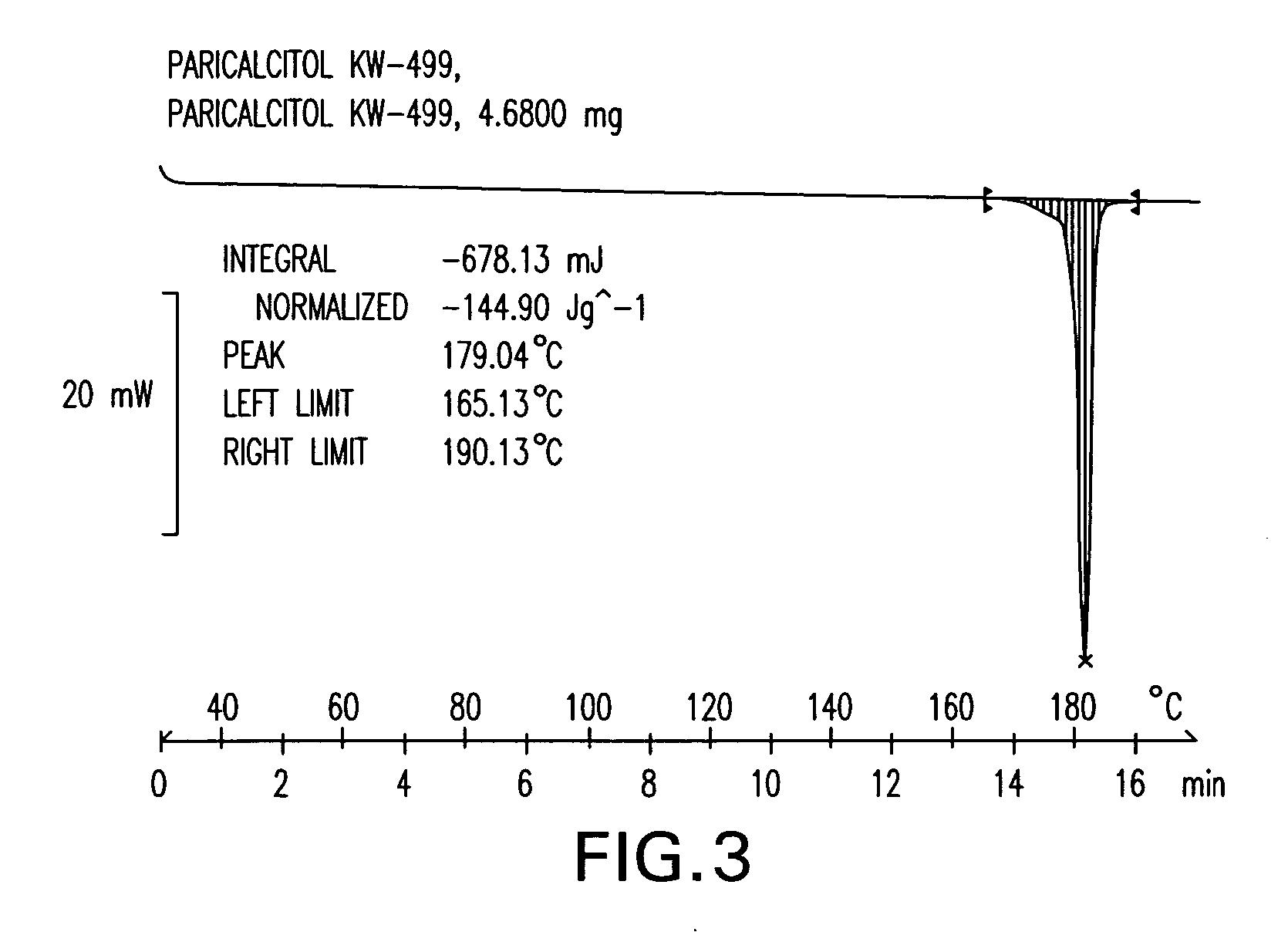
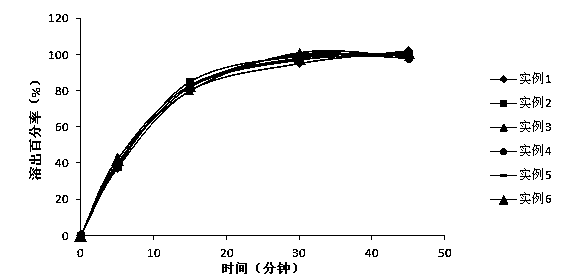
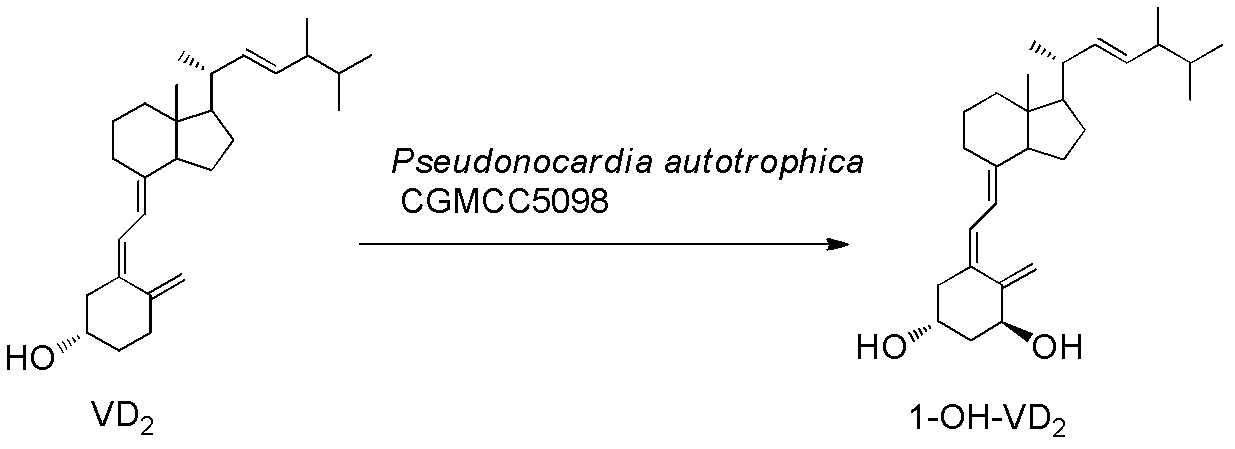
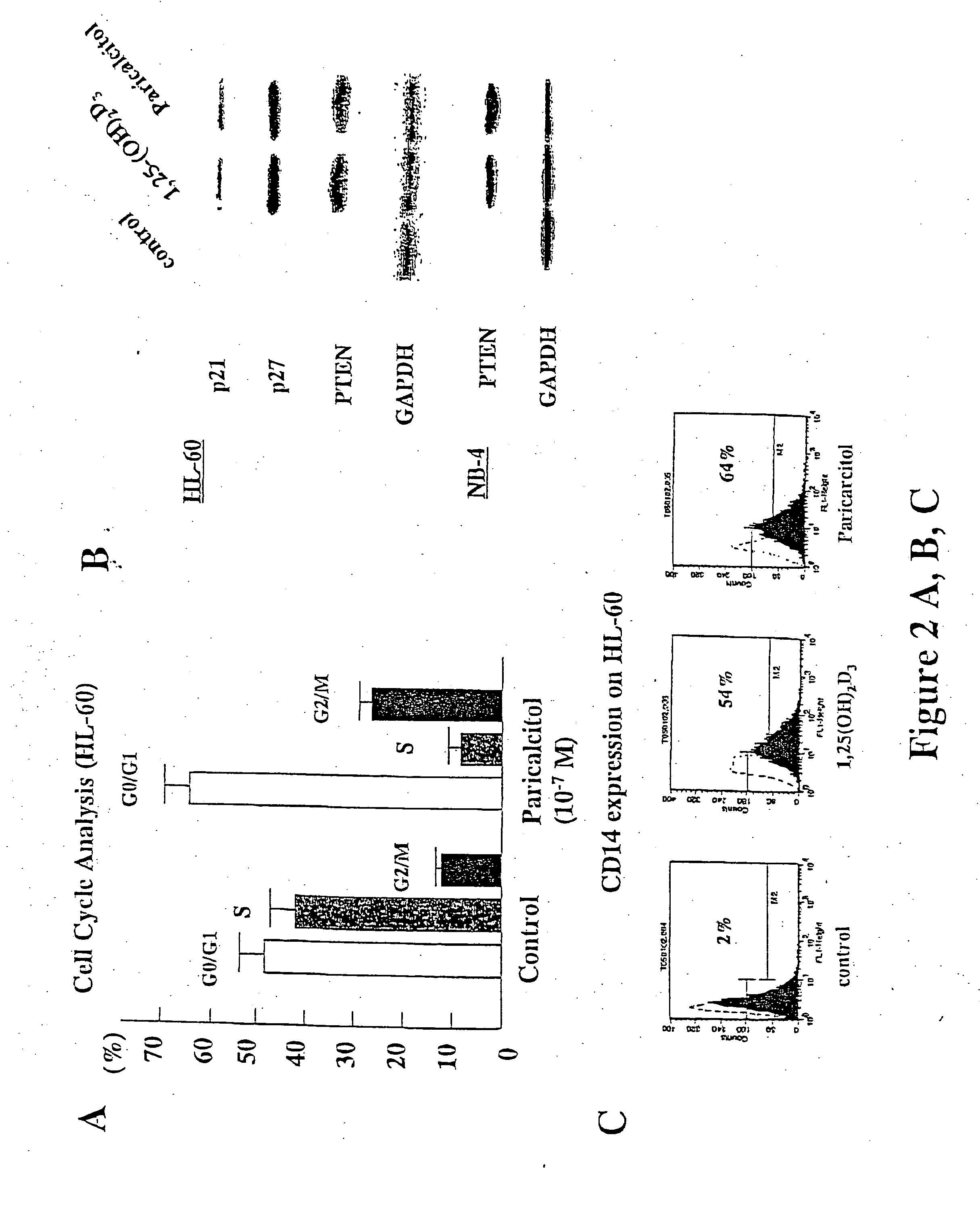
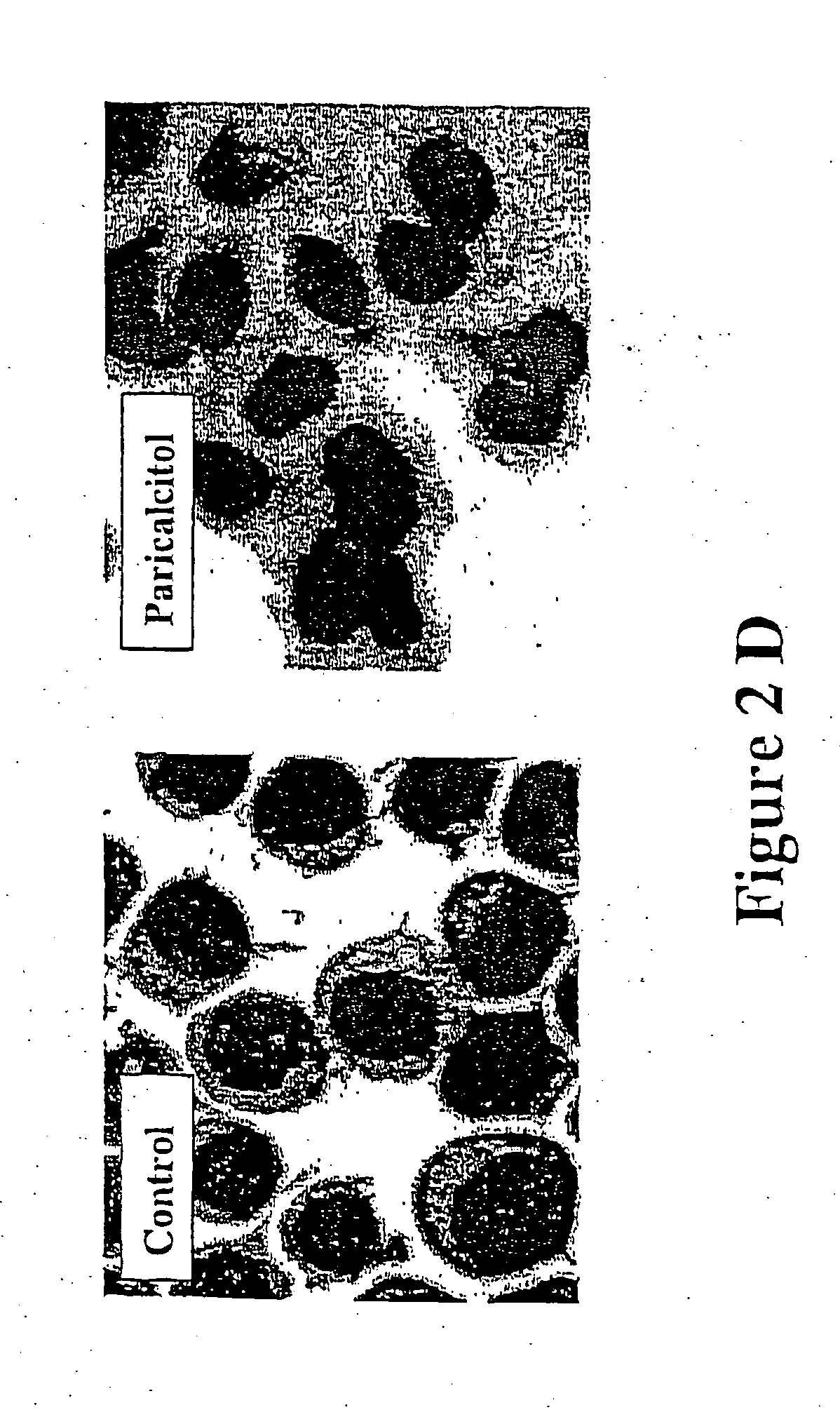
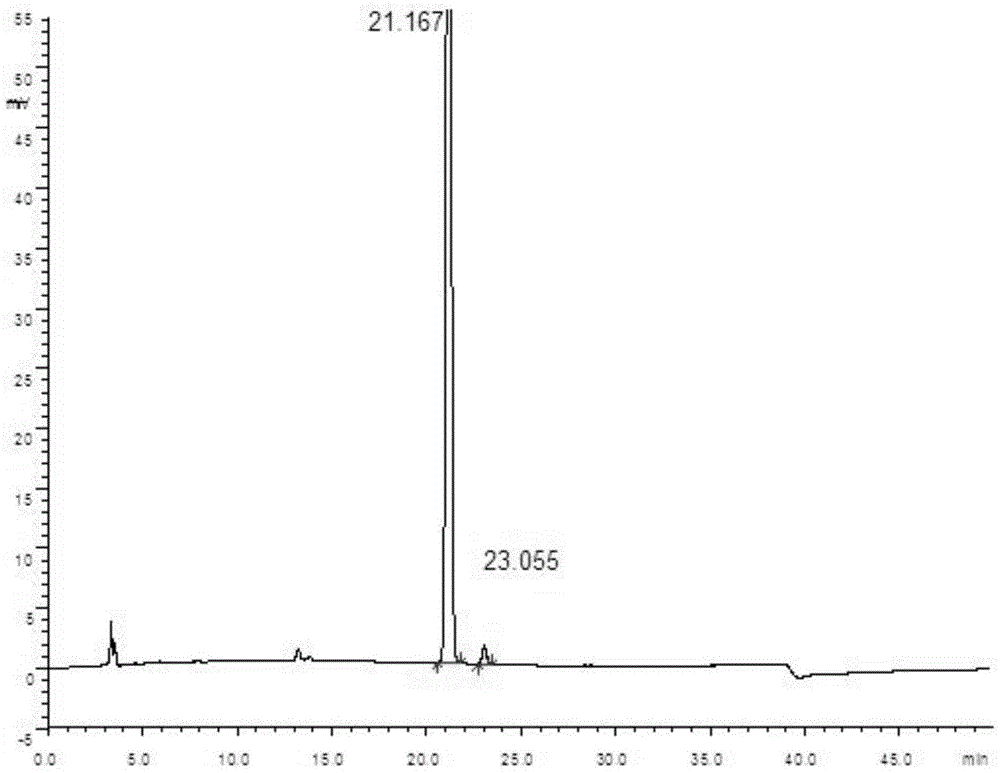
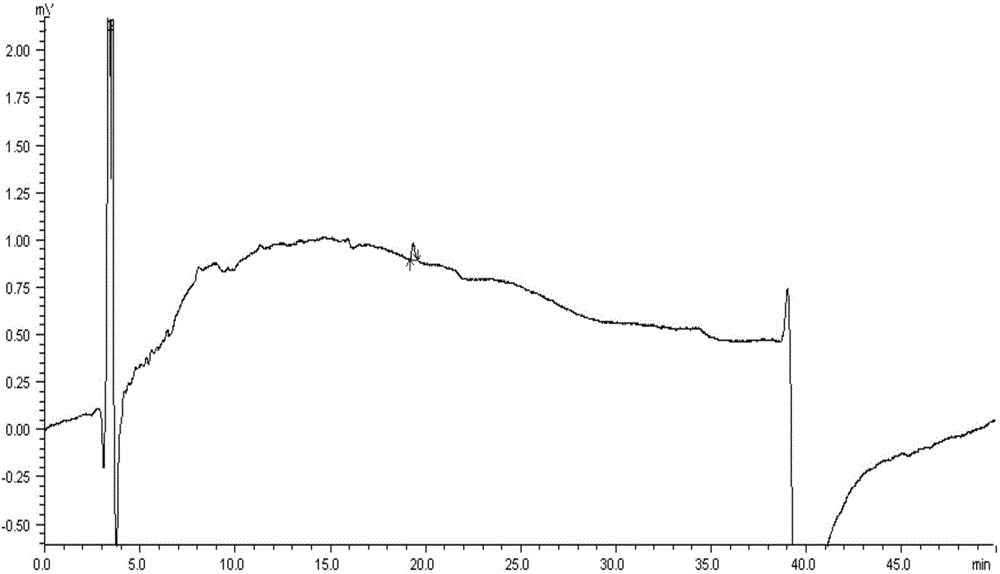
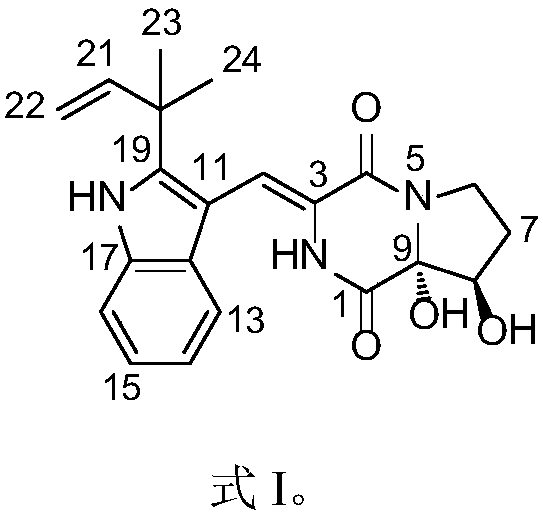
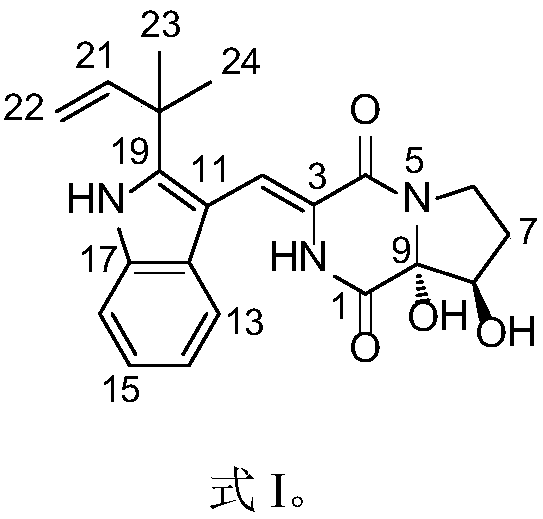
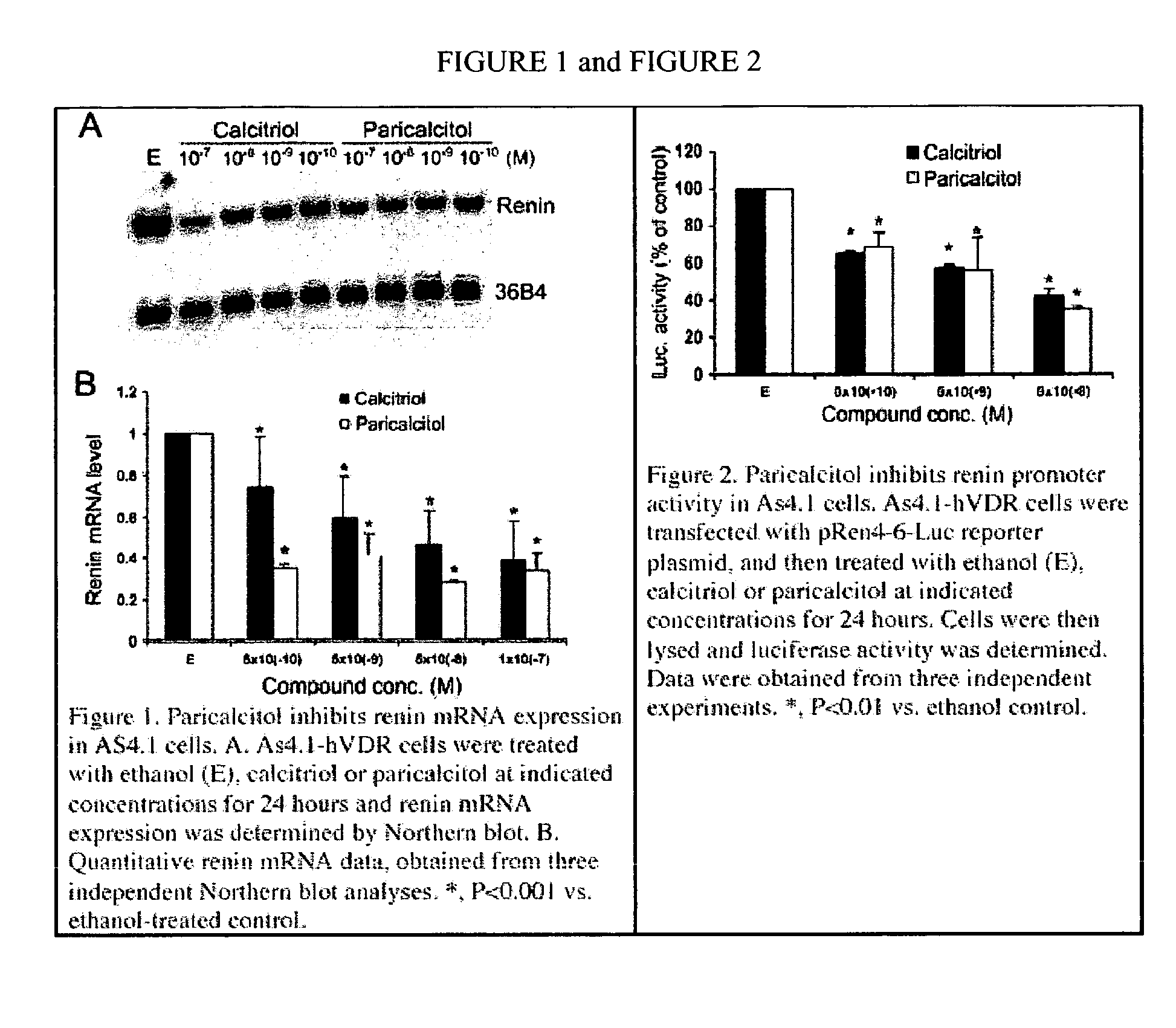
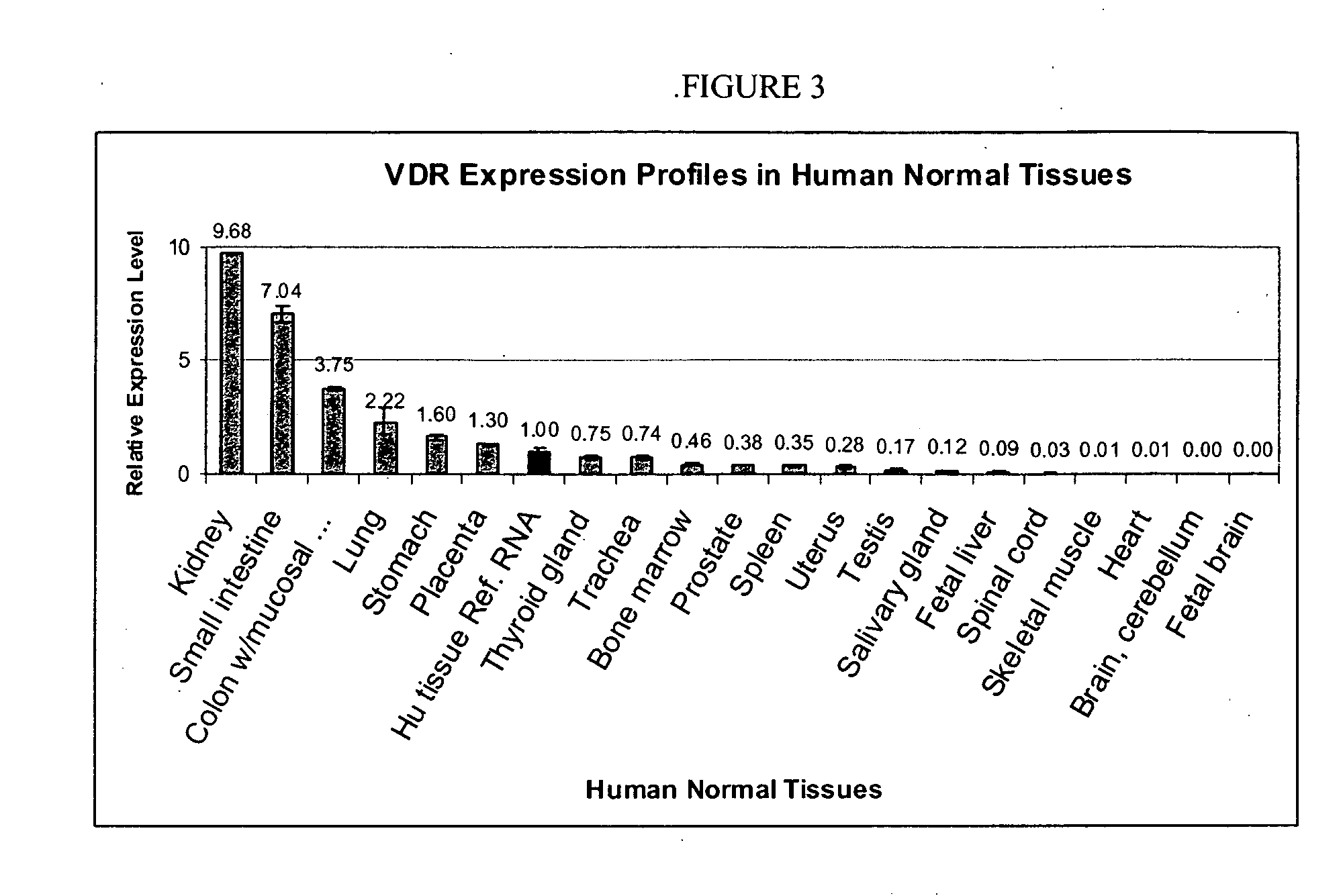
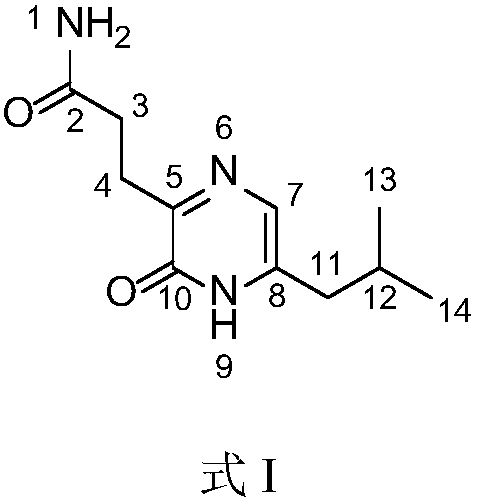
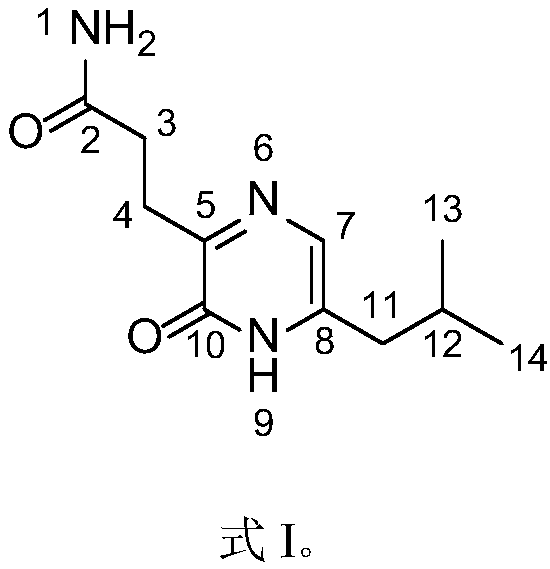
Patents
Literature
