditional Chinese medicine
InactiveCN103070943ASignificant effectCompatibility is simpleCardiovascular disorderPlant ingredientsDiseaseChronic heart disease
The invention discloses a traditional Chinese medicine for treating cardiovascular and cerebrovascular diseases and a preparation method of the traditional Chinese medicine. The traditional Chinese medicine comprises three raw materials, namely salvia miltiorrhiza, hawthorn and carthamus tinctorius. The traditional Chinese medicine can effectively alleviate myocardium conditions, has significant treatment effects on the cardiovascular and cerebrovascular diseases, particularly on chronic heart diseases, and has the advantages of simple preparation technology, lower cost, pure traditional Chinese medicine preparation, no toxic or side effects, and the like.
Owner:NORTHWEST A & F UNIV
Combination growth factor therapy and cell therapy for treatment of acute and chronic heart disease
InactiveUS20100303769A1Increase the number ofBiocidePeptide/protein ingredientsChronic heart diseaseHeart disease
Acute and chronic heart disease is treated using a rational, multi-tier approach. A patient is pretreated with growth factor proteins or gene therapy, followed by the administration of adult stem cells. The progress of treatment is continuously monitored by echo-cardiogram with growth factor treatment and / or stem cell administration adjusted according to the results of the echo-cardiogram or clinical status of the patient. Heart disease is also treated by a method that comprises administration of a therapeutically effective amount of a growth factor protein by oral inhalation therapy.
Owner:FRANCO WAYNE P
Drug combination for treating coronary heart diseases caused by myocardial ischemia
InactiveCN102526449AImprove the level ofLower levelAnthropod material medical ingredientsCardiovascular disorderChronic heart diseaseSalvia miltiorrhiza
The invention relates to a drug combination for treating coronary heart diseases caused by myocardial ischemia. The drug combination is prepared from the following active ingredients by weight portion: salvia miltiorrhiza accounting for 15 to 20 parts, allium macrostemon accounting for 5 to 8 parts, panax notoginseng accounting for 8 to 12 parts, Indian buead accounting for 10 to 15 parts, white paeony roots accounting for 10 to 15 parts, angelica accounting for 8 to 12 parts, honey accounting for 20 to 30 parts, shizandra berries accounting for 4 to 6 parts and cowherb seeds accounting for 5to 8 parts. The drug combination can achieve obvious curative effect on the coronary heart diseases caused by myocardial ischemia, and can achieve higher safety.
Owner:白玲强
Growth factor therapy mobilization of stem cells into the peripheral blood
InactiveUS7291597B2Good yieldReduce in quantityBiocidePeptide/protein ingredientsChronic heart diseaseCoronary heart disease
Stem cells are mobilized into the peripheral blood by a method comprising the steps of: (i) administering to an individual at least one of the following: FGF1 and FGF2, and also at least one of the following: VEGF, VEGFA and VEGF165; (ii) isolating the peripheral blood stem cells (PBSC) by apheresis; and (iii) injecting the PBSC into a patient suffering from chronic heart disease. Alternatively, gene therapy is used for the induction of the stem cells into the peripheral blood, wherein the gene therapy formulation comprises AD5FGF-4 or VEGF165 plasmid DNA. Additionally, the progress of the treatment of the chronic heart disease is monitored by using an echocardiogram unit.
Owner:FRANCO WAYNE P
Complex of angiotensin receptor antagonist and neutral endopeptidase inhibitor
ActiveUS10537555B2Easy to controlLow hygroscopicityOrganic chemistry methodsCardiovascular disorderChronic heart diseasePropanoic acid
Provided a complex of formula is [3-((1S, 3R)-1-biphenyl-4-ylmethyl-3-ethoxycarbonyl-1-butylcarbamoyl) propionate-(S)-3′-methyl-2′-(pentanoyl {2″-(tetrazol-5-ylate) biphenyl-4′-ylmethyl}amino) butyrate]6.XCa2+.YNa+.ZH2O, wherein X=1-3, Y=12-16, Z=9-18, and 2X+Y=18, and represented by formula (I). Also disclosed are the method of preparing the complex and the method of treating chronic heart disease using a medicament comprising the complex.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Chinese medicament for treating coronary heart disease
InactiveCN102940805AQuick resultsGood curative effectAmphibian material medical ingredientsMammal material medical ingredientsChronic heart diseaseCoronary artery disease
The invention discloses a Chinese medicament for treating coronary heart disease. The Chinese medicament comprises the following raw materials in parts by mass: 20 to 40 parts of root of red-rooted salvia, 14 to 20 parts of musk, 10 to 16 parts of storax, 18 to 20 parts of ginseng, 10 to 22 parts of astragalus root, 8 to 14 parts of safflower, 6 to 10 parts of kudzuvine root, 10 to 12 parts of Chinese angelica, 6 to 8 parts of trichosanthes kirilowii maxim, 10 to 20 parts of cinnamon, 8 to 12 parts of toad venom, 8 to 14 parts of honey-fried licorice root, 7 to 11 parts of immature bitter orange, 6 to 8 parts of rosewood heart wood, 6 to 8 parts of wheat head, 9 to 13 parts of fushen, and 16 to 20 parts of rehmannia. The Chinese medicament for treating the coronary heart disease has the advantages of quick response, good treatment effect, low cost, safety and reliability, and does not have toxic or side effect.
