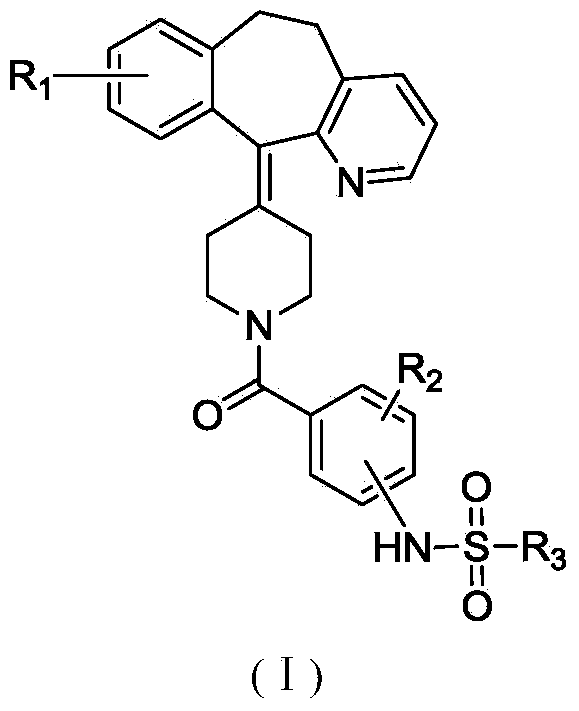
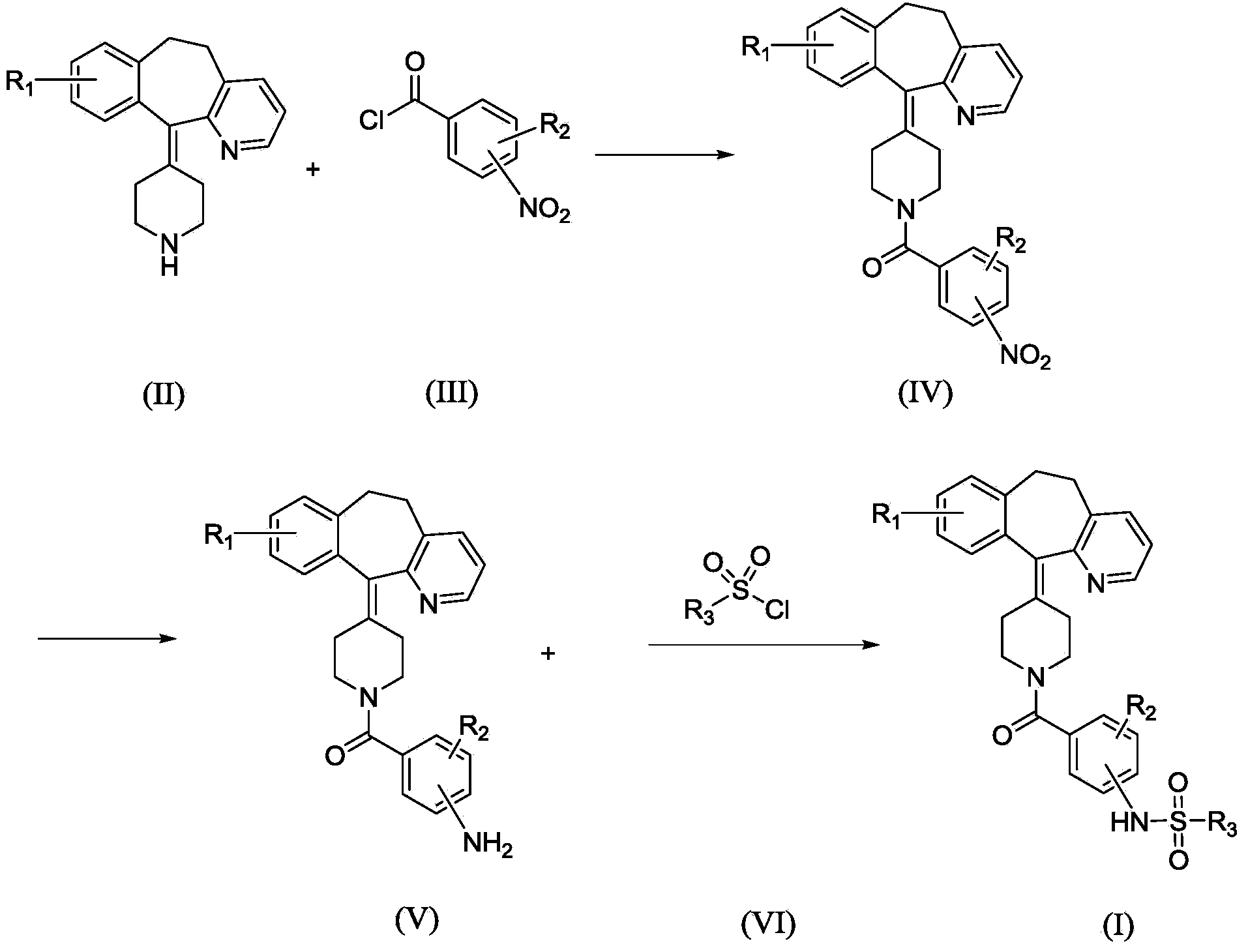
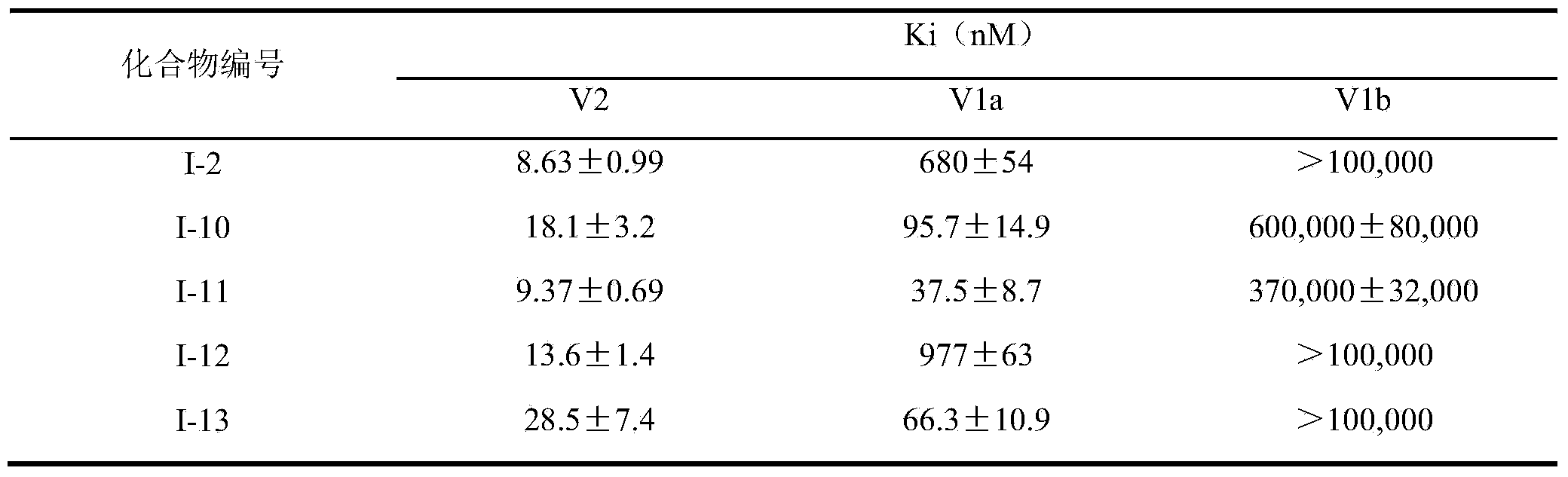
Sulfonamide compound, and preparation method and application thereof
A compound and low-level technology, applied in the field of medicine, can solve the problems of insufficient physicochemical properties of benzoazepines in terms of activity and side effects, and achieve the effect of obvious antagonism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055]
[0056] II-1 (100g, 322mmol) was placed in a 2000mL reaction flask, and CH was added 2 Cl 2 (1000mL) was stirred to make it dissolve, added triethylamine (50g, 490mmol), stirred at room temperature, added intermediate III-1 (59.8g, 322mmol) in batches, kept the temperature and stirred for 8h, TLC detection showed that the reaction ended ( Developing agent ethyl acetate:petroleum ether=1:3).
[0057] The reaction solution was poured into 500ml of cold water, fully shaken to separate the layers, and the organic layer was separated and washed three times in succession. The organic layer was dried over anhydrous sodium sulfate and left overnight. After filtration, the solvent was evaporated to dryness under reduced pressure to obtain a light yellow solid crude product. The obtained crude product was recrystallized from ethanol to obtain 140.2 g of light yellow solid. The purity is 98.9% (HPLC normalization method), and the yield is 94.7%. ESI-MS: 460.1.
Embodiment 2
[0059]
