Benzylamino-oxoethyl benzamide analogs and methods of use
a technology of benzamide and oxoethyl benzamide, applied in the field of benzylaminooxoethyl benzamide analogs and methods of use, can solve the problems of affecting the health of patients, affecting the effect of treatment, and accumulating unfolded or misfolded proteins, etc., and achieves the effect of low efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0077]Certain novel embodiments of the present disclosure, having now been generally described, will be more readily understood by reference to the following examples, which are included merely for purposes of illustration of certain aspects and embodiments of the present disclosure, and are not intended to be limiting. The following detailed examples are to be construed, as noted above, only as illustrative, and not as limiting of the present disclosure in any way whatsoever. Those skilled in the art will promptly recognize appropriate variations from the various compositions, structures, components, procedures and methods.
[0078]Methods
[0079]Synthesis of Analogs
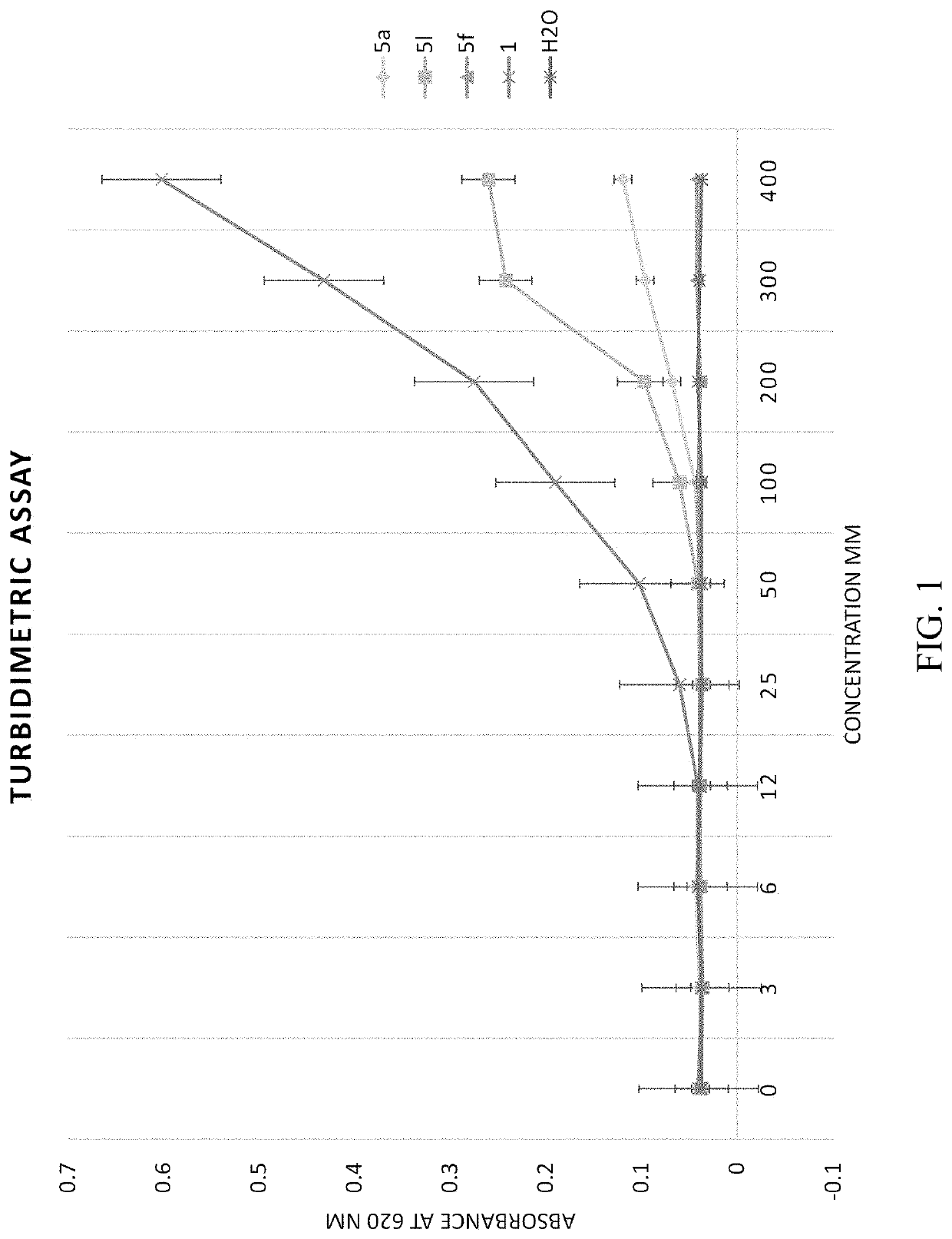
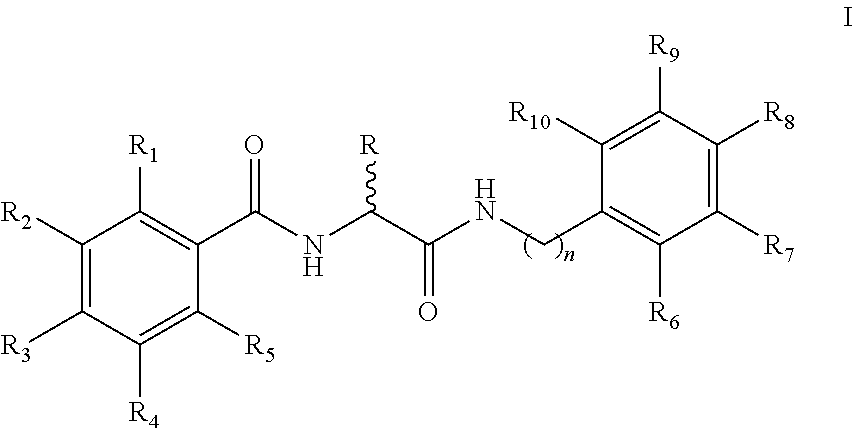
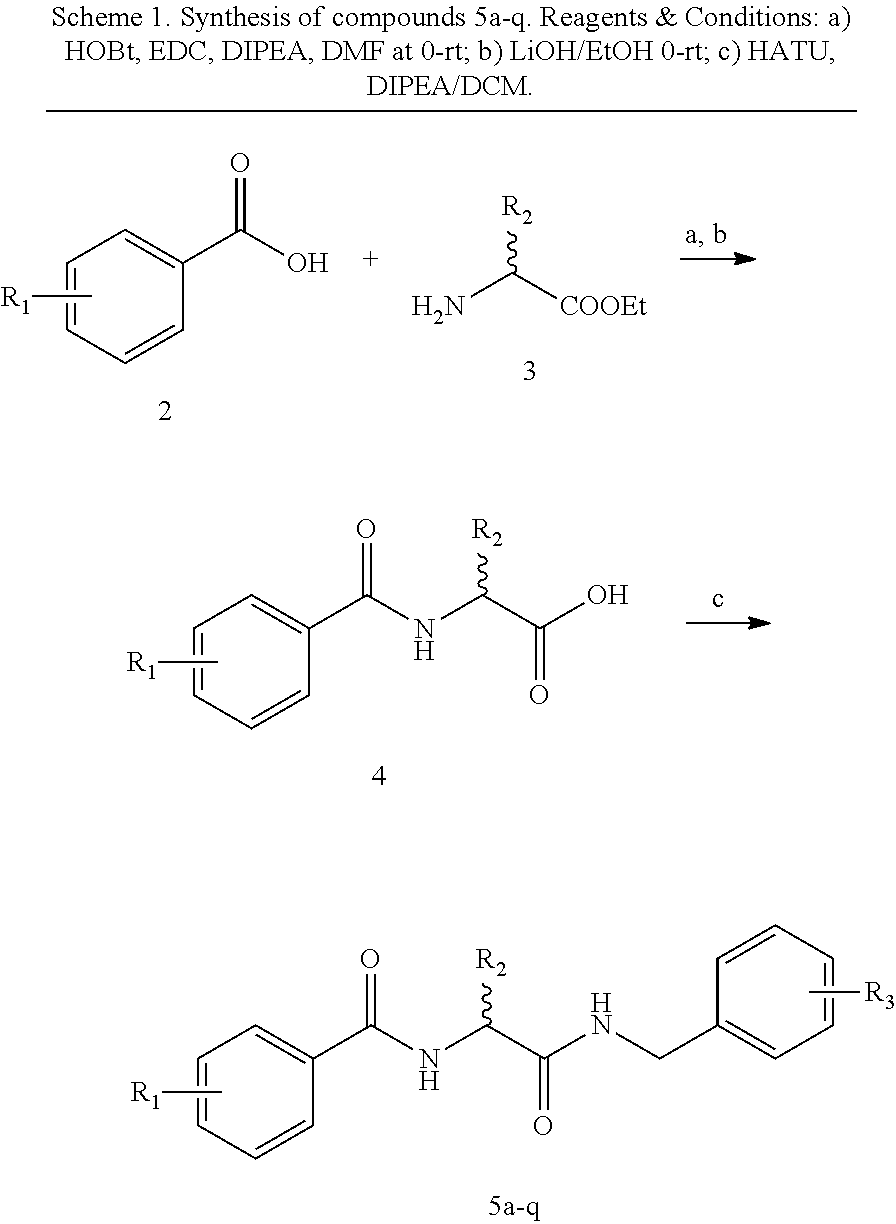
[0080]Synthesis of N-(2-(Benzylamino)-2-oxoethyl)benzamide 5a-q analogs is outlined in Scheme 1 and substituents of the various analogs are listed in Table 1. Commercially available substituted benzoic acids 2 were coupled with corresponding substituted ethyl glycinates 3 in the presence of EDC / HOBt in dimethylformamide to y...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com