Owner:JIANGSU ZHONGWEI HEAVY IND MACHINERY
Chinese medicinal formulation for treating coronary heart disease and cerebral thrombosis
InactiveCN102526173ALow costEasy to useCardiovascular disorderPlant ingredientsChronic heart diseaseCoronary artery disease
The invention discloses a Chinese medicinal formulation for treating coronary heart disease and cerebral thrombosis and aims to provide a Chinese medicinal formulation for treating coronary heart disease, cerebral thrombosis and myocardial infarction, which has the advantages of low cost, good use effect and less side effect. The invention adopts the technical scheme that the preparation method comprises the following steps: (1), preparing 120 g to 350 g of Panax ginseng, 400 g to 600 g of Rhizoma Ligustici Chuanxiong, 400 g to 600 g of Angelica sinensis, and 400 g to 600 g of Astragalus membranaceus, weighing 60% the prescription dose of Panax ginseng and 25% the prescription dose of Astragalus membranaceus, and grinding to 100 mesh fine powders; (2), grinding the remaining dose of Panaxginseng the remaining dose of Astragalus membranaceus and other medicinal materials to 10 mesh fine powders, decocting with water twice (2 to 3 hours each time), mixing decoctions, filtering, concentrating the filtrate until the relative density of the resulting concentrate is 1.15 to 1.30 and the weight is 3 kg (80 DEG C); and (3), adding the fine powders obtained in the step (1) and starch intothe concentrate, thoroughly mixing, drying, grinding, oven-drying, and sieving with a 14 mesh sieve. The preparation method has a relatively-low cost and is convenient to operate, and the Chinese medicinal formulation has better use effects.
Owner:NO 371 HOSPITAL PLA
Drug for treating coronary heart disease
InactiveCN102846911AQuick resultsGood curative effectCardiovascular disorderPlant ingredientsChronic heart diseaseCoronary artery disease
The invention discloses a drug for treating coronary heart disease. The drug is prepared from the following raw materials of traditional Chinese medicines, by weight: 12-15 parts of Fructus Trichosanthis, 12-15 parts of Allium macrostemon, 10-12 parts of cassia twig, 10-12 parts of magnolia obavata, 12-15 parts of safflower, 15-18 parts of Angelica sinensis, 14-18 parts of the root of red-rooted salvia, 10-12 parts of banksian rose, 12-15 parts of Ligusticum wallichii, 8-10 parts of polygala root, 15-20 parts of fried jujube kernel, 10-12 parts of Fructus aurantii, 10-12 parts of rhizoma anemones altaicae, and 10-12 parts of honey-fried licorice. The medicines are mixed and decocted with water for administration, or can also be ground. The drug has efficacies of nourishing yin for suppressing hyperactive yang, promoting blood circulation and warming heart yang, and has good therapeutic effect on coronary heart disease, with a total effective rate reaching 94.6%.
Owner:高海元
Medication for treating coronary heart disease
InactiveCN1872044ASmall molecular weightFast dissolutionOrganic active ingredientsPill deliveryChronic heart diseaseCoronary artery disease
A medicine for treating coronary heart disease is disclosed. Its advantages are high natural level and safety and low toxic by-effect.
Owner:TIANJIN TASLY PHARMA CO LTD
Sphingosine 1-phosphate receptor antagonists
InactiveUS9663511B2Improve featuresImproved pharmacokinetic parametersOrganic active ingredientsSenses disorderChronic heart diseaseDiabetic retinopathy
The present invention relates to sphingosine-1-phosphate (S1P) receptors and compounds of the general formula:that are useful in the treatment and prevention of conditions associated with such receptors. More specifically, the present invention relates to the synthesis and use of sphingosine 1-phosphate receptor 2 (S1P2) antagonists that are useful in the treatment of cancer, atherosclerosis, diabetic retinopathy, and other inflammatory diseases. Among these inflammatory diseases that could be treated with these S1P2 antagonist are those characterized by fibrosis including chronic lung disease, chronic kidney and liver disease, chronic heart disease, and skin diseases such as sclerosis / scleroderma. The S1P2 antagonists can also be used in the treatment of glioblastoma multiforme (brain cancer), pediatric neuroblastoma, and other cancers.