78 results about "Paricalcitol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
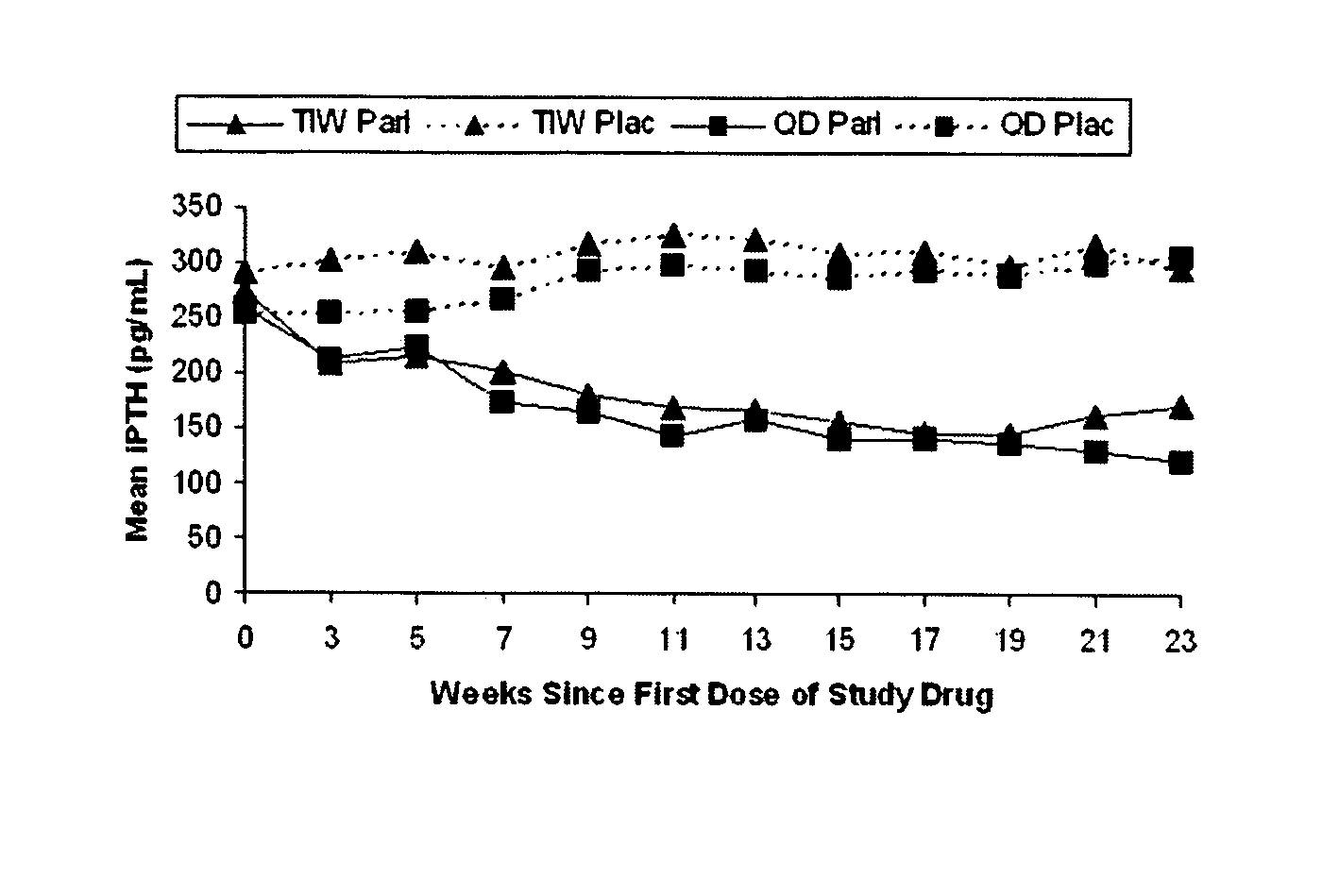
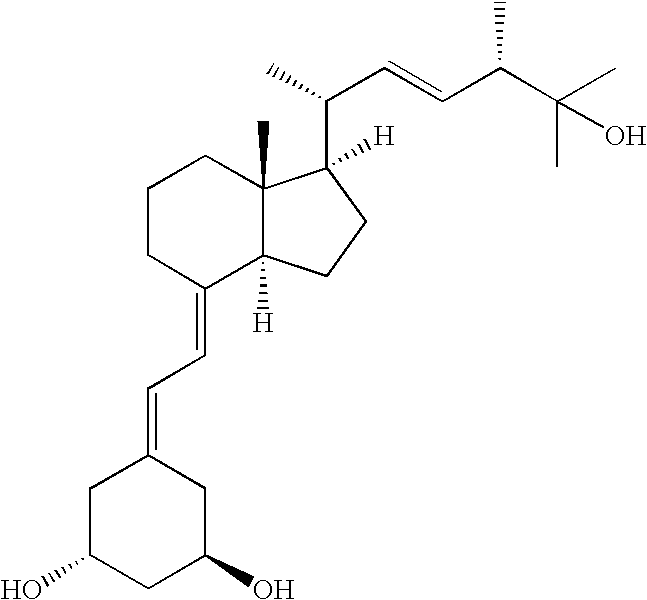
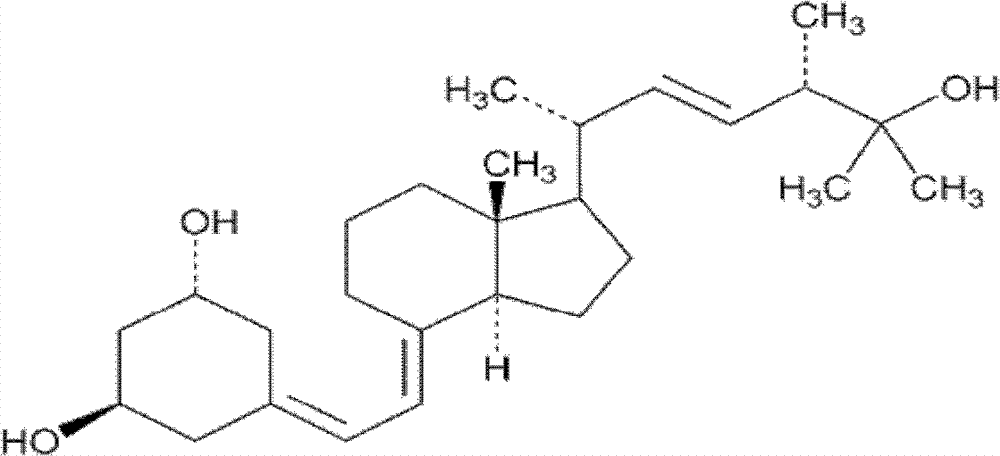
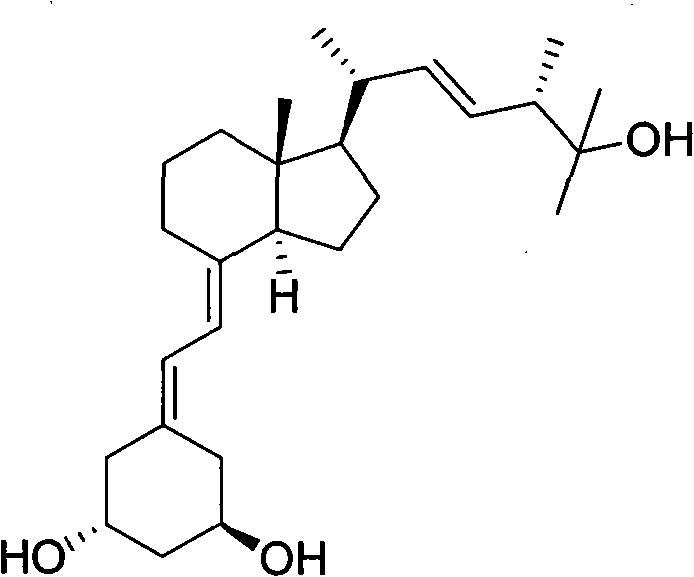
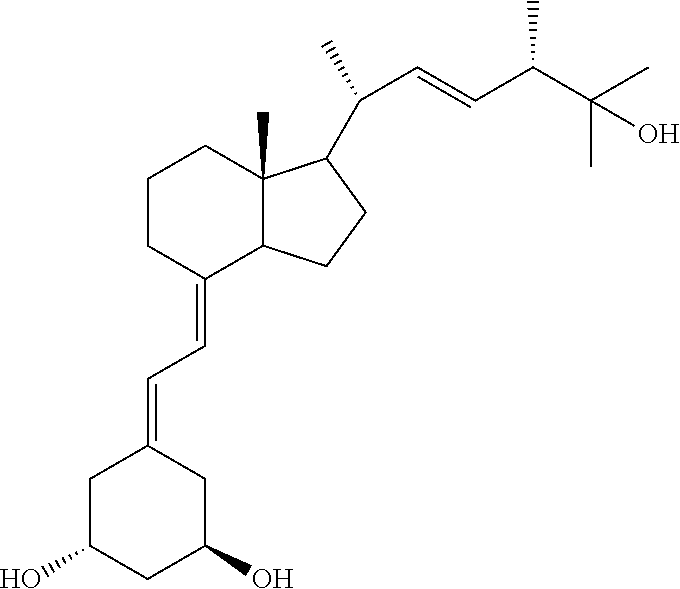
Paricalcitol is used to treat and prevent high levels of a certain natural substance made by the body (parathyroid hormone) in patients with long-term kidney disease. In these patients, the high level of parathyroid hormone is caused by a low level of calcium and a certain kind of vitamin D.