[0060] II-1 (20g, 64mmol) was placed in a 250mL reaction flask, and CHCl was added 3 (100mL) stirred to dissolve, added pyridine (7.8g, 98mmol), stirred at 50°C, added intermediate III-2 (12.5g, 67mmol) in batches, kept the temperature and stirred for 5h, TLC detection showed that the reaction was complete (developed Agent ethyl acetate: petroleum ether = 1:3).
[0061] The reaction solution was poured into 100ml of cold water, fully shaken to separate the layers, and the organic layer was separated and washed three times in succession. The organic layer was dried over anhydrous sodium sulfate and allowed to stand overnight. After filtration, the solvent was evaporated to dryness under reduced pressure to obtain a light yellow solid crude product. The obtained crude product was purified by silica gel column chromatography to obtain 23.7 g of a white solid. The purity is 99.9% (HPLC normalization method), and the yield is 80.6%. ESI-MS: 460.1.
Embodiment 3
[0063]
[0064] Put II-2 (20g, 72mmol) in a 250mL reaction flask, add pyridine (60mL), stir to dissolve, stir at -5°C, add intermediate III-3 (15.2g, 76mmol) in batches, keep stirring at the temperature After 24 hours, TLC detection showed that the reaction was complete (developing agent ethyl acetate:petroleum ether=1:3).
[0065] The reaction solution was poured into 300ml of cold water, stirred, and solids were precipitated. After filtering, the filter cake was washed with water and dried to obtain a yellow crude product. The crude product was recrystallized from ethanol to obtain 29.5 g of white solid. The purity is 98.3% (HPLC normalization method), and the yield is 93.1%. ESI-MS: 440.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com