Owner:ARROYO BIOSCI L L C
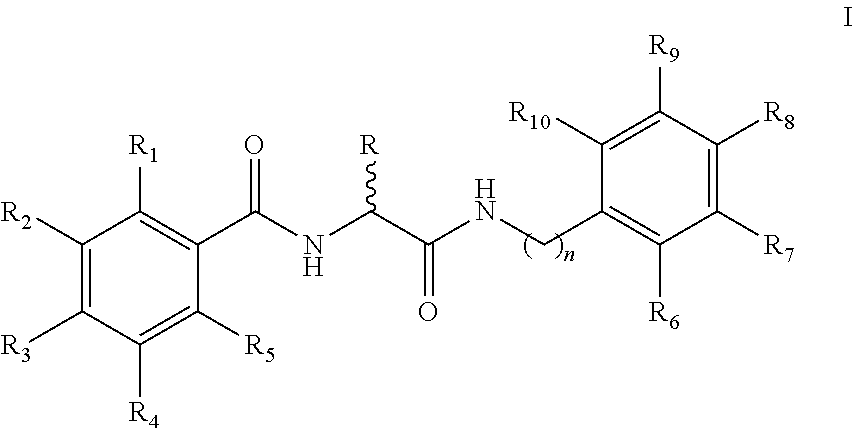
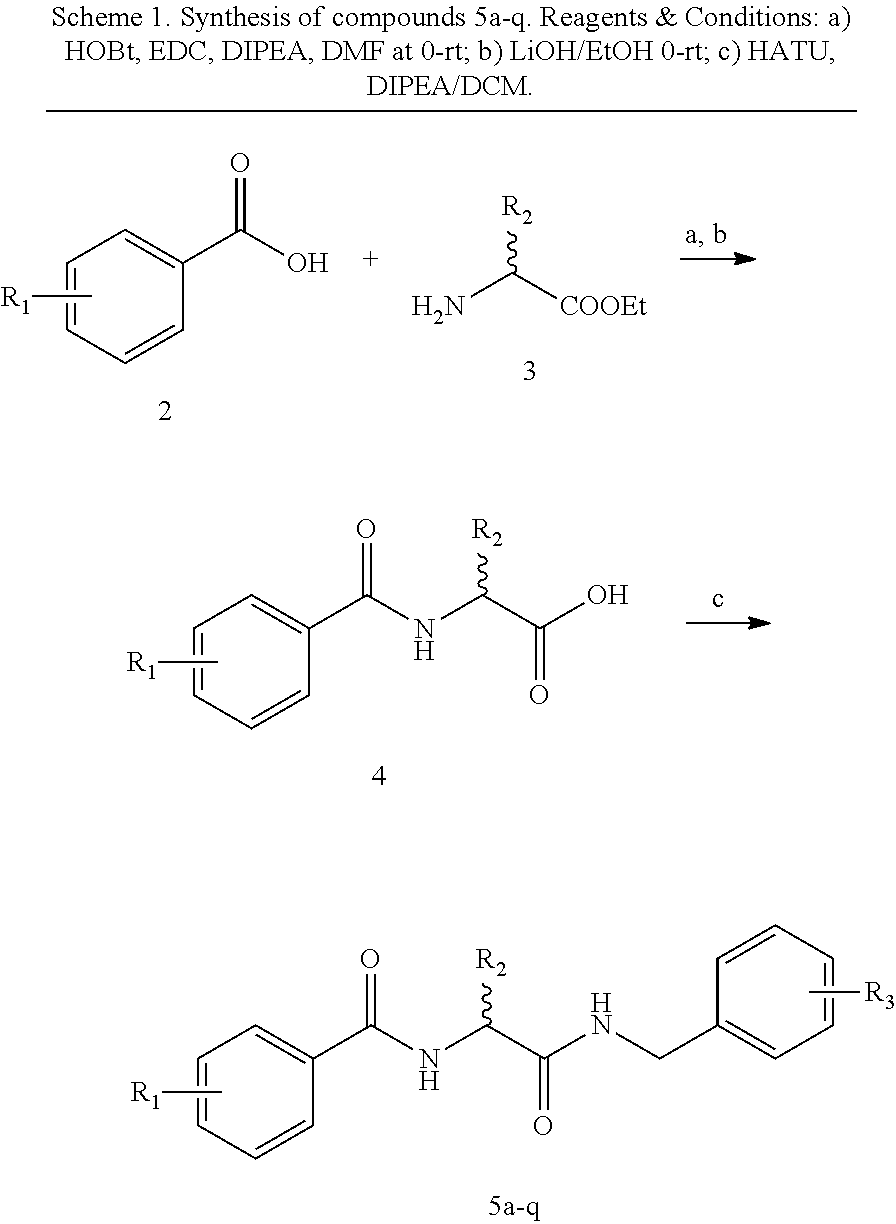
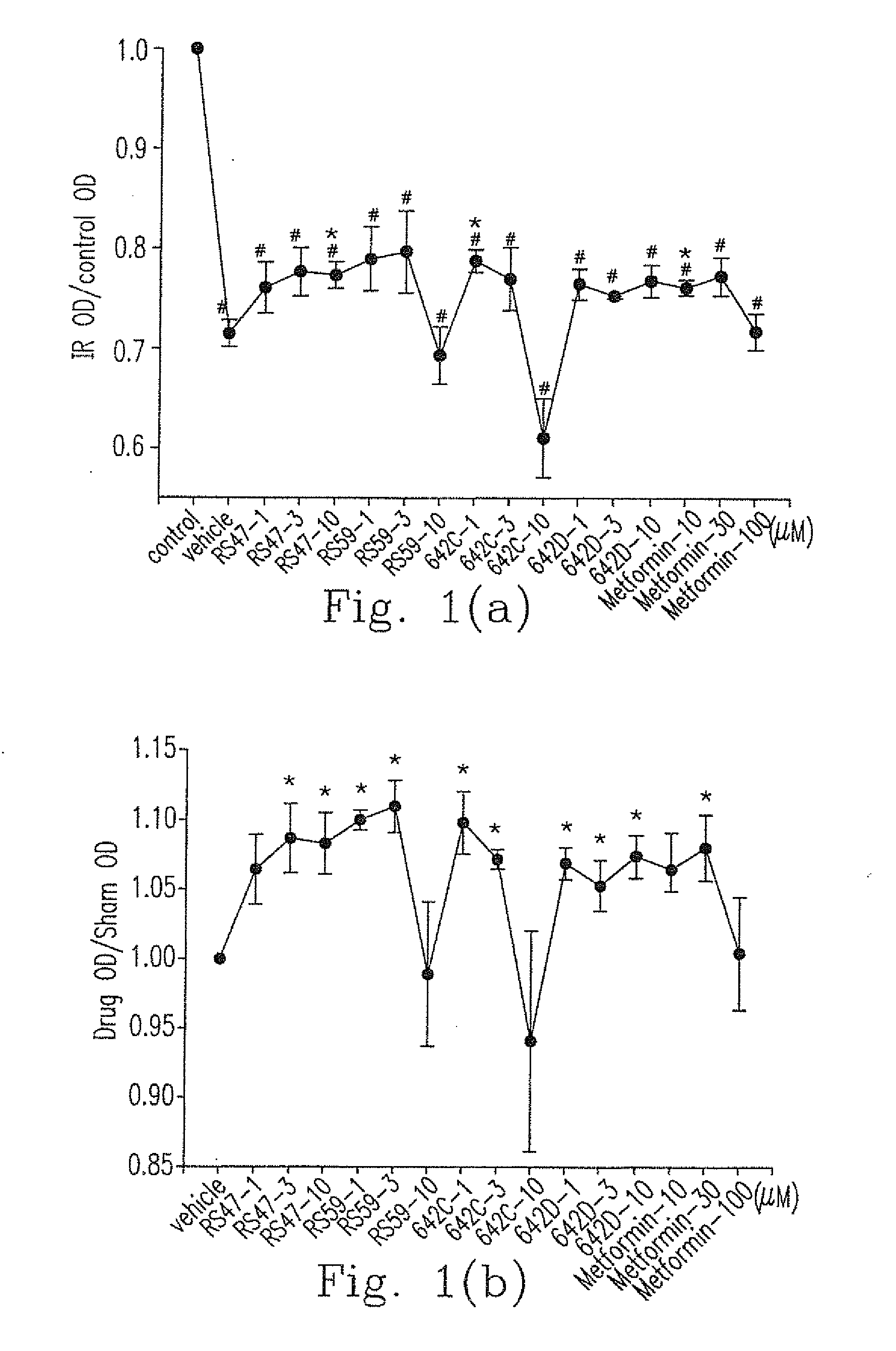
Benzylamino-oxoethyl benzamide analogs and methods of use
Benzylamino-oxoethyl benzamide compounds for use in treating diseases and conditions associated with abnormal cell function related to endoplasmic reticulum (ER) stress. For example, the compounds can be used as suppressors of ER stress-induced pancreatic β-cell dysfunction and death, for example in the treatment of Type 1 and Type 2 diabetes. The compounds can also be used in treatments for chronic heart disease, neurodegenerative diseases, retinal degeneration, and other metabolic disorders associated with ER stress.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Medicine for treating coronary heart diseases
InactiveCN106266474ASimple recipeEasy to prepareInanimate material medical ingredientsConiferophyta medical ingredientsChronic heart diseaseSalvia miltiorrhiza
The invention discloses a medicine for treating coronary heart diseases. The medicine is prepared from the following raw materials in parts by weight: 6-8 parts of allium macrostemon, 3-5 parts of sweetgum, 3-5 parts of ampelopsis humulifolia, 5-9 parts of lindera glauca, 3-5 parts of safflower, 5-15 parts of ligusticum wallichii, 12-18 parts of radix pseudostellariae, 8-12 parts of poria cocos, 12-18 parts of polygala tenuifolia, 12-18 parts of salvia miltiorrhiza, 4-8 parts of schisandra chinensis, 12-18 parts of rhizoma corydalis, 12-18 parts of keel and 12-18 parts of semen boitae. According to the medicine for treating coronary heart diseases disclosed by the invention, 14 selected natural traditional Chinese medicines are scientific in compatibility, the formula is simple, a synergistic effect of the raw medicinal materials achieves the effects of regulating vital energy and activating blood and activating meridians to stop pain, and the medicine is fast in effectiveness and does not have any toxic or side effect. Moreover, the medicine is simple in preparation method, convenient to take and low in cost, and the effective rate is 93.3% or over.