Use of vitamin Ds to treat kidney disease
InactiveUS20050124591A1Reduce inflammationSuppression problemBiocideUrinary disorderDiseaseAngiotensin Receptor Blockers
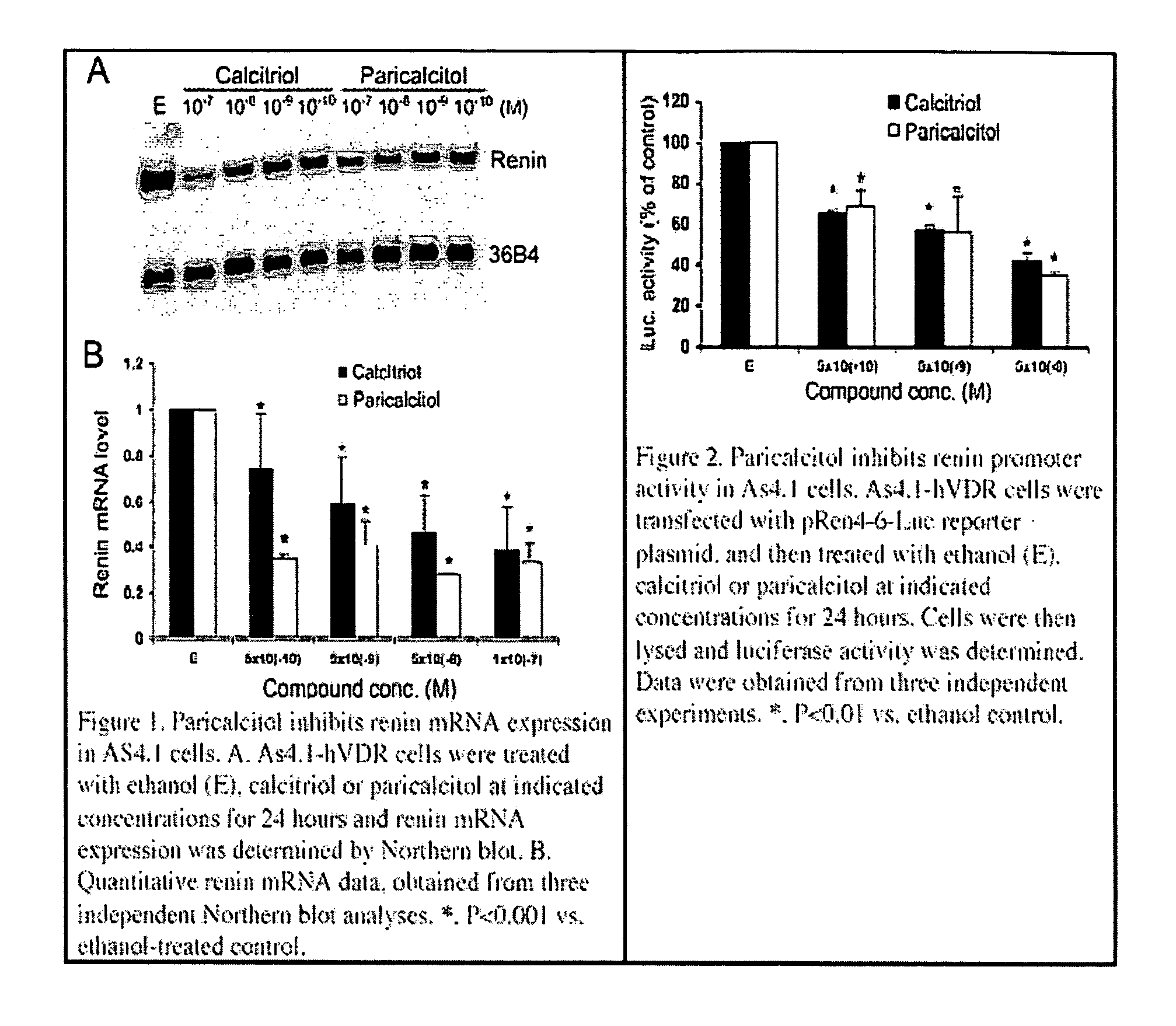
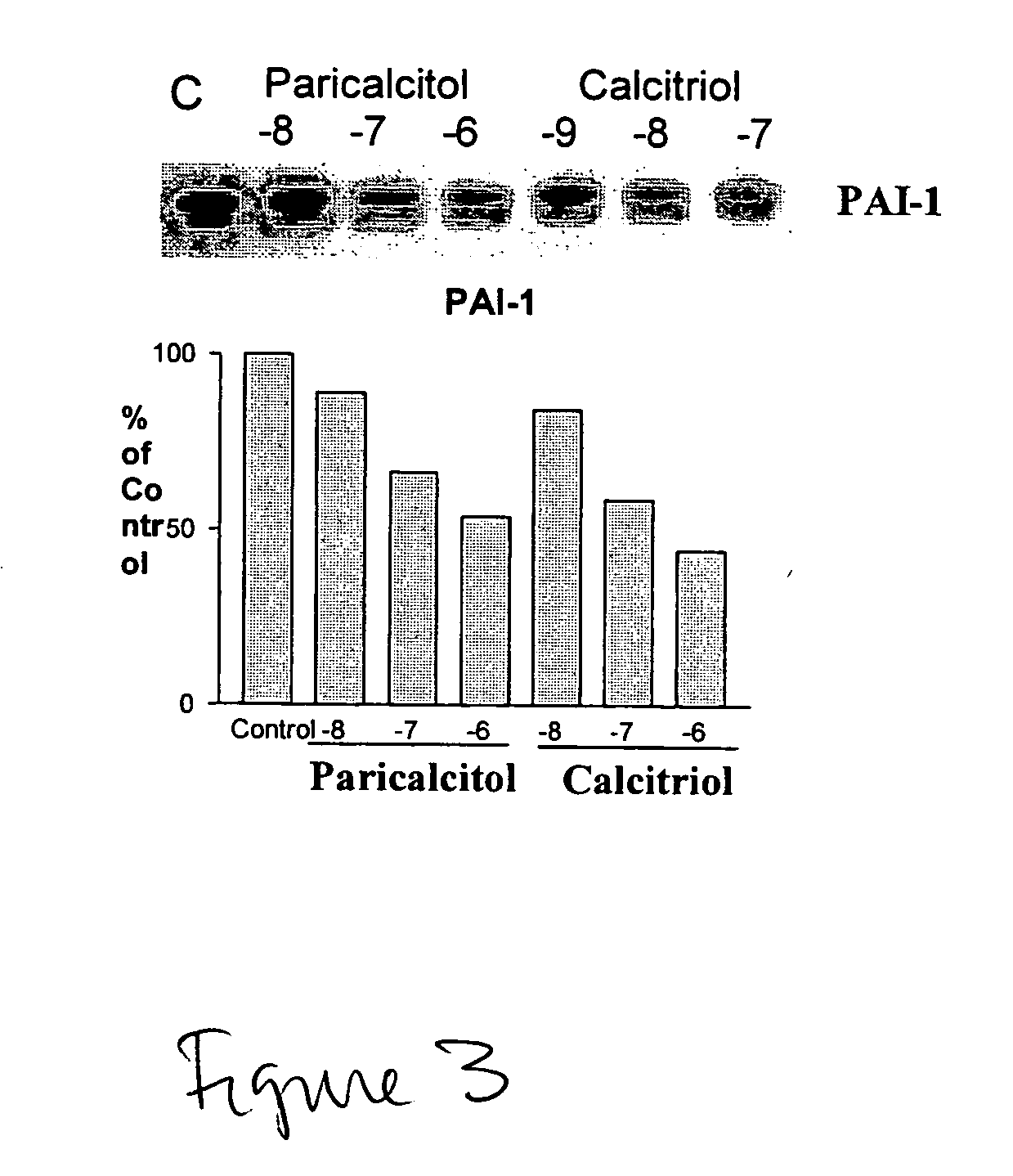
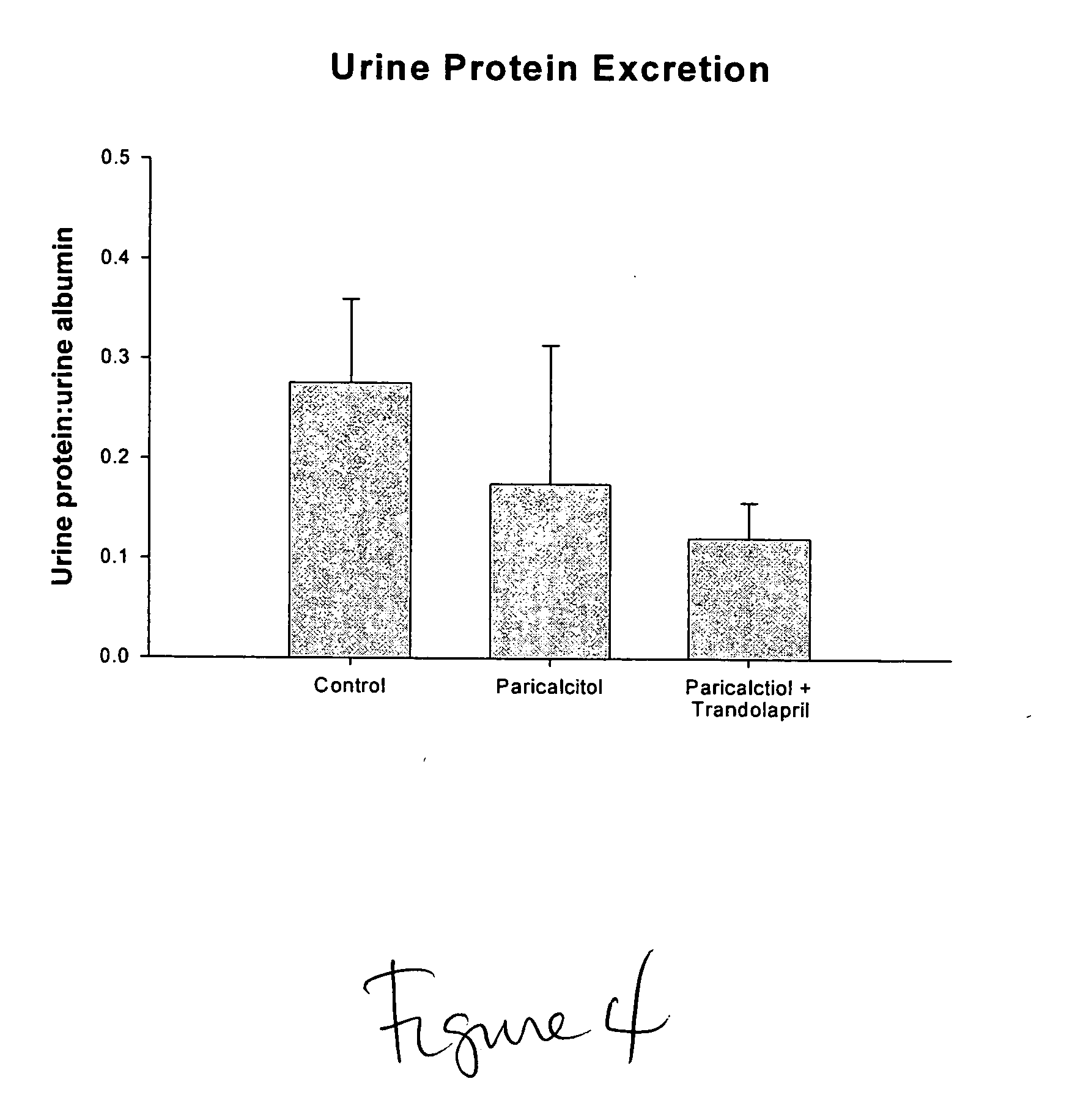
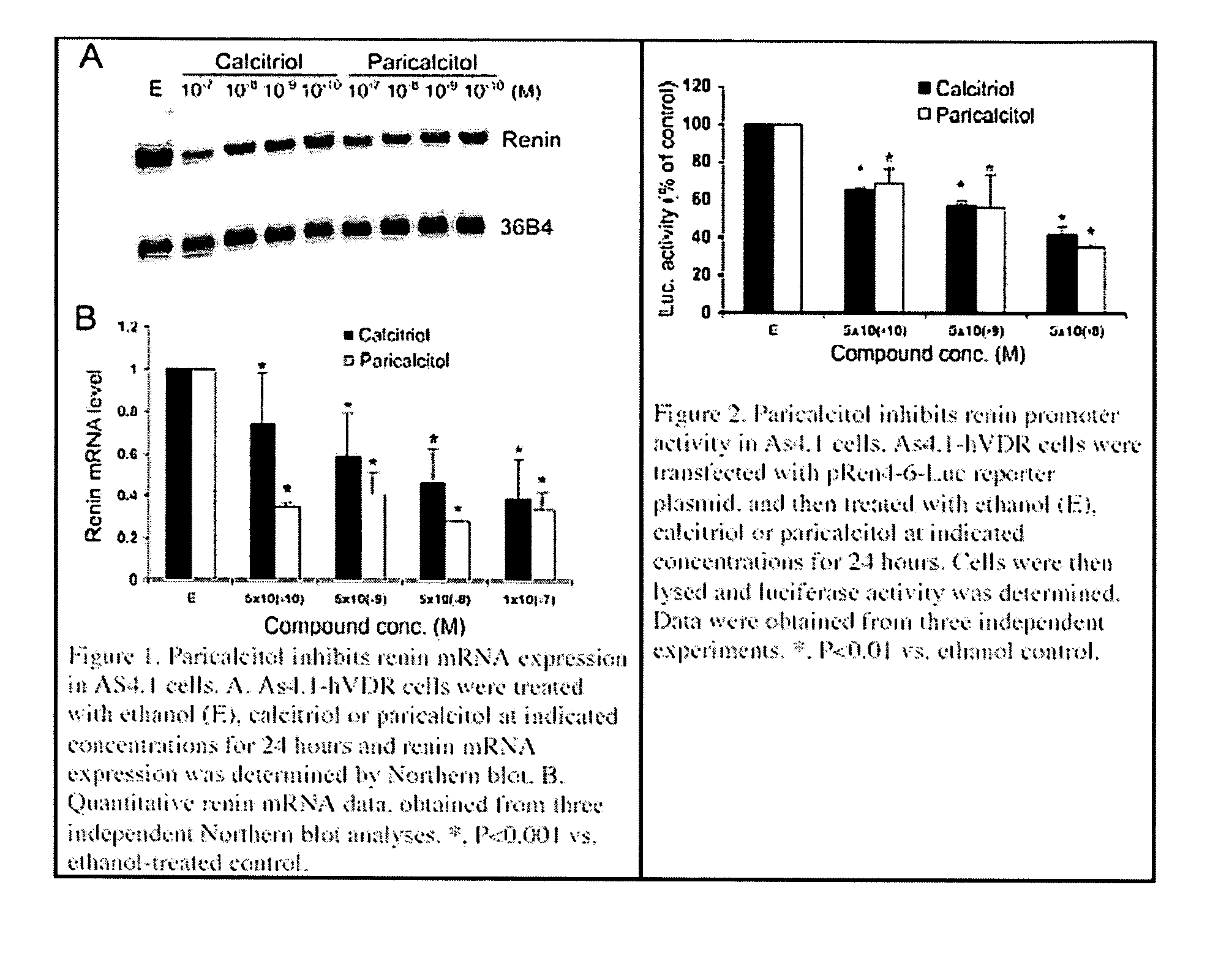
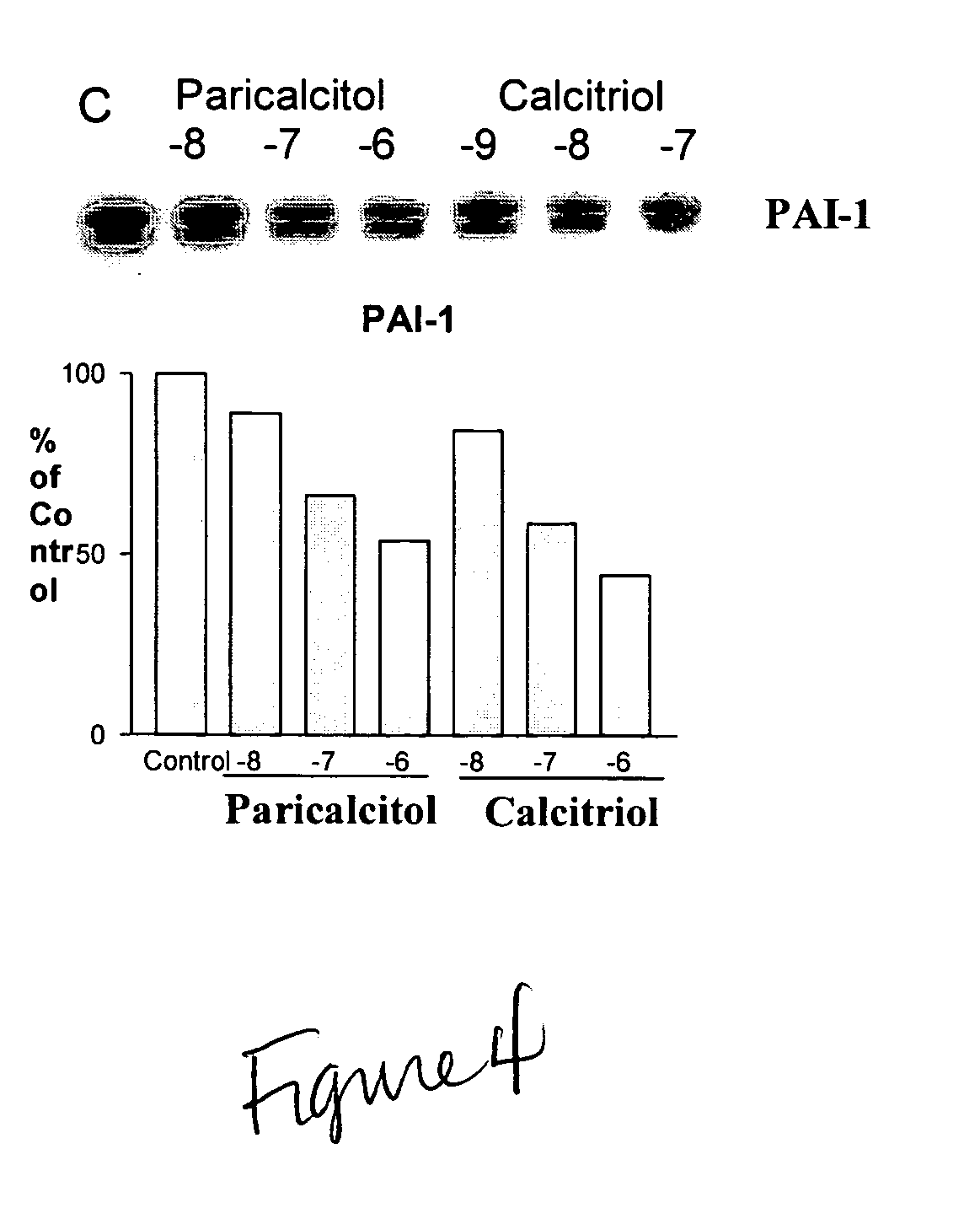
Disclosed are compositions containing a VDRA / Vitamin D analog to treat or prevent kidney disease, including chronic kidney disease. The present invention also relates to methods of treating kidney disease by administering to a patient a pharmaceutical composition containing a therapeutically effective amount of a VDRA / Vitamin D analog. Compositions according to the invention include a VDRA / Vitamin D analog and at least one of the following agents: an ACE inhibitor, an angiotensin (II) receptor blocker (ARB) and aldosterone blocker in therapeutically effective amounts to inhibit renin production or inhibit activation of the renin-angiotensin-aldosterone system. Preferred compositions contain paricalcitol with at least one of these other agents. Such combinations can avoid ACE inhibition escape and aldosterone escape with subsequent increase in angiotensin (II) and aldosterone generation.