Owner:黄佩丽
Capsule for treating coronary heart disease
InactiveCN101073588APrecision medicineTake it safely to overcome other drug deficienciesInanimate material medical ingredientsCapsule deliveryChronic heart diseaseCoronary artery disease
The invention is concerned with the capsule for treat coronary heart disease, the compounding is: gen-seng, notoginseng, amber, cunpu, nard. It is: processes to parch, grinds and sifts by 120 meshes - 150 meshes, puts into capsule. The invention is safety, easy to taking, and simple technics.
Owner:凌子春
Drug for treating coronary heart disease
InactiveCN103417830AGood curative effectLittle side effectsHeavy metal active ingredientsCardiovascular disorderHerbal preparationsChronic heart disease
The invention discloses a drug for treating coronary heart disease. The drug is characterized by comprising the following components: 20-40 g membranous milkvetch root, 35-45 g Chinese angelica, 20-40 g suberect spatholobus stem, 35-45 g Chinese cabbage root, 35-45 g potentilla discolor, 20-40 g dwarf lilyturf turber, 20-40 g kudzuvine root and the like. The invention provides a traditional Chinese medicine for treating coronary heart disease, which is excellent in curative effect and less in side effects, can be manufactured into tablets, powder and other compound Chinese herbal preparations as required by adopting conventional methods. The invention builds a technical platform for innovative traditional Chinese medicine research and development in the aspects of safety, drug effect evaluation, pharmacodynamics, pharmacokinetics and the like of compound traditional Chinese medicines, and can further improve the new medicine creation level and international competitiveness in China.
Owner:王新程
Combination growth factor therapy and cell therapy for treatment of acute and chronic heart disease
Owner:FRANCO WAYNE P
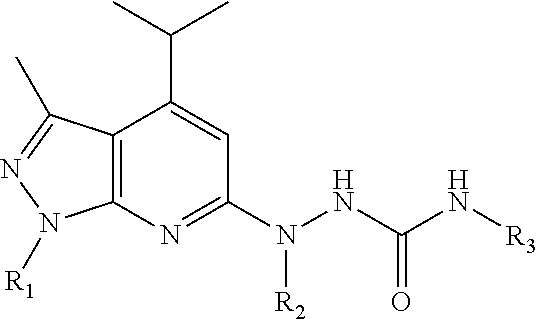
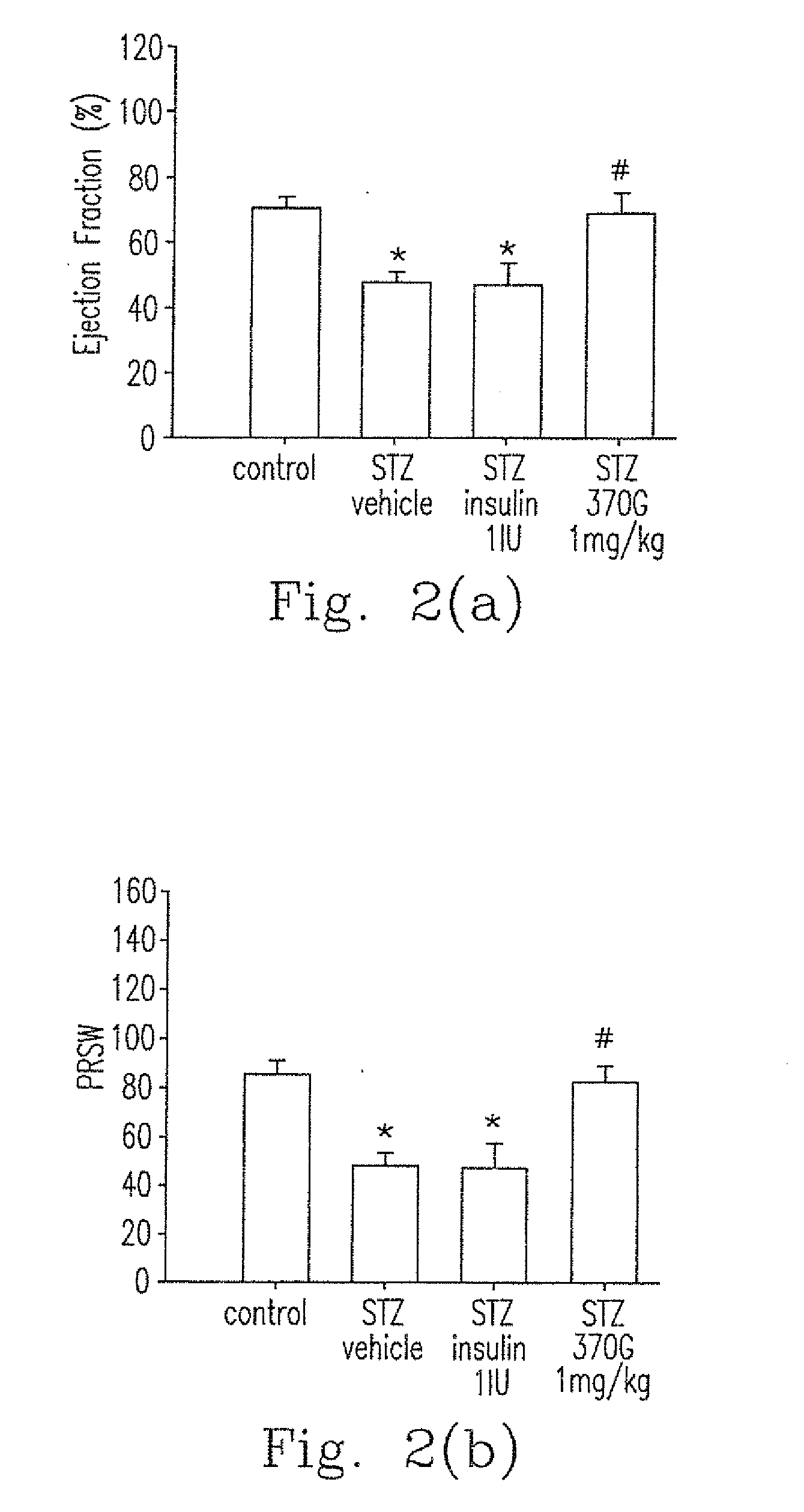
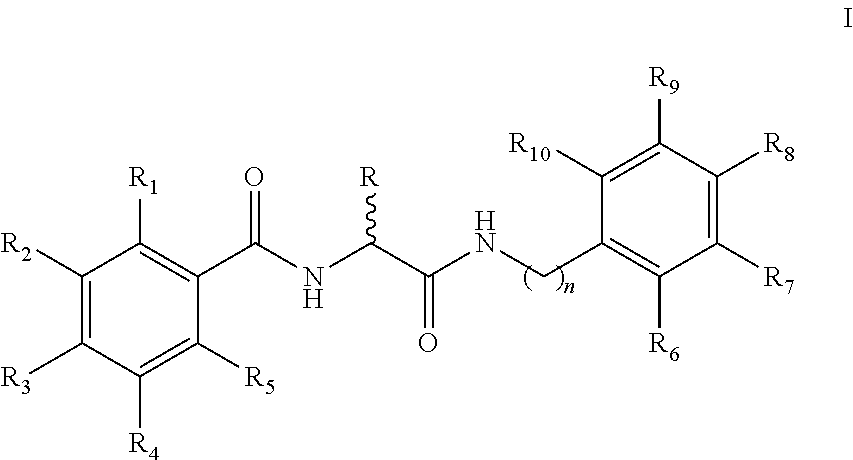
Pharmaceutical composition and method for preventing or treating chronic heart disease
The present invention provides a pharmaceutical composition for preventing or treating a chronic heart disease, comprising a compound of a formula (I):wherein:R1 is one independently selected from a group consisting of a hydrogen, a methyl and an ethyl;R2 is one of a hydrogen and a methyl; andR3 is one selected from a group consisting of a hydrogen, (CH2)nAr and (CH2)nArR′R″, wherein n is one of 1 and 2, R′ and R″ is located at C-3 and C-4 positions, respectively, R′ is a hydrogen and R″ is one of a hydroxy, a fluorine, a bromine and a OMe, or R′+R″=—OCH2O—; orR2+R3 is one ofwherein n is one of 4 and 5.