Owner:ABBOTT LAB INC
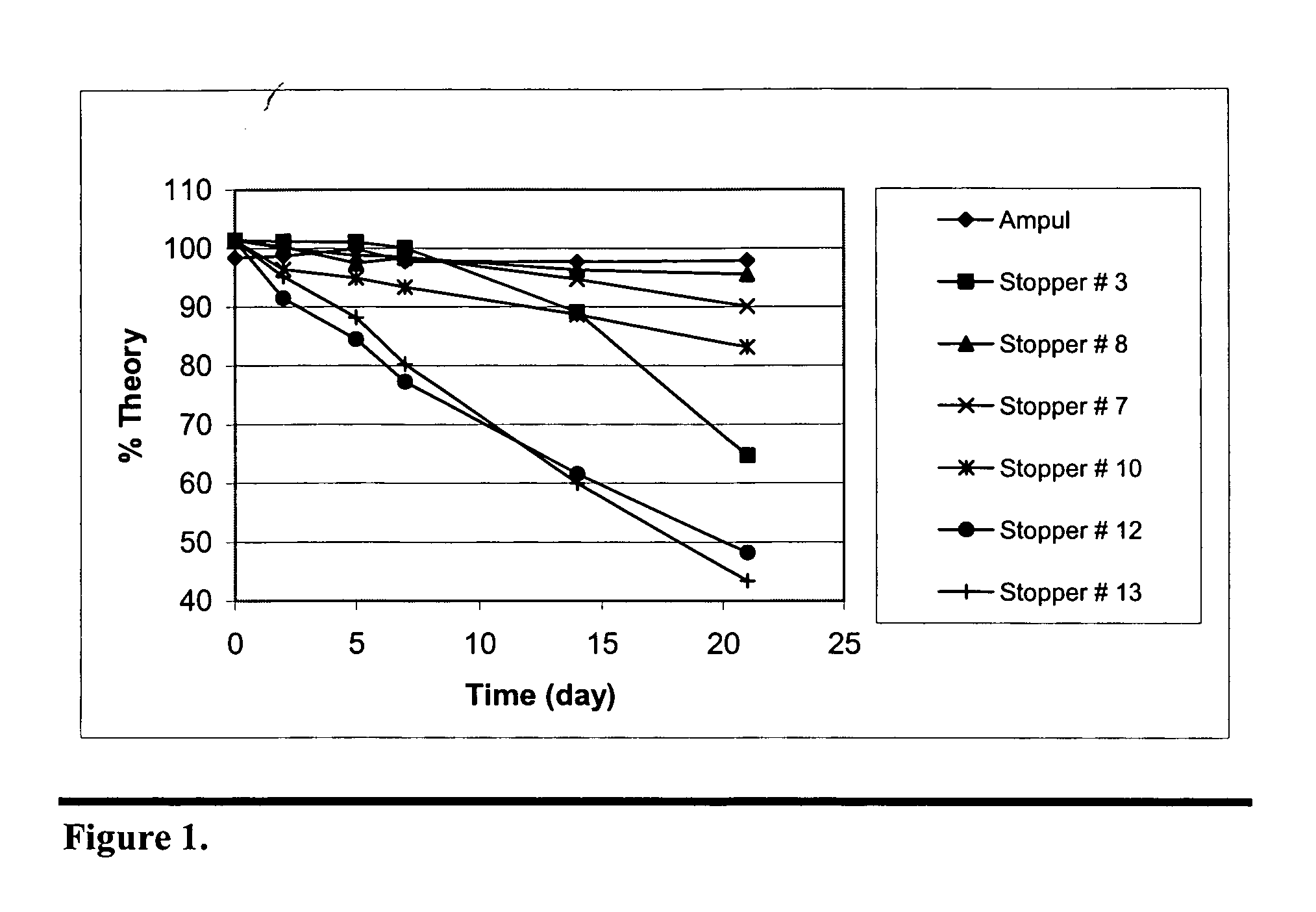
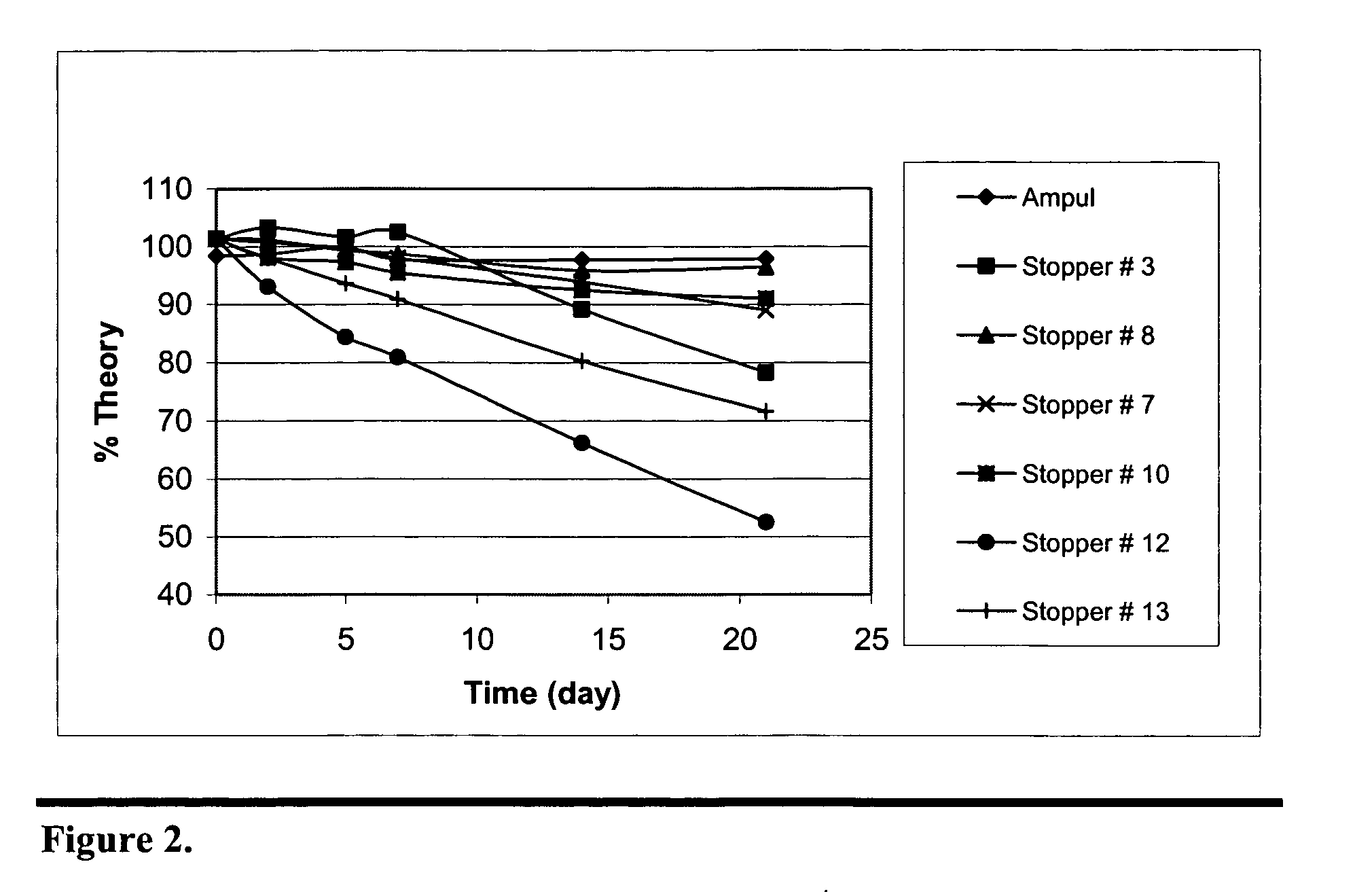
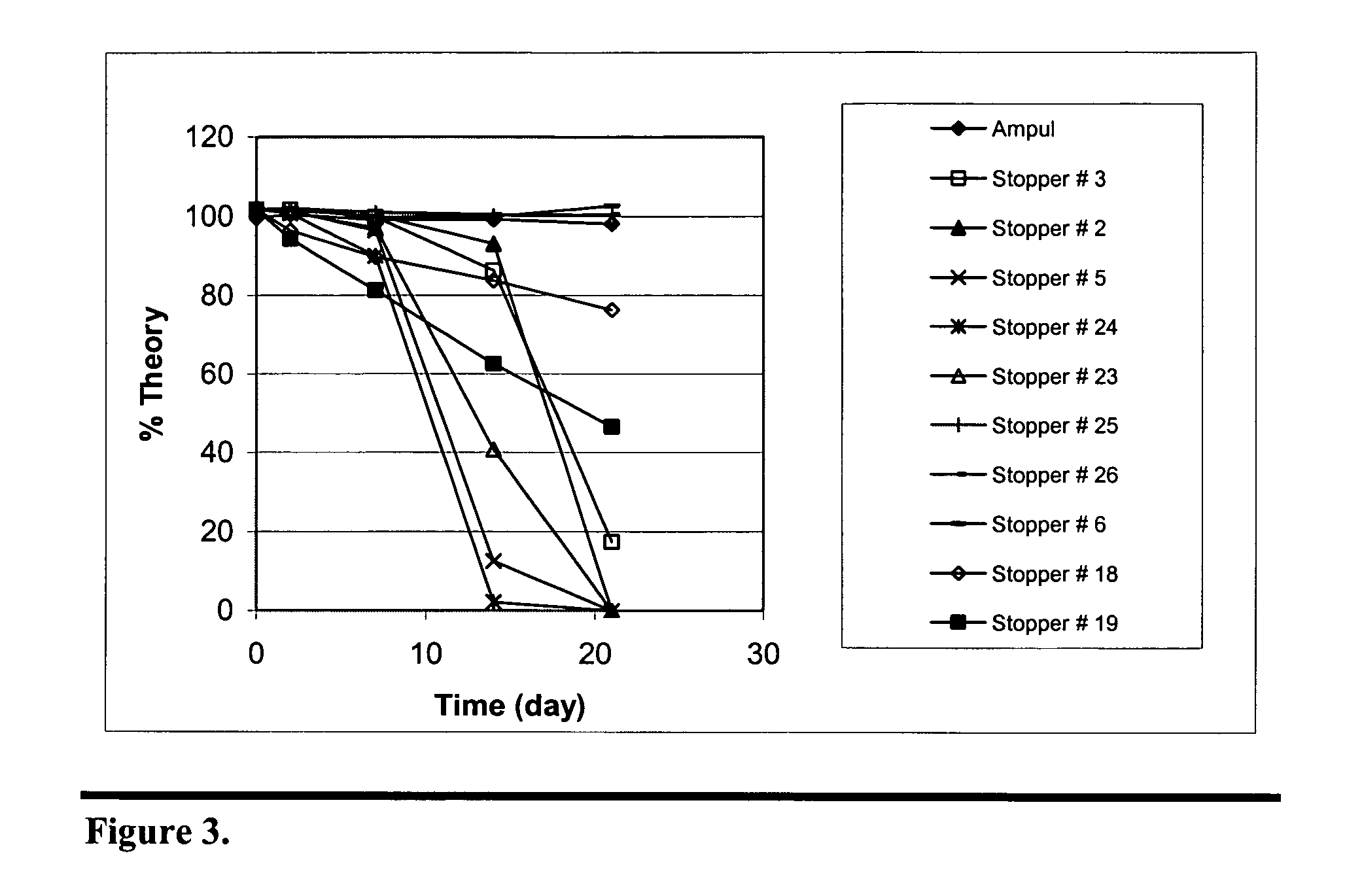
Stabilization of paricalcitol using chlorobutyl or chlorinated butyl stoppers
InactiveUS20070166187A1Extended shelf lifeImprove drug stabilityPharmaceutical containersPharmaceutical delivery mechanismParicalcitolEnvironmental chemistry
This invention relates to a method of enhancing the stability of paricalcitol solution in a container by using a chlorobutyl or chlorinated butyl stopper in the container.
Owner:ABBOTT LAB INC
Use of Vitamin Ds to treat kidney disease
InactiveUS20050148557A1Reduce inflammationSuppression problemBiocideUrinary disorderDiseaseAldosterone escape
Disclosed are compositions containing a VDRA / Vitamin D analog to treat or prevent kidney disease, including chronic kidney disease. The present invention also relates to methods of treating kidney disease by administering to a patient a pharmaceutical composition containing a therapeutically effective amount of a VDRA / Vitamin D analog. Compositions according to the invention include a VDRA / Vitamin D analog and at least one of the following agents: an ACE inhibitor, an angiotensin (II) receptor blocker (ARB) and aldosterone blocker in therapeutically effective amounts to inhibit renin production or inhibit activation of the renin-angiotensin-aldosterone system. Preferred compositions contain paricalcitol with at least one of these other agents. Such combinations can avoid ACE inhibition escape and aldosterone escape with subsequent increase in angiotensin (II) and aldosterone generation.
Owner:UNIVERSITY OF CHICAGO
Oral formulations of paricalcitol
The present invention relates to oral formulations comprising paricalcitol that are available in a variety of different dosage forms that are bioequivalent to one another.
Owner:ABBOTT LAB INC
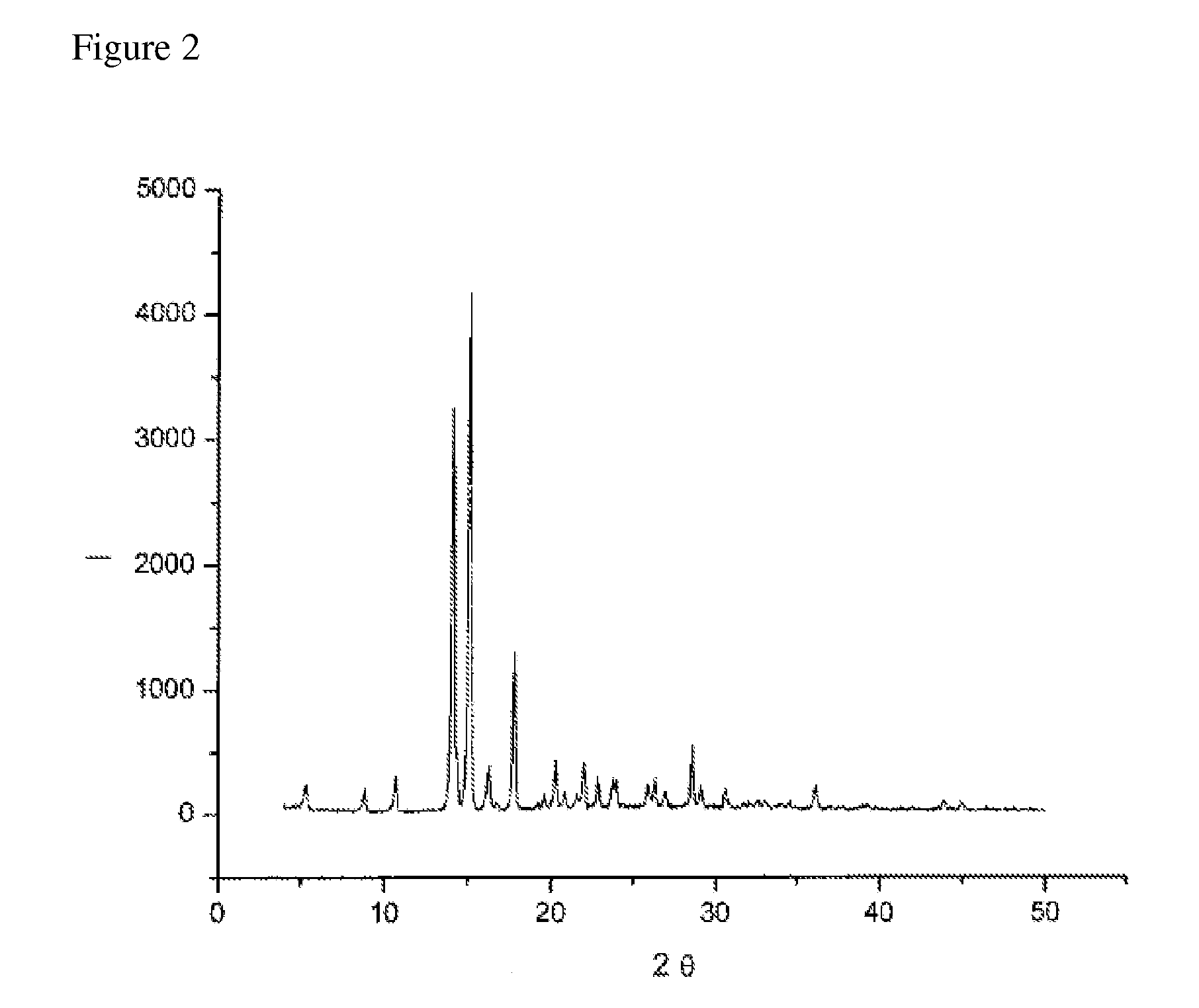
Preparation of paricalcitol and crystalline forms thereof
The present invention is directed to a novel process for preparing Paricalcitol wherein Paricalcitol, dissolved in a solvent, is precipitated from a concentrated or seeded solution. The present invention also provides crystalline forms of paricalcitol and processes for their preparations.
Owner:TEVA PHARM USA INC
Stable paricalcitol pharmaceutical composition and preparation method thereof
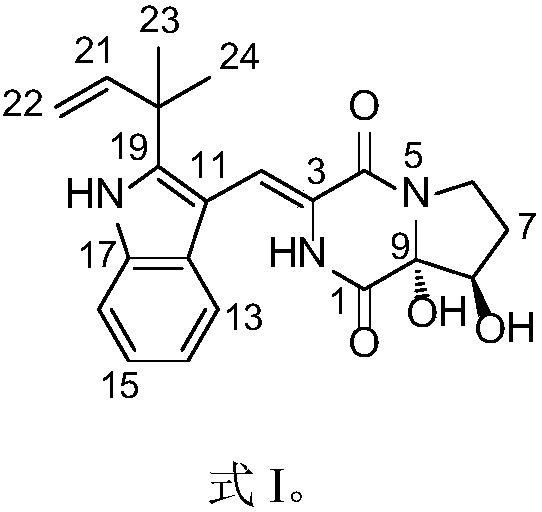
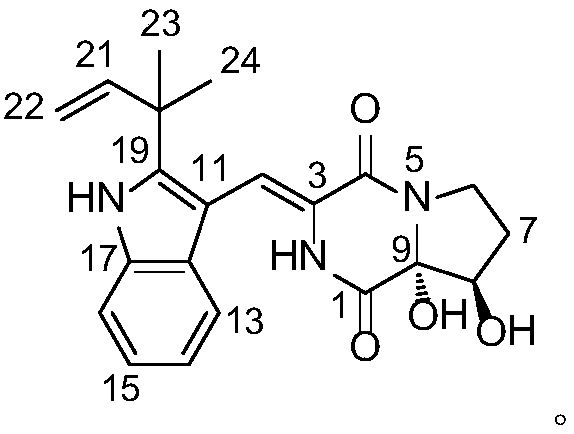
The invention discloses a high-stability paricalcitol pharmaceutical composition and a preparation method thereof. The medicinal composition comprises a cyclodextrin inclusion compound containing an active component paricalcitol and other pharmaceutically acceptable carriers, and is prepared by adopting a freeze-drying dry granulation process and strictly controlling temperature and humidity in the preparation process. The paricalcitol is included by cyclodextrin, and the freeze-drying and dry granulating preparation process is adopted, so that the defects that the main drug paricalcitol is extremely easily hygroscopic and extremely poor in stability and cannot be prepared into oral preparations can be greatly overcome, and the disintegration or main drug component dissolution cannot be influenced; and the paricalcitol pharmaceutical composition is not a coated tablet, so that the defect of slow dissolution of a conventional coated tablet can be avoided.