Owner:NAT TAIWAN UNIV +1
Complex of angiotensin receptor antagonist and neutral endopeptidase inhibitor
ActiveUS20180318259A1Easy to controlGood chemical stabilityOrganic chemistry methodsCardiovascular disorderPropionateChronic heart disease
Provided a complex of formula is [3-((1S, 3R)-1-biphenyl-4-ylmethyl-3-ethoxycarbonyl-1-butylcarbamoyl) propionate-(S)-3′-methyl-2′-(pentanoyl {2″-(tetrazol-5-ylate) biphenyl-4′-ylmethyl}amino) butyrate]6.XCa2+.YNa+.ZH2O, wherein X=1-3, Y=12-16, Z=9-18, and 2X+Y=18, and represented by formula (I). Also disclosed are the method of preparing the complex and the method of treating chronic heart disease using a medicament comprising the complex.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
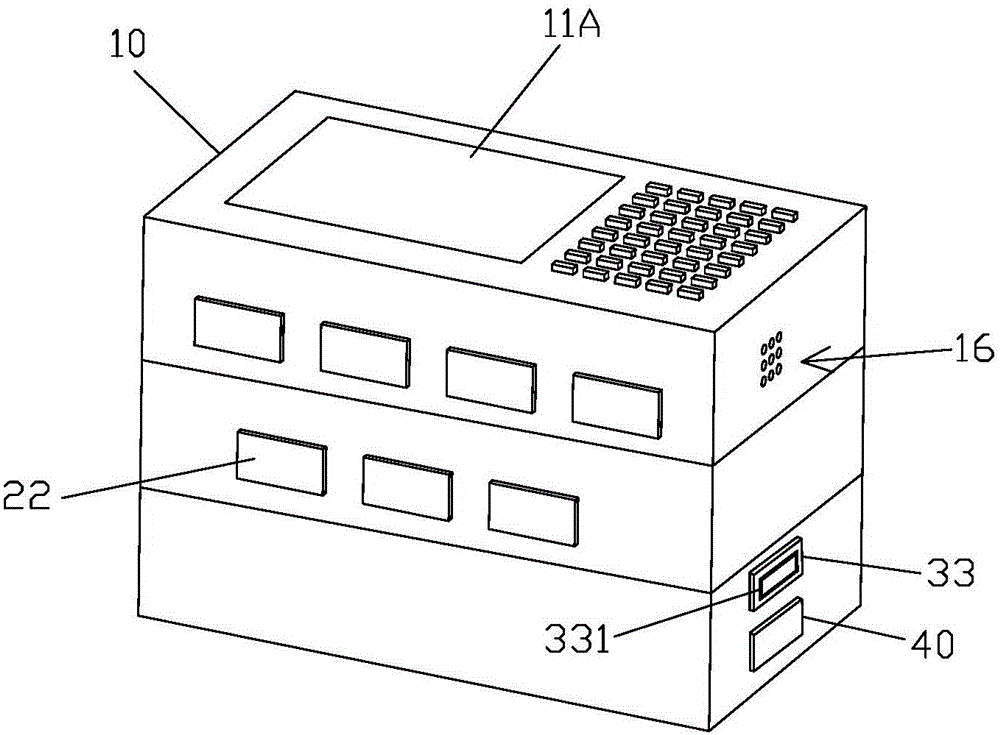
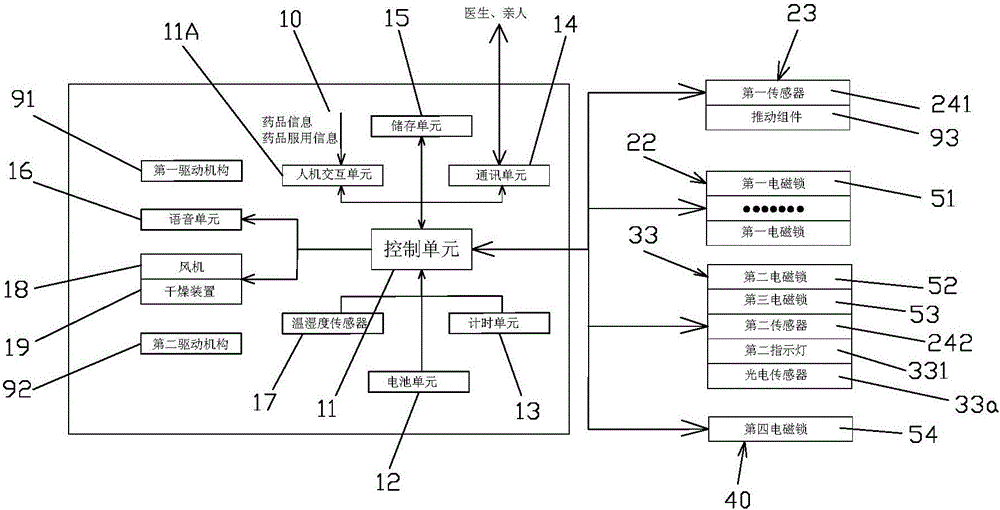
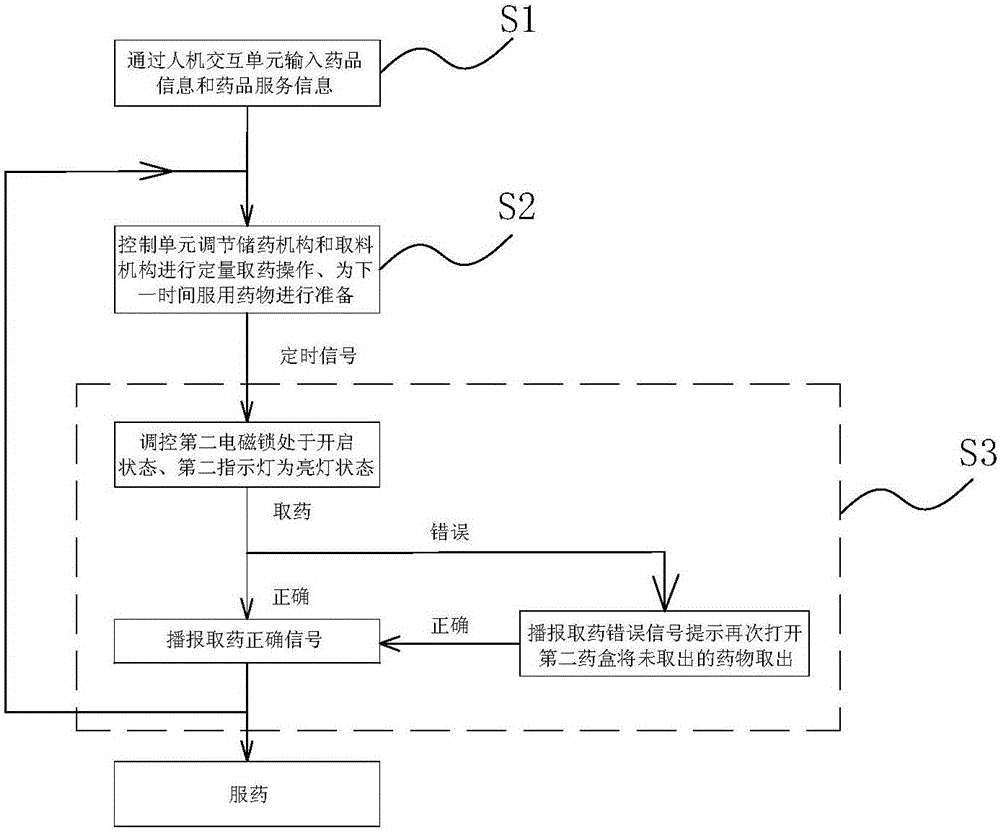
Medicine chest for accurately regulating and controlling medicine taking of elderly people with chronic diseases
InactiveCN105662884APrecision medicineAvoid confusionSmall article dispensingOral administration deviceChronic heart diseaseMedicine
The invention relates to a medicine chest for accurately regulating and controlling medicine taking of elderly people with chronic diseases.The medicine chest comprises a chest body, the chest body is provided with medicine storage units used for storing and taking various kinds of medicine respectively and a medicine collecting unit used for collecting the medicine taken from the medicine storage units to allow a patient to take; each medicine storage unit comprises a medicine storage mechanism used for storing the medicine and a medicine taking mechanism used for taking out the medicine in the medicine storage mechanism in dosage.The patient only needs to take out all the medicine in a second medicine box and take the medicine when the patient takes the medicine every time, therefore, it is guaranteed that the patient whose consciousness is not quite clear can accurately get and take the medicine, and the phenomena that less medicine is got, less medicine is taken, and more medicine is taken are prevented from occurring.