Owner:BEIJING TIDE PHARMA
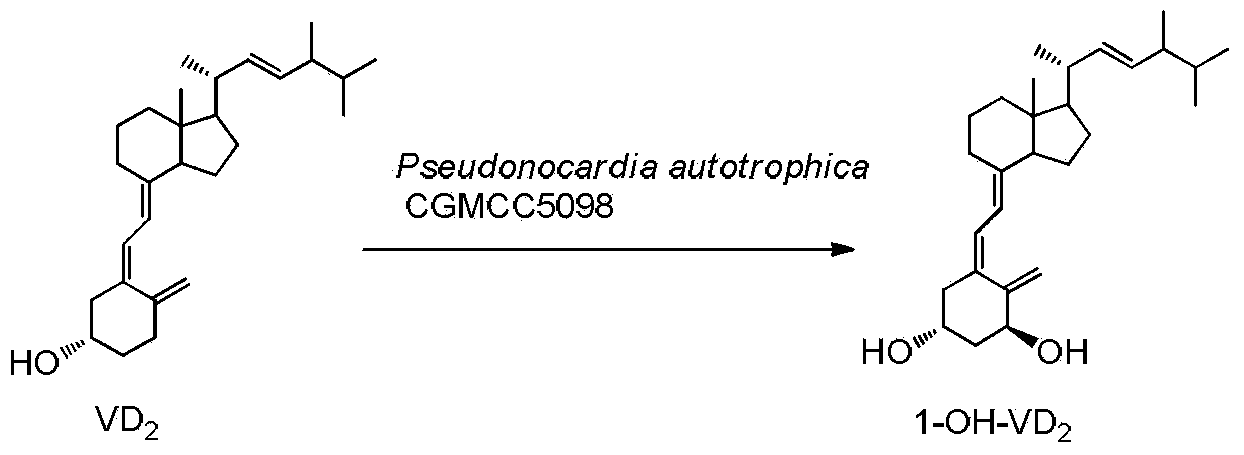
Method for preparing 1alpha-hydroxyl vitamin D through microbial transformation
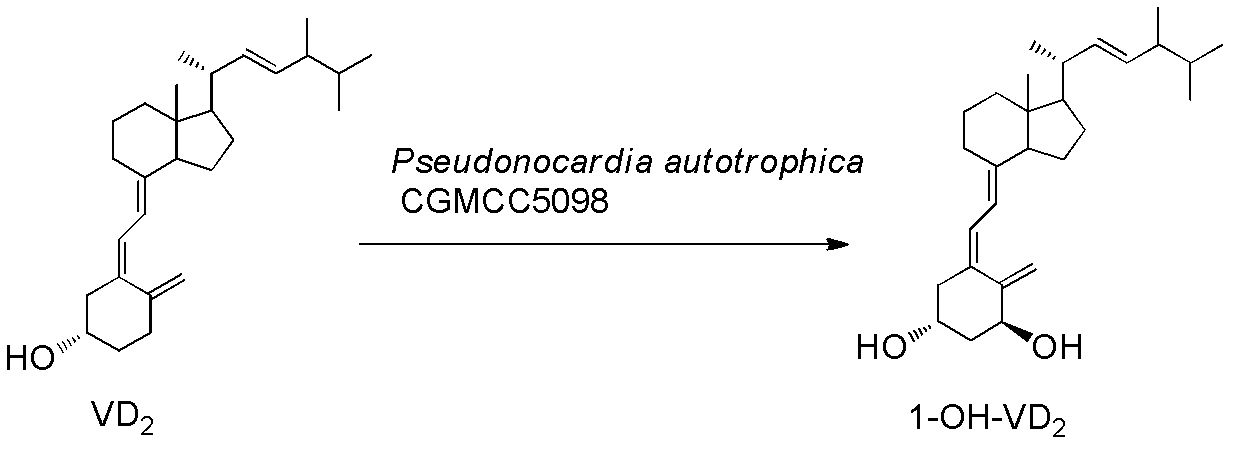
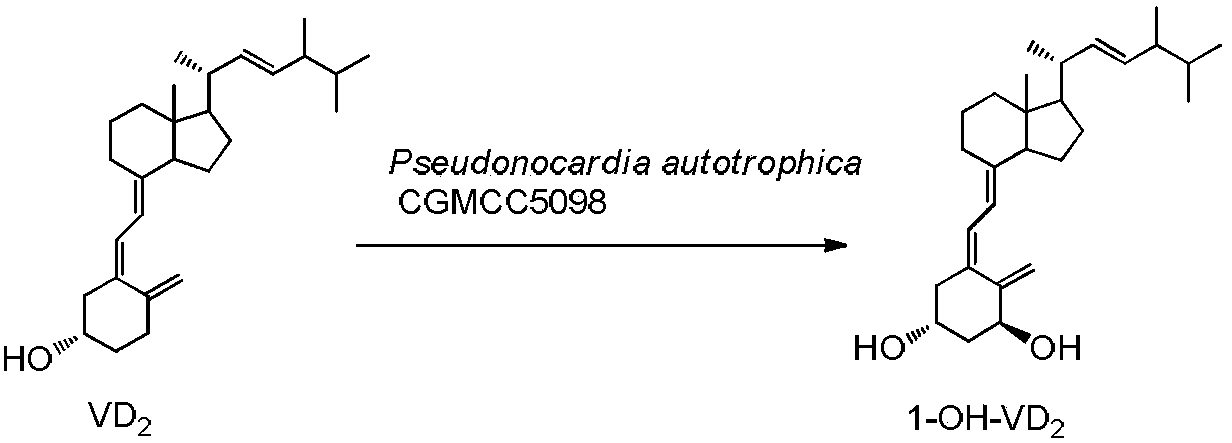
InactiveCN102321678AMild transformation conditionsRegionally selectiveMicroorganism based processesFermentationManufacturing cost reductionChemical reaction
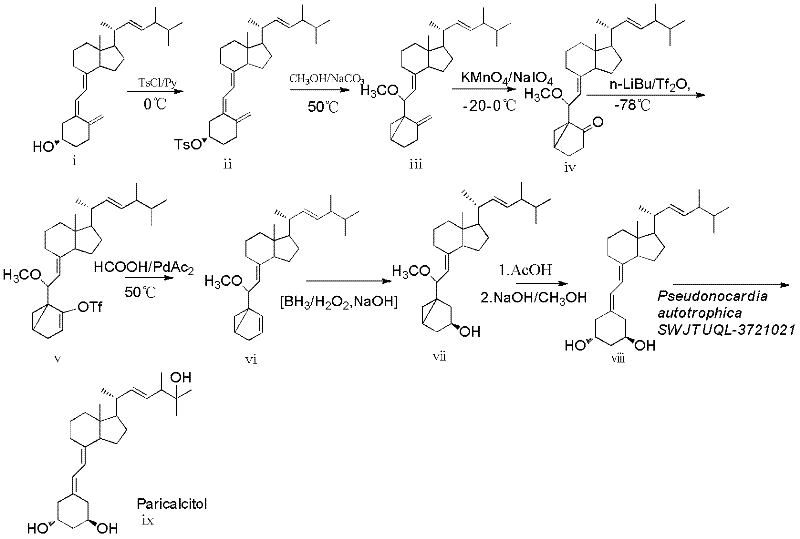
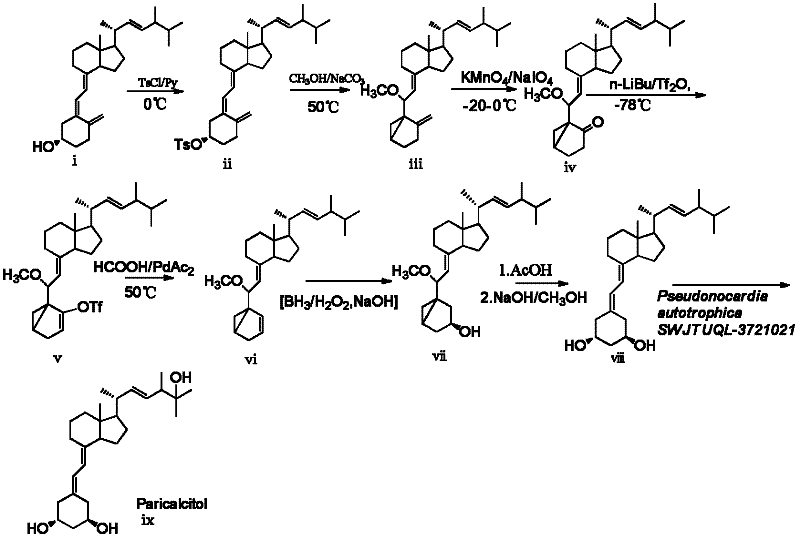
The invention discloses a novel industrial preparation method for a medicament 1-alpha-hydroxyl vitamin D2 or D3, namely paricalcitol and doxercalciferol, for treating osteoporosis. Site 1 of the vitamin D is subjected to microbial transformation by using pseudonocardia CGMCC No. 5098 to directly obtain 1-alpha-hydroxyl vitamin D in one step. The method is milder than a chemical method; protection is not required and toxic selenium dioxide is not required to be used for oxidation; the method has regional selectivity; radical protection is not required; the biotransformation yield reaches 40-50 percent; the method is environmentally-friendly; the process route is greatly shortened through one-step transformation reaction, the yield is improved, and the preparation cost is reduced; the known chemical reaction of about six steps for synthesizing the paricalcitol and the doxercalciferol is shortened into one-step reaction; and the total yield is increased to be 40-50 percent.
Owner:SOUTHWEST JIAOTONG UNIV
Pharmaceutical composition containing paricalcitol
InactiveCN106474086AOvercoming the problem of disintegration time delayHigh dissolution rateOrganic active ingredientsCapsule deliveryAlcoholGlycerol
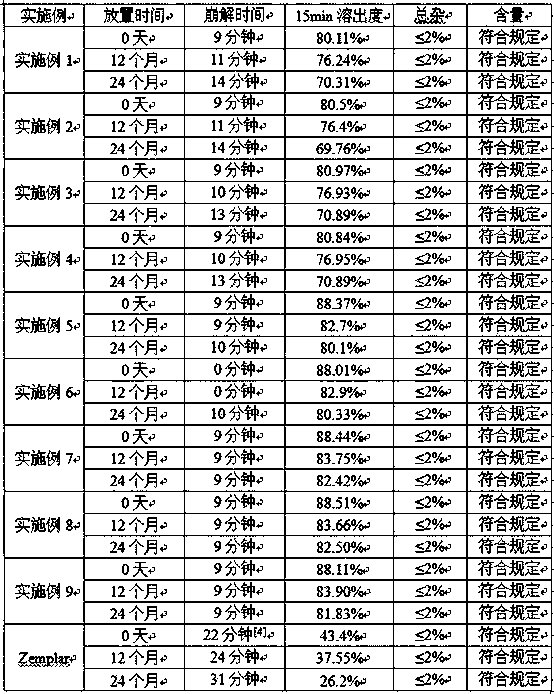
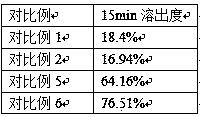
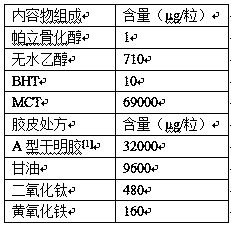
The invention relates to a soft capsule pharmaceutical composition containing paricalcitol. The prescription is characterized in that a content consists of the paricalcitol, absolute ethyl alcohol, BHT and MCT, and a rubber shell consists of dried gelatin, glycerol, titanium dioxide and yellow ferric oxide, wherein in the content, the weight ratio of the paricalcitol to the absolute ethyl alcohol is at 1 to (600-800), the weight ratio of the paricalcitol to the BHT is at 1 to (8.5-15), and the weight ratio of the paricalcitol to the MCT is at 1 to (71000-80000); in the rubber shell, the weight ratio of the various components is at 1 to (0.3-0.8) to (0.005-0.04) to (0.001-0.008) of the dried gelatin to the glycerol to the titanium dioxide to the yellow ferric oxide; and the adopted gelatin is type A gelatin. In addition, the invention also discloses a preparation method of the soft capsule. The paricalcitol soft capsule provided by the invention can get disintegrated and dissolved rapidly, and the paricalcitol soft capsule is good in disintegrating stability and high in disintegrating uniformity in a long-term storage process; and the preparation method is simple and applicable to large-scale industrial production.
Owner:CHENGDU GUOHONG PHARMA
Fat emulsion of Paricalcitol, its preparation and preparation methods thereof
ActiveCN102772364ALess irritatingSafe and stable intravenous injectionPowder deliveryOrganic active ingredientsOrganic solventInjection emulsion
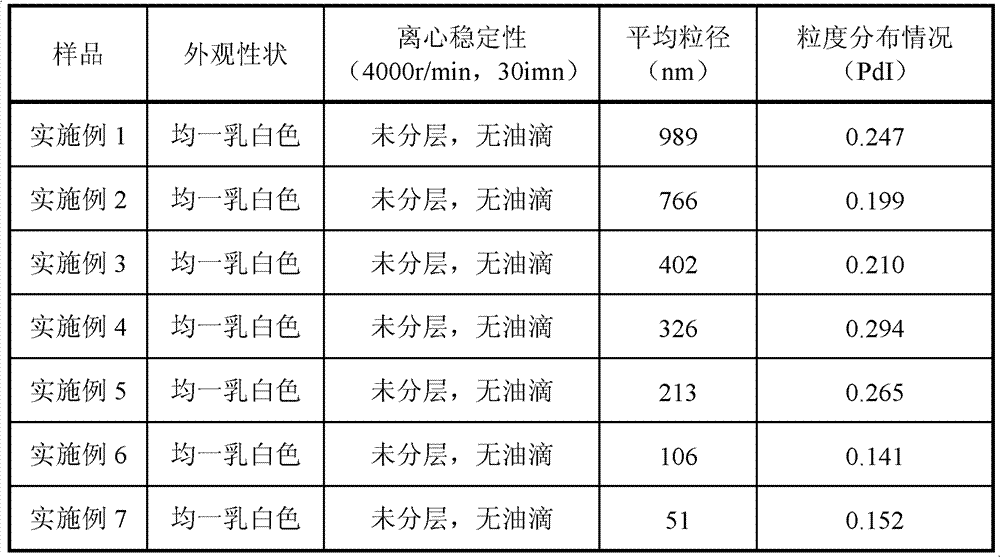
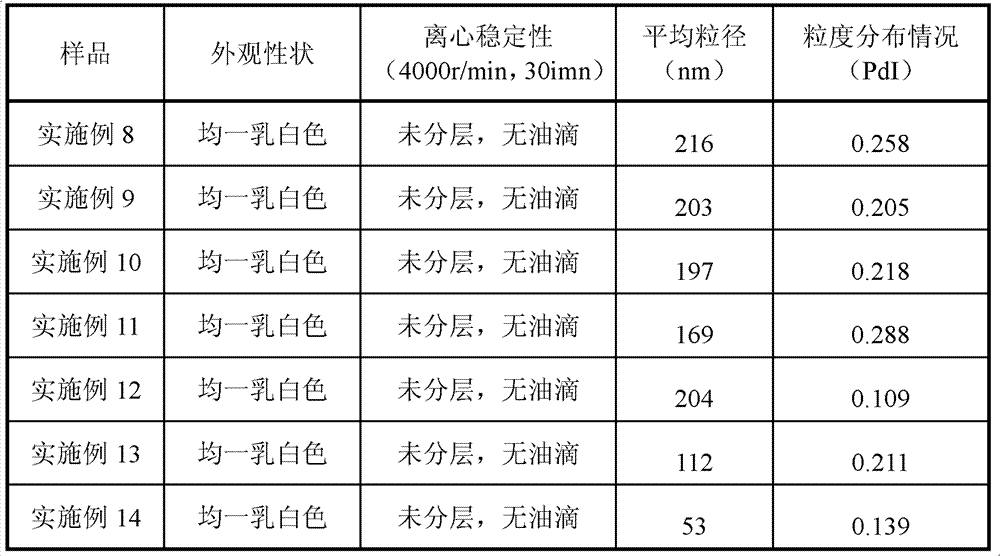
The invention relates to a fat emulsion of Paricalcitol. The fat emulsion comprises oil for injection, an emulsifier, a stabilizer, an isoosmotic adjusting agent, and a pH adjusting agent. Emulsion droplets have an average diameter of 50nm-1000nm. The fat emulsion can be prepared into injection emulsions, freeze-dried emulsions or capsules. Specifically, organic solvents cannot be employed as injections so as to reduce stimulation on blood vessels during injection and avoid injection package material impurity leaching caused by organic solvents, thus ensuring product quality and safety, and reducing the difficulty of product quality control.
Owner:CHONGQING HUAPONT PHARMA
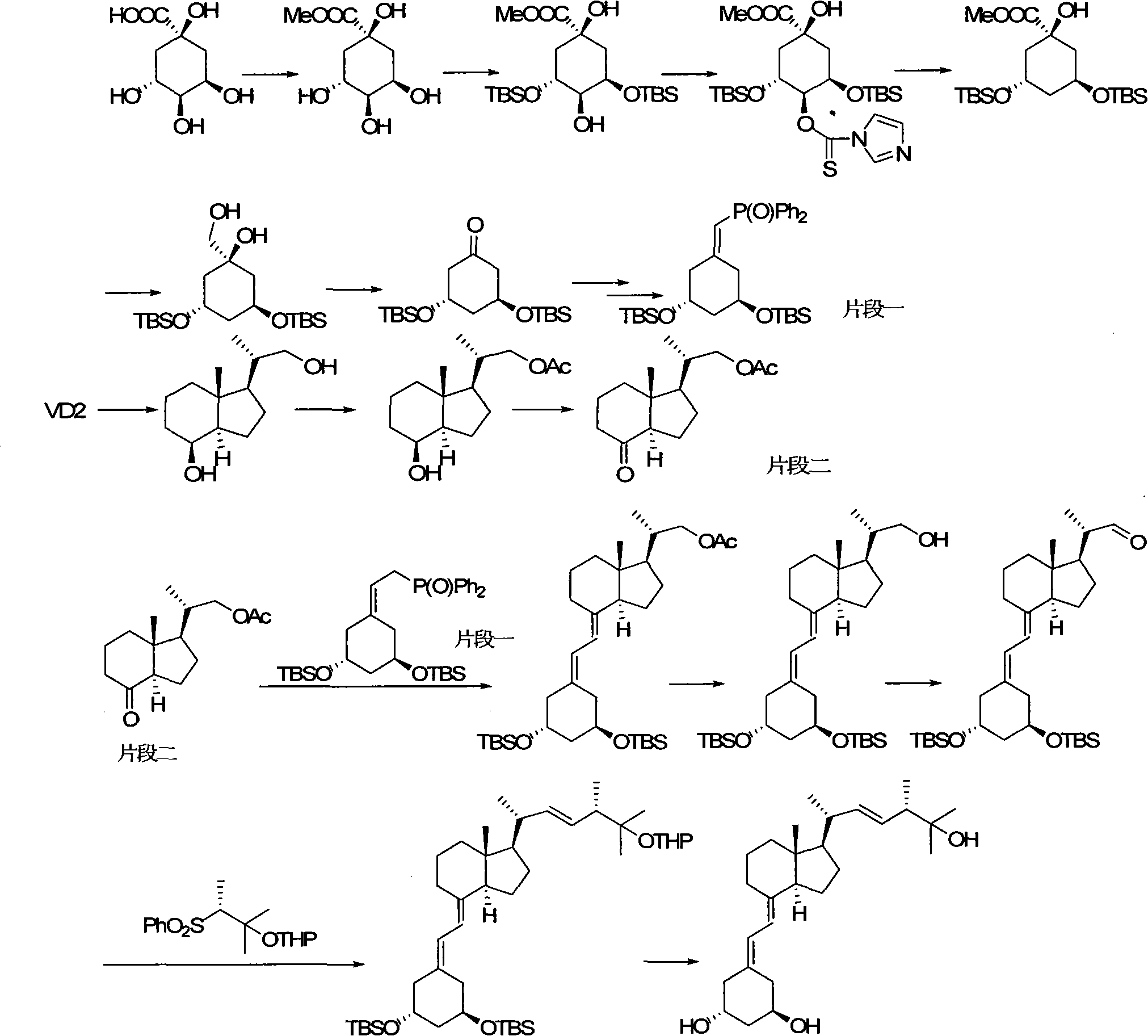
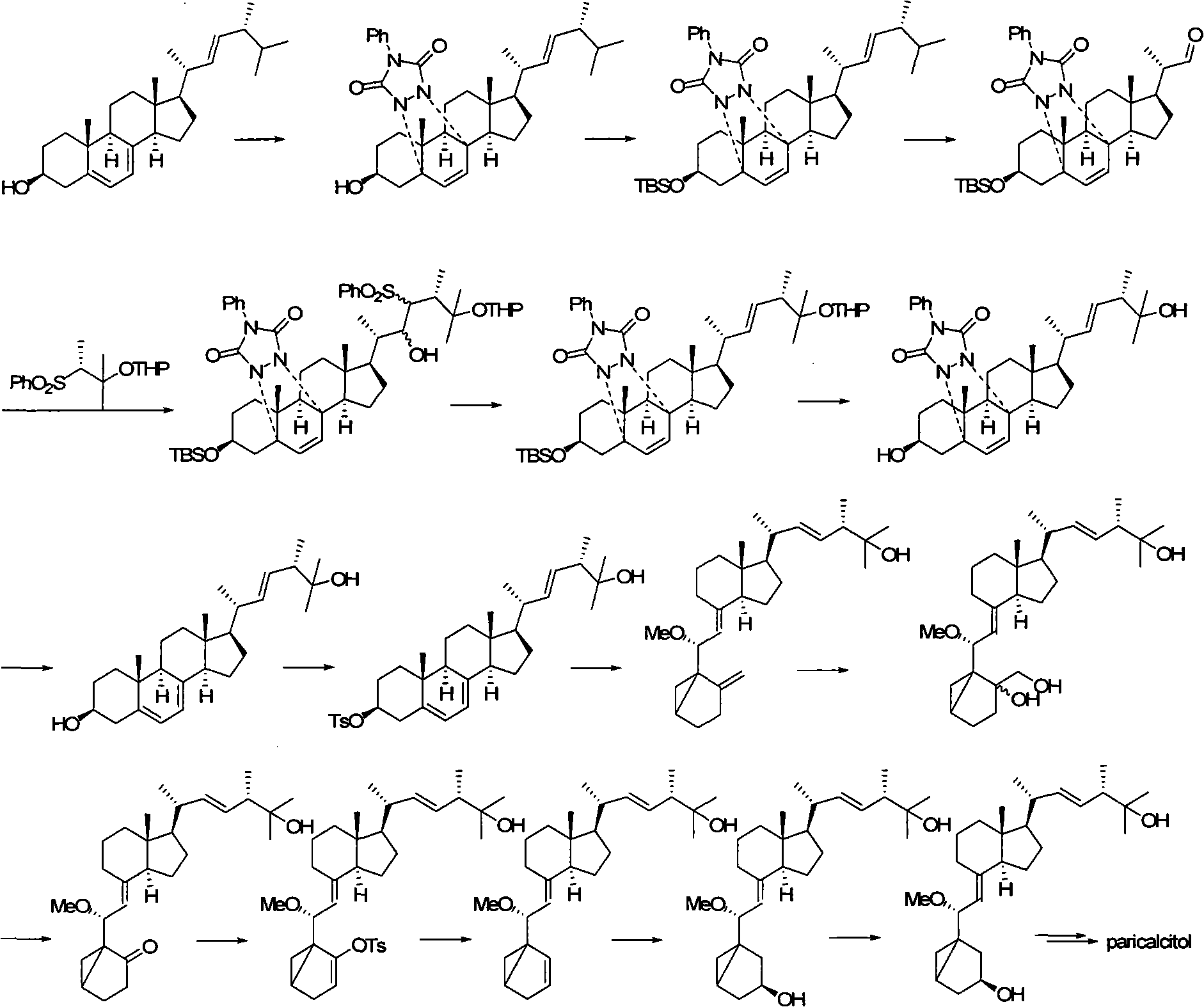
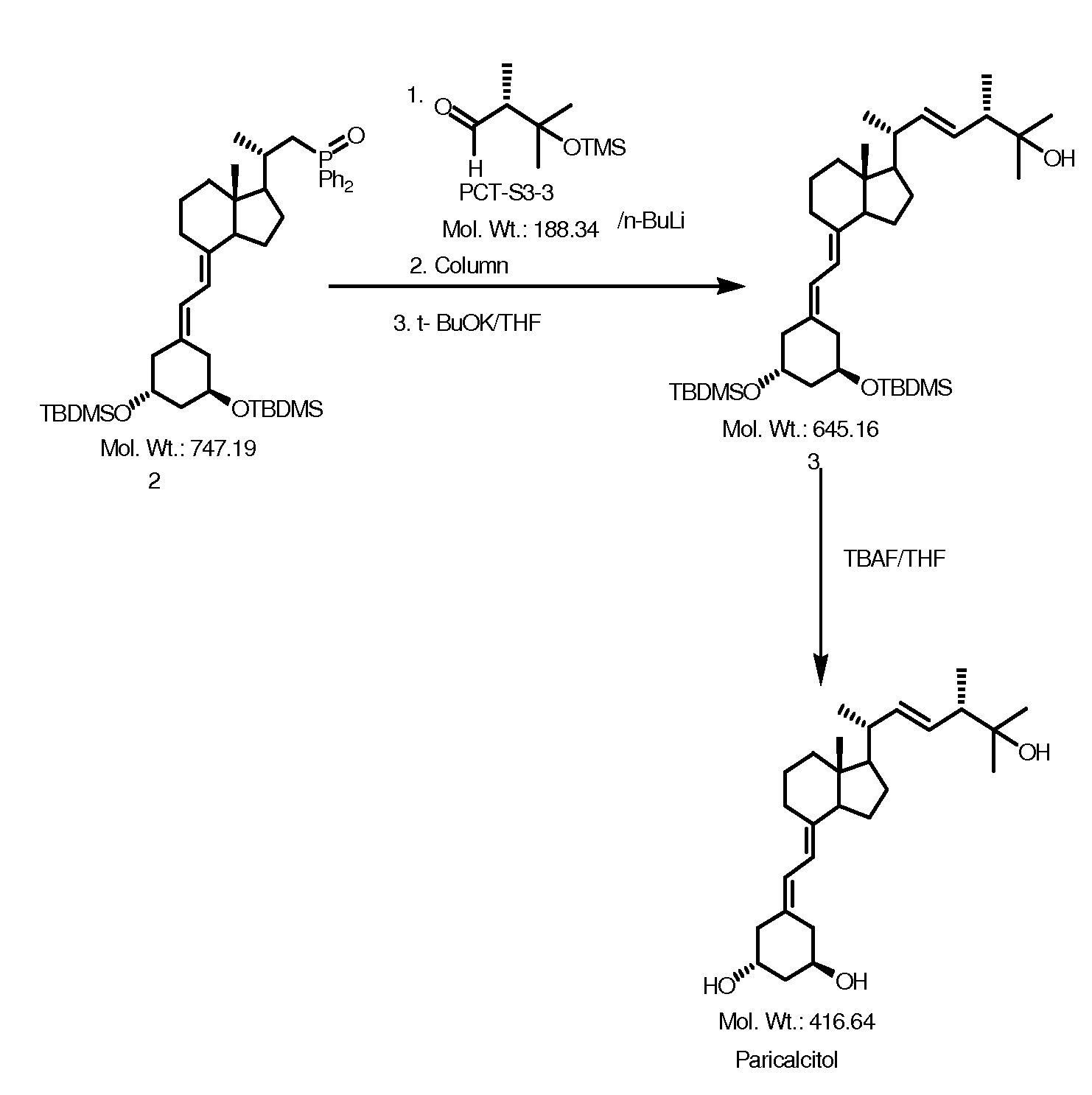
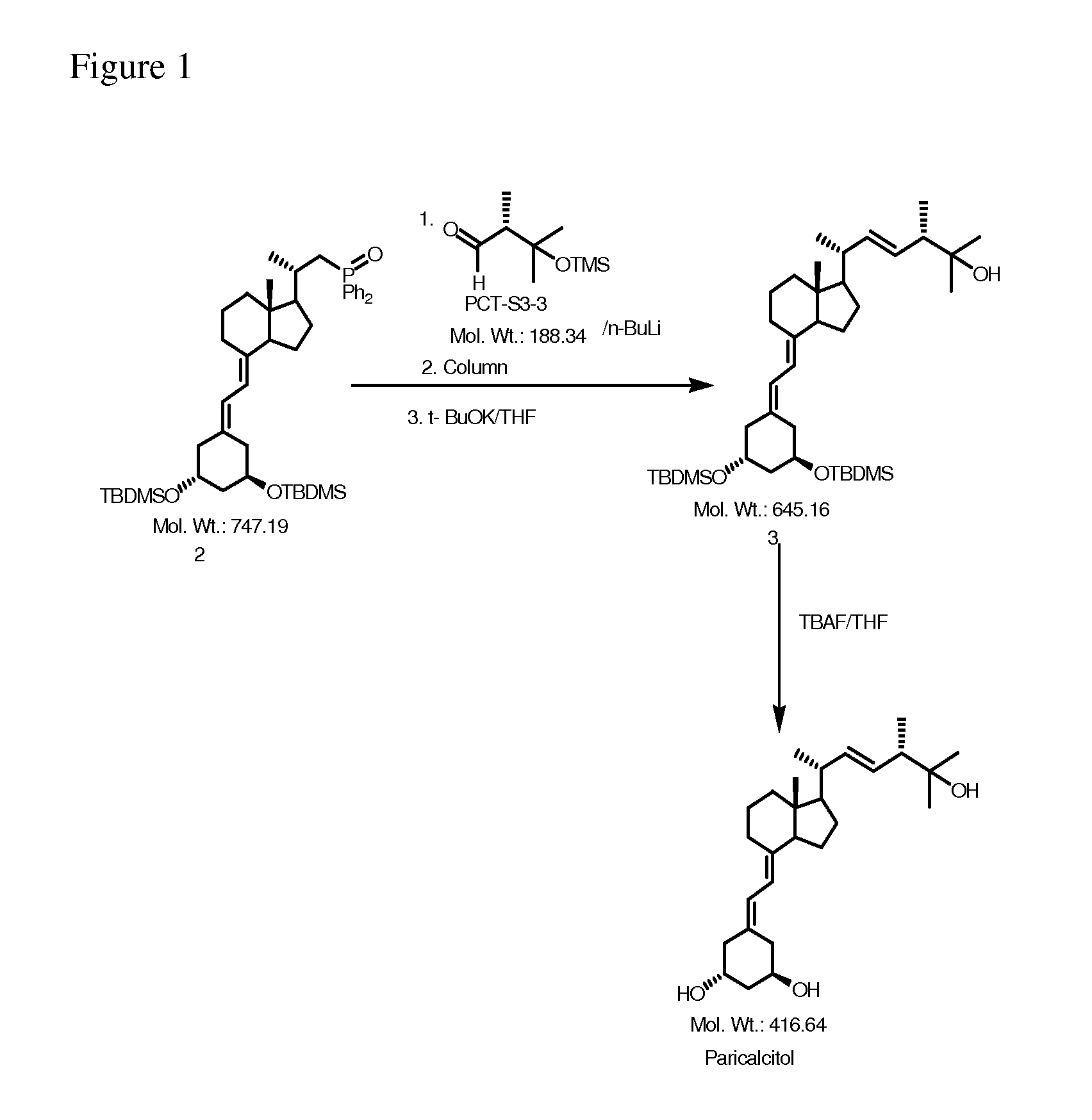
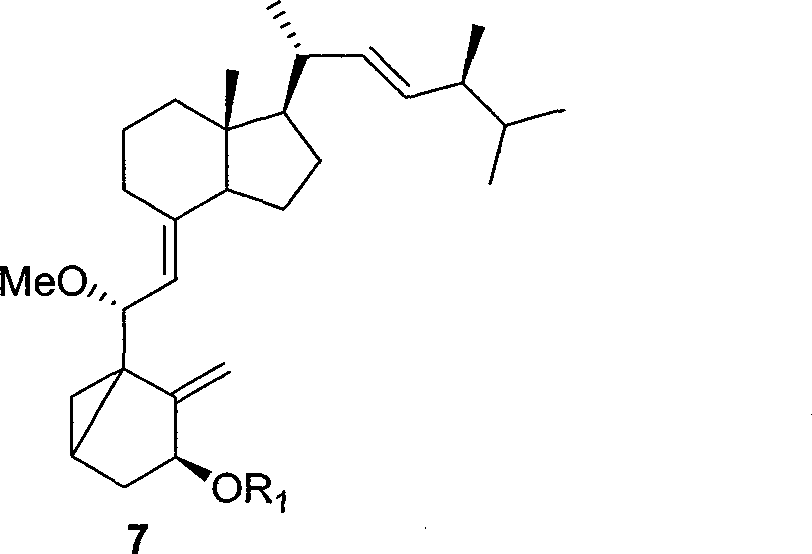
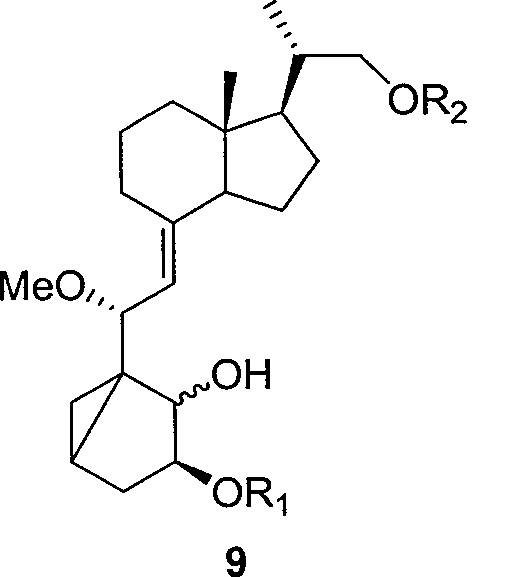
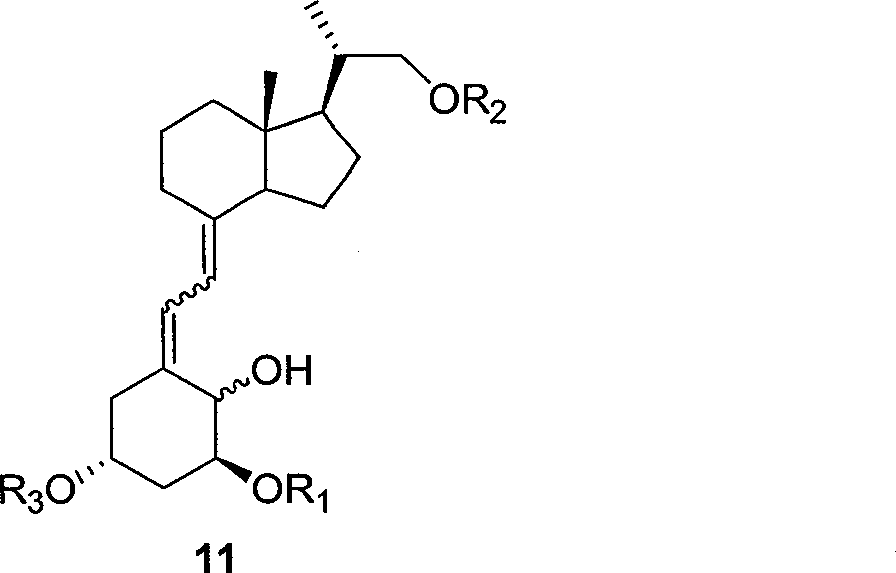
Preparation method of paricalcitol
InactiveCN101880253AEasy to operateLess regional selectivityOrganic chemistryBulk chemical productionSide chainDouble bond
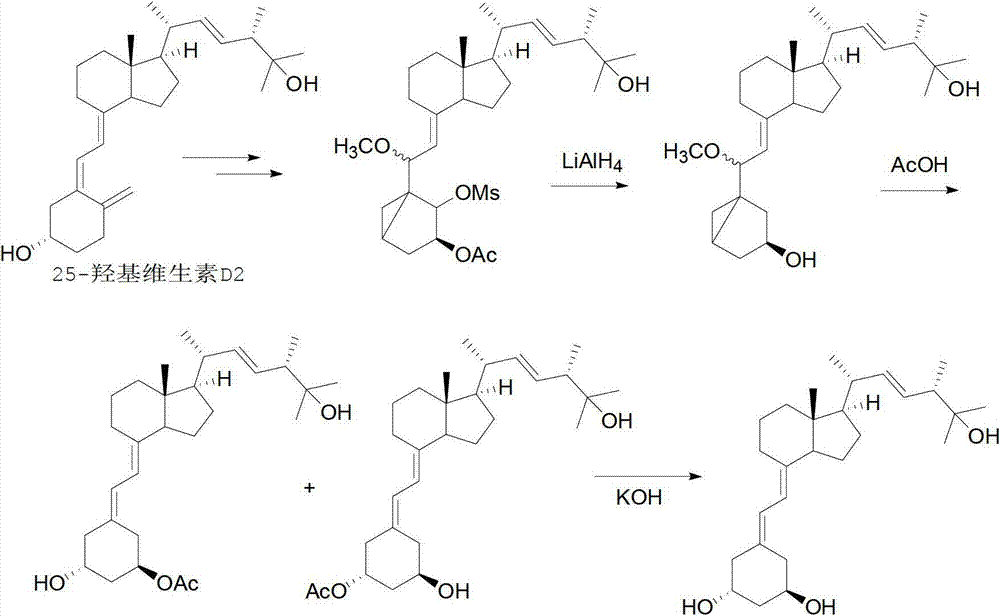
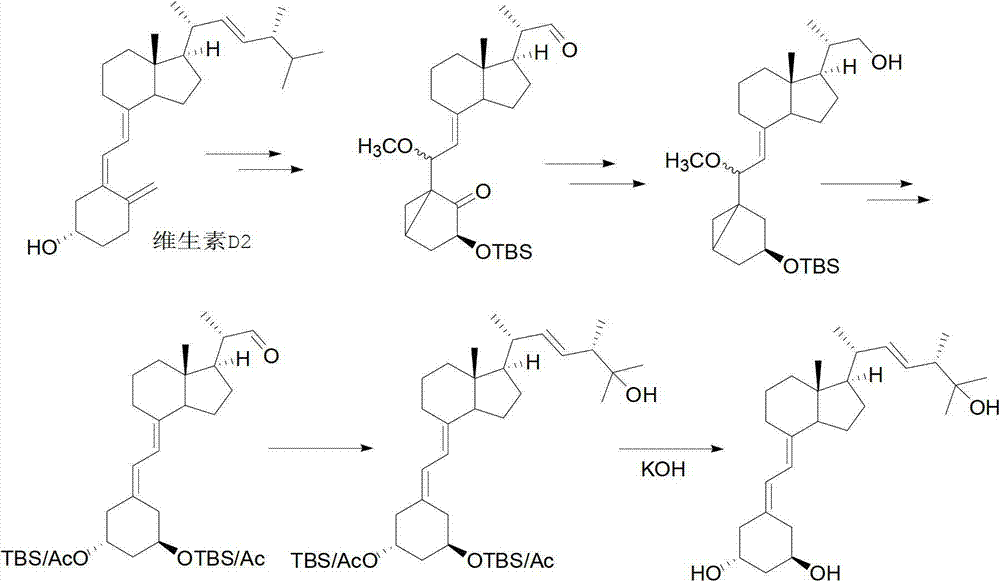
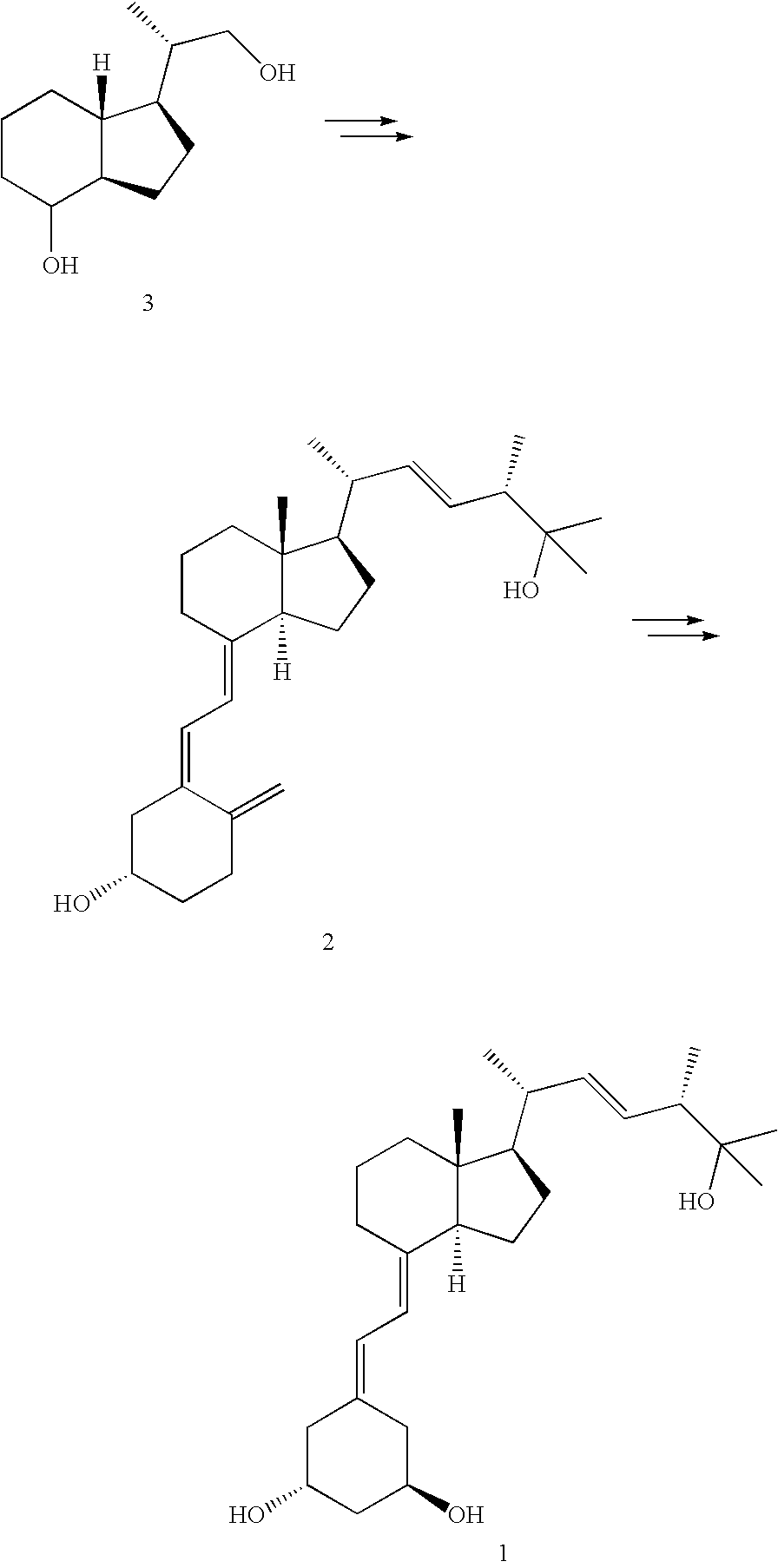
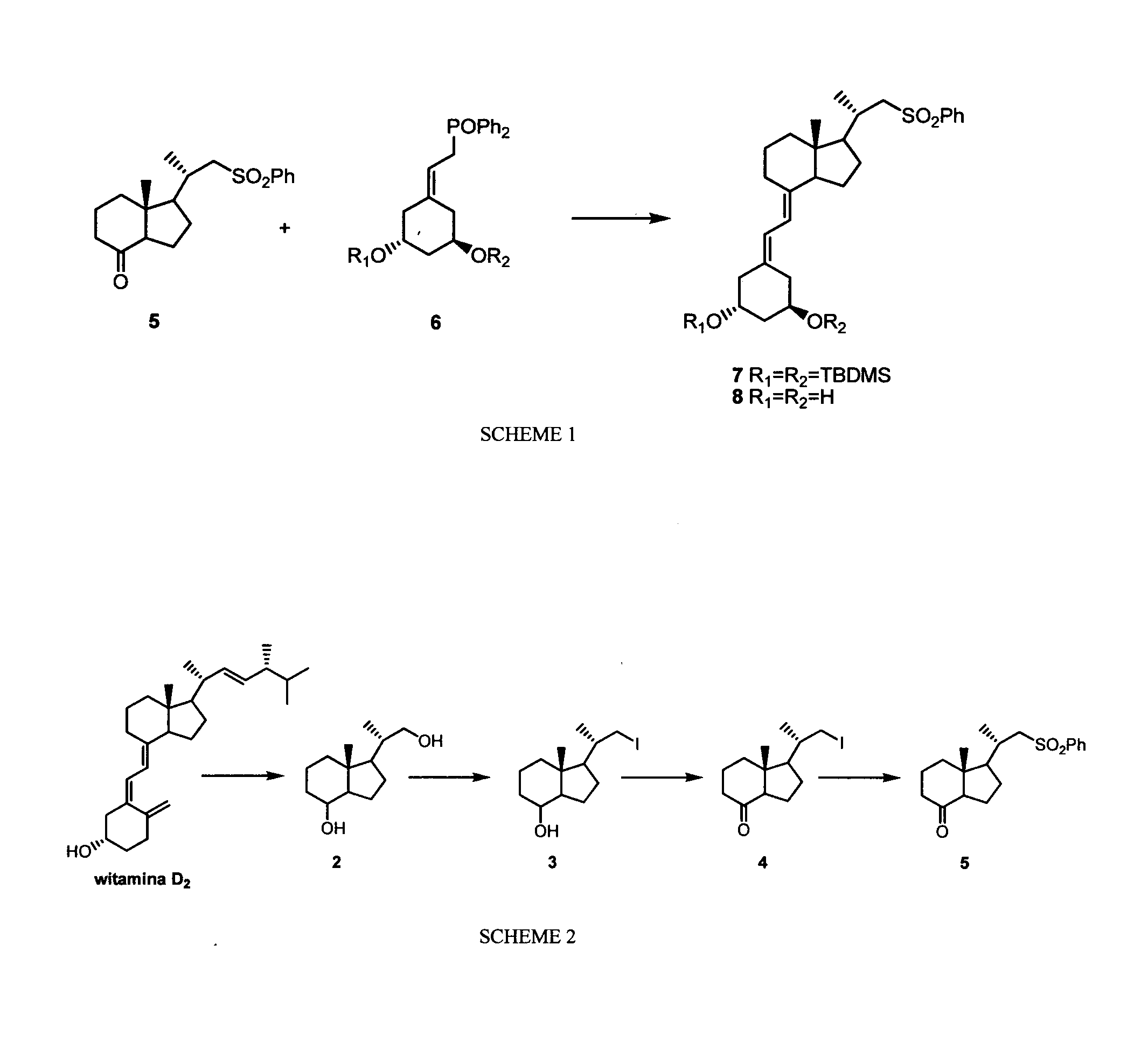
The invention relates to a preparation method of paricalcitol, which is characterized in that after hydroxyl in the vitamin D2 is protected by p-toluenesulfonates, in the presence of alkali, intramolecular cyclization reaction happens in methanol to generate a compound 5; the compound 5 undergoes allylic oxidation and hydroxyl is protected to obtain a key intermediate 7; in the presence of ozone,the side chains and exocyclic terminal double bonds of the key intermediate 7 are cut off to obtain a compound 8; the primary hydroxyl in the compound 8 is selectively protected, a three-membered ring is opened in the presence of acid and then hydroxyl is protected to obtain a key intermediate 11; after the secondary hydroxyl in the key intermediate 11 is protected by sulphonate, a compound 12 isobtained through reduction by LiAlH4; a compound 13 is obtained after the compound 12 is subjected to Swern oxidation and carries out Wittig reaction with the compound 12 to obtain a compound 14; andthe target compound can be obtained by removing the protective group in the compound 14. The reagents used in the method are simple and are convenient to operate, the reactions concerning regioselectivity and stereoselectivity are few, the route is shorter and 12 steps of reactions are carried out.
Owner:CHONGQING TAIHAO PHARM CO LTD
Preparation of paricalcitol
The present invention is directed to a novel process for preparing Paricalcitol wherein Paricalcitol, dissolved in a solvent, is precipitated from a concentrated or seeded solution.