Owner:王丽
Benzylamino-oxoethyl benzamide analogs and methods of use
Benzylamino-oxoethyl benzamide compounds for use in treating diseases and conditions associated with abnormal cell function related to endoplasmic reticulum (ER) stress. For example, the compounds can be used as suppressors of ER stress-induced pancreatic β-cell dysfunction and death, for example in the treatment of Type 1 and Type 2 diabetes. The compounds can also be used in treatments for chronic heart disease, neurodegenerative diseases, retinal degeneration, and other metabolic disorders associated with ER stress.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Injection for treating coronary heart disease and cerebral infarction
ActiveCN1298348CIncrease cerebral blood flowHigh activityPharmaceutical delivery mechanismCardiovascular disorderChronic heart diseaseCoronary artery disease
Owner:西安万隆制药股份有限公司
Bean curd for prevention and treatment of coronary heart disease
InactiveCN106234610AImprove disease resistancePrevent cancerCheese manufactureFood scienceChronic heart diseaseCoronary artery disease
The invention discloses bean curd for prevention and treatment of coronary heart disease, wherein the bean curd is prepared from, in parts by weight, 80-90 parts of soybeans, 5-7 parts of a grape seed powder, 3-6 parts of leek, 6-12 parts of kelp, 6-8 pars of sweet potato leaves and other raw materials. Through scientific collocation of the raw materials, the obtained bean curd has the efficacies of lowering blood lipid, lowering blood pressure, maintaining beauty, keeping young and protecting eyesight with long-term eating.
Owner:张可池
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com