Owner:TEVA PHARM USA INC
Method of determining low-content paricalcitol through high performance liquid chromatography-tandem mass spectrometry method and application thereof
InactiveCN105372340AStrong specificityHigh sensitivityComponent separationInternal standardDissolution
The invention belongs to the technical field of analytical chemistry and particularly relates to a method of determining low-content paricalcitol through a high performance liquid chromatography-tandem mass spectrometry method and application thereof. According to the method, the high performance liquid chromatography-tandem mass spectrometry method is adopted for determination, an internal standard method is also adopted, and dutasteride is used as an internal standard substance. The method is high in specificity, sensitivity and accuracy, the detection limit of the method can reach 40 pg / ml, and the quantization limit can reach 80 pg / ml. The method can be widely applied to testing of the content and dissolution rate of a low-content paricalcitol preparation, and particularly testing of the dissolution rate of a paricalcitol soft capsule.
Owner:CHONGQING HUAPONT PHARMA
Method for synthesizing paricalcitol
InactiveCN103086937ADoes not involve the use ofEase of industrial productionOrganic chemistryBulk chemical productionProtecting groupFluorine containing
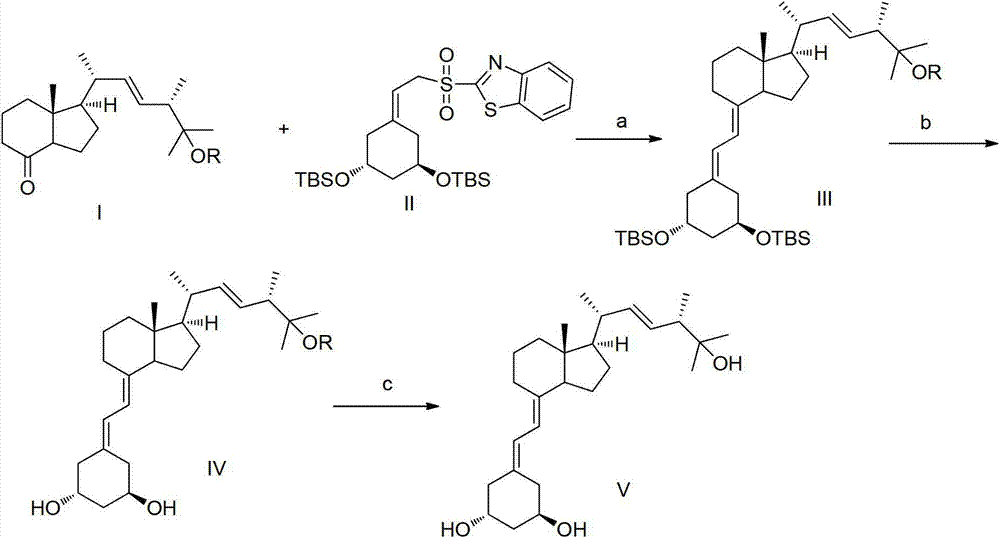
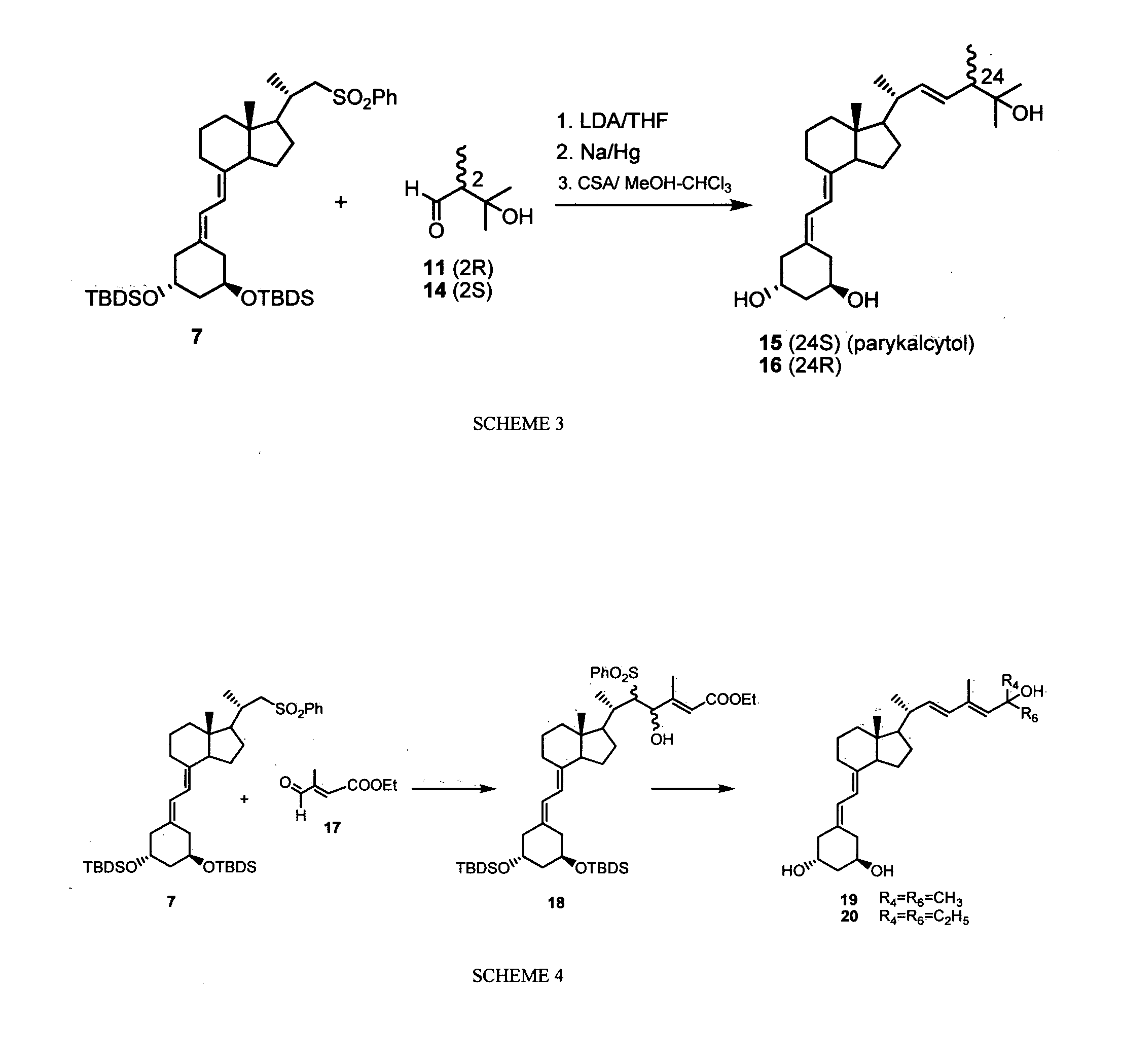
The invention provides a new method for synthesizing paricalcitol, comprising the following steps of: a. reacting a compound II with alkali, then adding a compound I, carrying out a Julia reaction to generate a compound III; b. reacting the compound III with a fluorine-containing reagent to remove the protecting group so as to obtain a compound IV; c. removing the protecting group of the compound IV under acidic condition to obtain the product of paricalcitol. The method for synthesizing paricalcitol disclosed the invention has the advantages of low cost and high yield, and is simple in operation and easy in industrial production.
Owner:SHANGHAI PUYI CHEM CO LTD
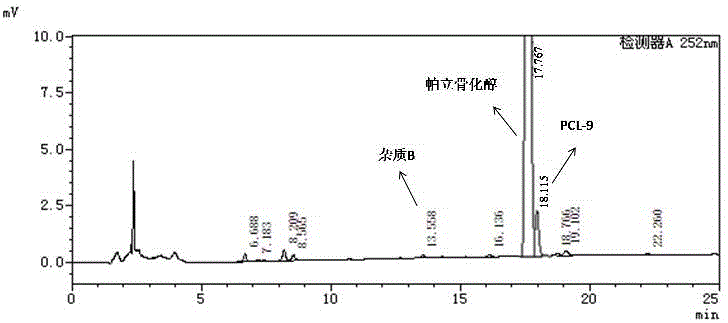
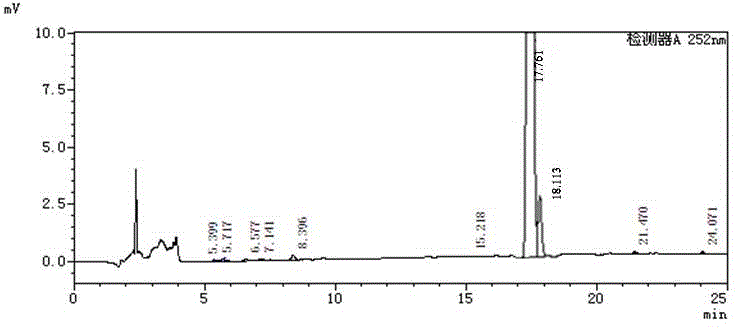
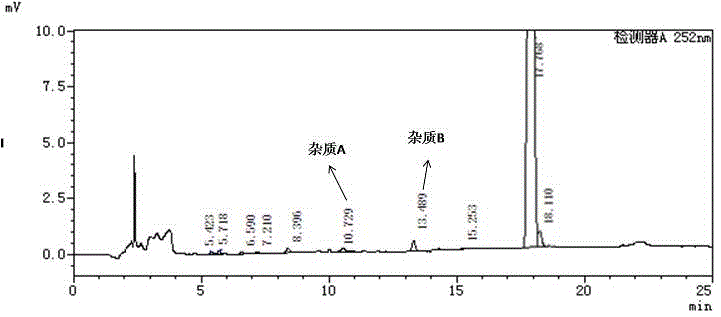
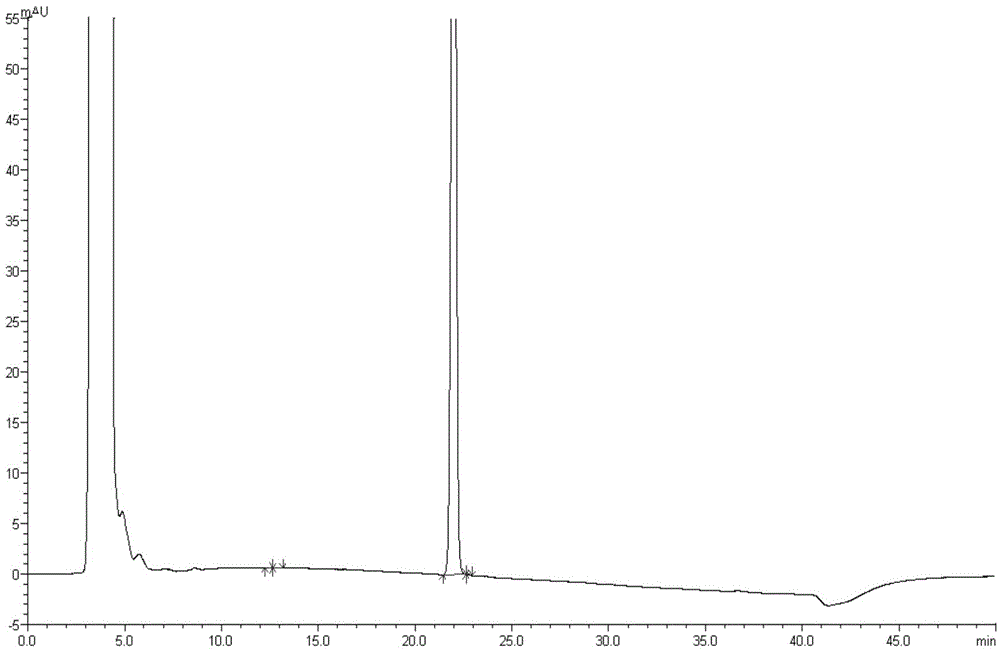
High performance liquid chromatography method for simultaneously separating and analyzing paricalcitol and isomer impurities in paricalcitol injection
ActiveCN109406695AGood separation and analysisImprove accuracyComponent separationParicalcitol InjectionMethanol water
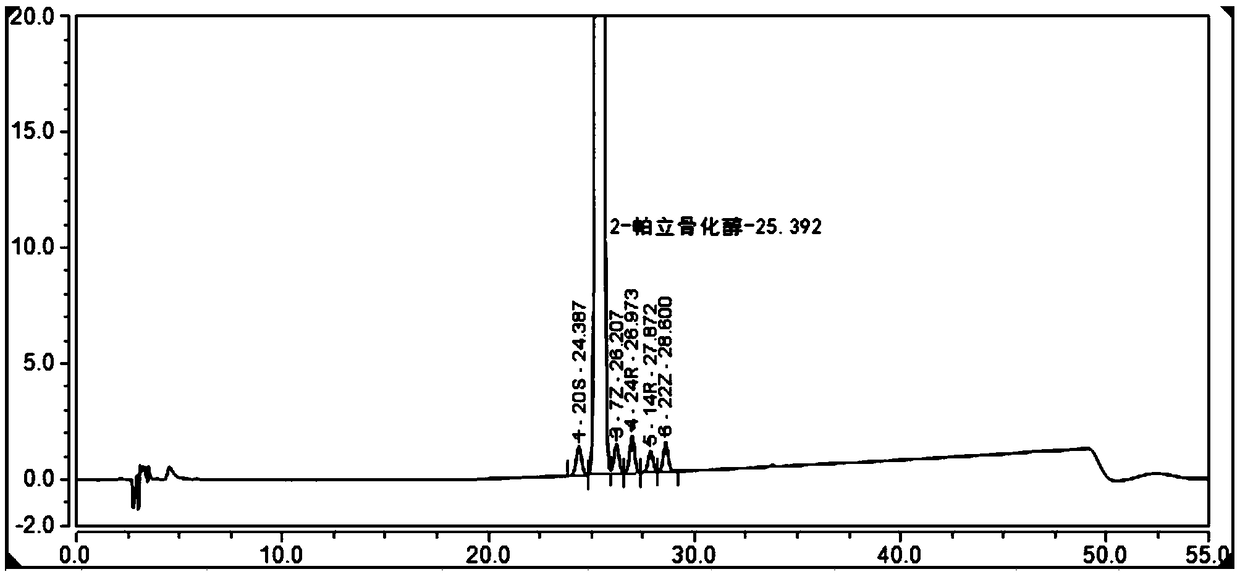
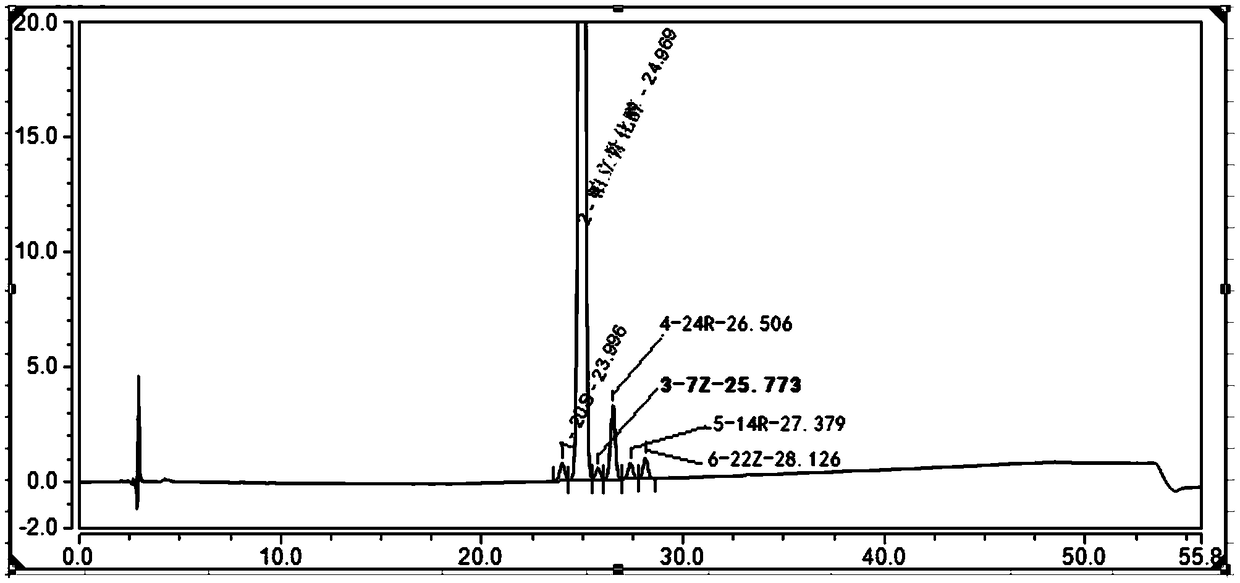
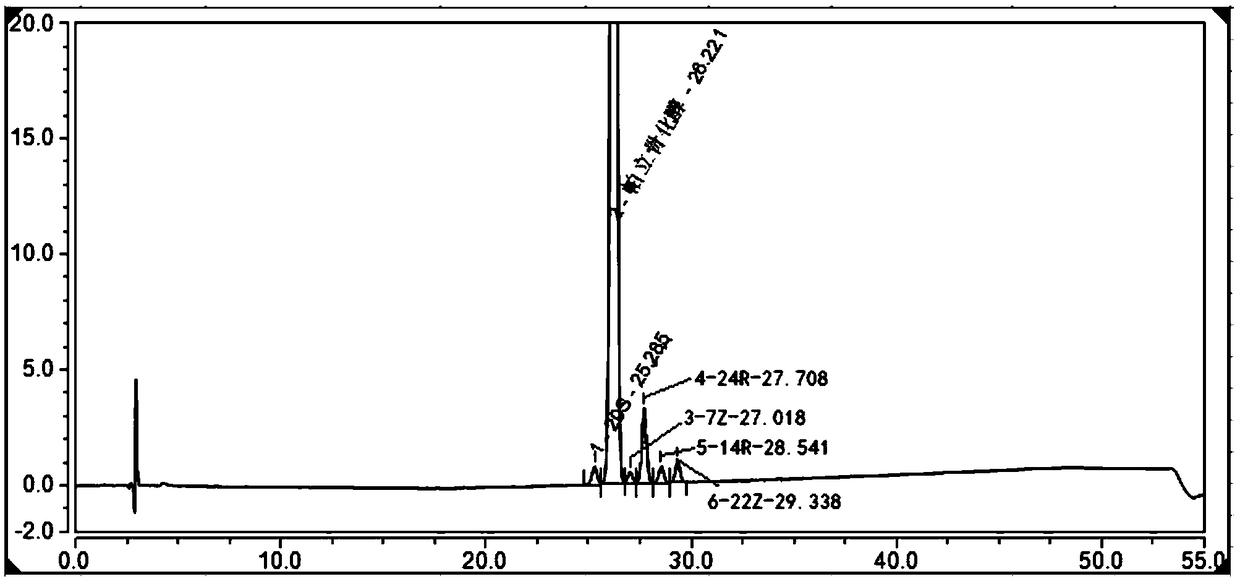
The invention belongs to the field of analysis chemistry, and particularly relates to a high performance liquid chromatography method for simultaneously separating and analyzing paricalcitol and isomer impurities in paricalcitol injection. The method is characterized in that a used chromatographic column uses octadecyl bonded silica gel as filling agents and uses methanol-water as a flowing phaseA and methanol as a flowing phase B for elution; the volume ratio of the flowing phase methanol-water is (20 to 30):(70 to 80); the method is used for simultaneously separating and analyzing the paricalcitol and isomer impurities in paricalcitol injection. The method can be used for better separating and analyzing the paricalcitol and isomer impurities 20S and / or impurities 24R and / or impurities 7Z and / or impurities 14R and / or impurities 22Z in the paricalcitol injection; the interference is avoided; the blank that the detection method is not provided by the existing quality standard or literature is made up; the method has the advantages of simplicity, high speed, high accuracy and the like; the technical support can be provided for the quality control of the paricalcitol injection.
Owner:CHONGQING HUAPONT PHARMA
Medicinal composition containing paricalcitol and preparation method of medicinal composition
ActiveCN106265492AEasy to manufactureNo precipitationOrganic active ingredientsPharmaceutical delivery mechanismActivated carbonAlcohol
The invention relates to a medicinal composition containing paricalcitol. The medicinal composition is composed of paricalcitol, alcohol, propanediol and water for injection. A preparation method of the medicinal composition comprises the following steps: uniformly mixing alcohol and propanediol, adding with 0.5% (w / v) activated carbon, carrying out adsorption for 15-75 min, carrying out decarburization, adding with paricalcitol, carrying out stirring so that the dissolving is complete, adding with the water for injection, carrying out uniform stirring, carrying out filling, carrying out sealing, and carrying out sterilization for 12 min at 121 DEG C. The invention further relates to a method for reducing the degradation impurities of the medicinal composition containing paricalcitol, which comprises the step of encapsulating the composition in an ampoule bottle, wherein the residual oxygen quantity of gas in the ampoule bottle is controlled to be 0.32-2 ml. The product is simple and rapid in preparation, no precipitation of raw materials occurs, the long-time stirring for redissolving of the materials is not needed, meanwhile, the residual oxygen quantity of the gas in an injection container is controlled, so that the content of the degradation impurity PCL-9 is reduced to be 0.1% or below after illumination, and thus the safety of the product is good.
Owner:CHENGDU GUOHONG PHARMA
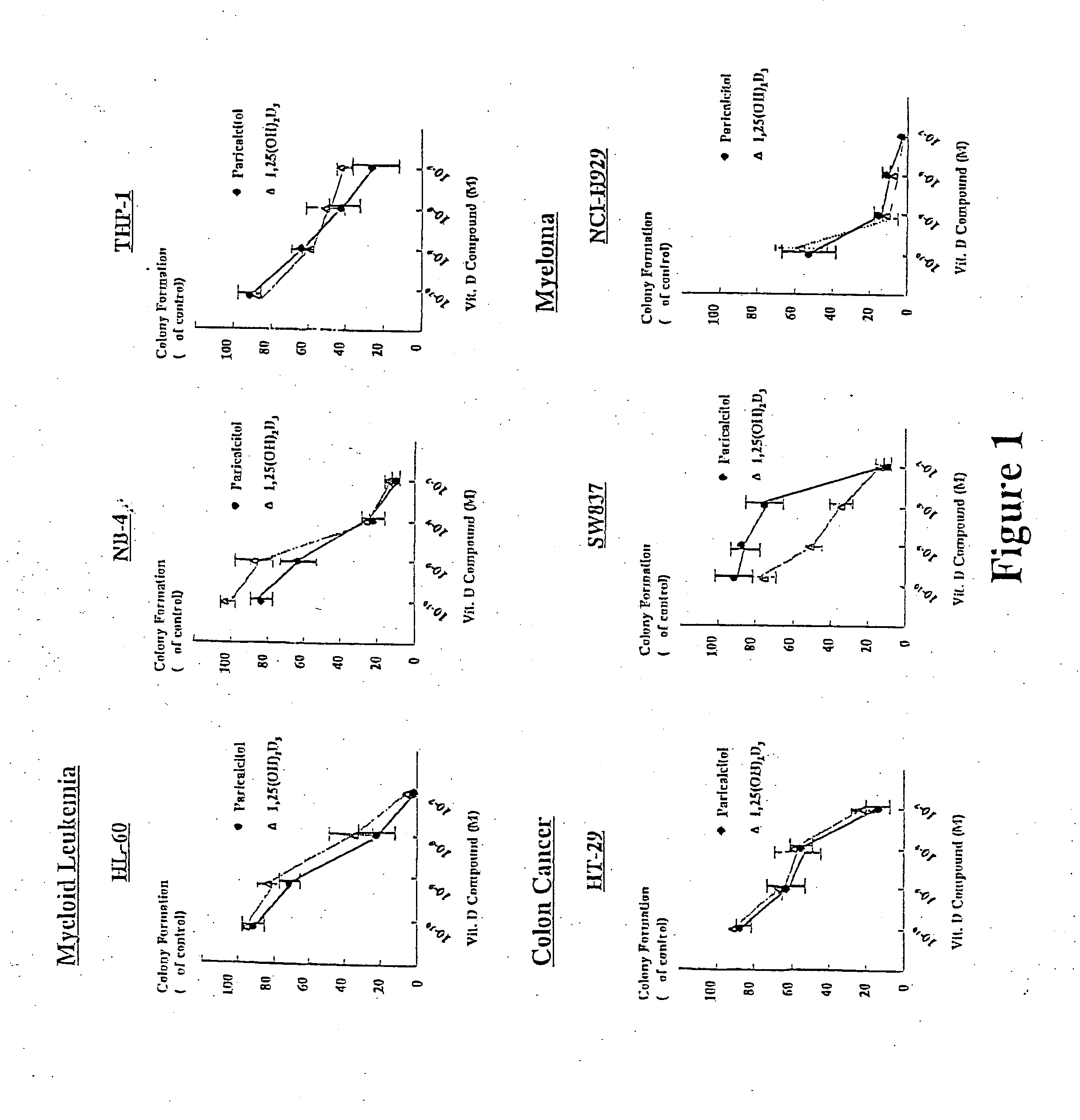
Paricalcitol as a chemotherapeutic agent
InactiveUS20050054620A1Reducing cancer recurrenceReduces cancer cell proliferationOrganic active ingredientsBiocideAnticarcinogenProstate cancer
The invention provides methods of reducing the severity of a proliferative disorder. One method involves administering to an individual having the proliferative disorder an effective amount of paricalcitol, wherein the paricalcitol reduces cellular proliferation, with the proviso that the cancer is not prostate cancer or head and neck squamous cell carcinoma. Another method of reducing the severity of a proliferative disorder provided by the invention involves administering to an individual having the proliferative disorder an effective amount of paricalcitol and an anti-cancer agent, wherein the combination of paricalcitol and the anti-cancer agent reduces cell proliferation, with the proviso that the proliferative disorder is not prostate cancer or head and neck squamous cell carcinoma.
Owner:CEDARS SINAI MEDICAL CENT
Method for separation determination of related substances in bulk drugs and preparations of paricalcitol through HPLC method
The present invention belongs to the field of analytical chemistry, and particularly relates to a method for separation and determination of related substances in bulk drugs and preparations of paricalcitol by using a high-performance liquid chromatography method. According to the method, a normal phase liquid chromatography method is used, the polarity of the stationary phase of the used chromatography column is greater than the polarity of the mobile phase, the peak areas of various substances in a sample solution are determined by using the normal phase liquid chromatography method, and the relative contents of various substances are calculated according to peak area normalization. Compared to the method in the prior art, the method of the present invention has characteristics of strong specificity, good paricalcitol and impurity separation effect and the like, and can be used for impurity detection and quality control during preparation process of paricalcitol bulk drugs and paricalcitol soft capsules.
Owner:CHONGQING HUAPONT PHARMA
Oral pharmaceutical paricalcitol formulations
Pharmaceutically acceptable liquid paricalcitol formulations intended for oral administration, processes for preparing such formulations, and methods of using the same. Embodiments relate to liquid paricalcitol formulations filled into gelatin capsules.
Owner:DR REDDYS LAB LTD +1
Paricalcitol purification
ActiveUS20100063330A1Reduce the amount requiredRecovery of paricalcitol isOrganic compound preparationOrganic chemistry methodsFiltrationVacuum drying
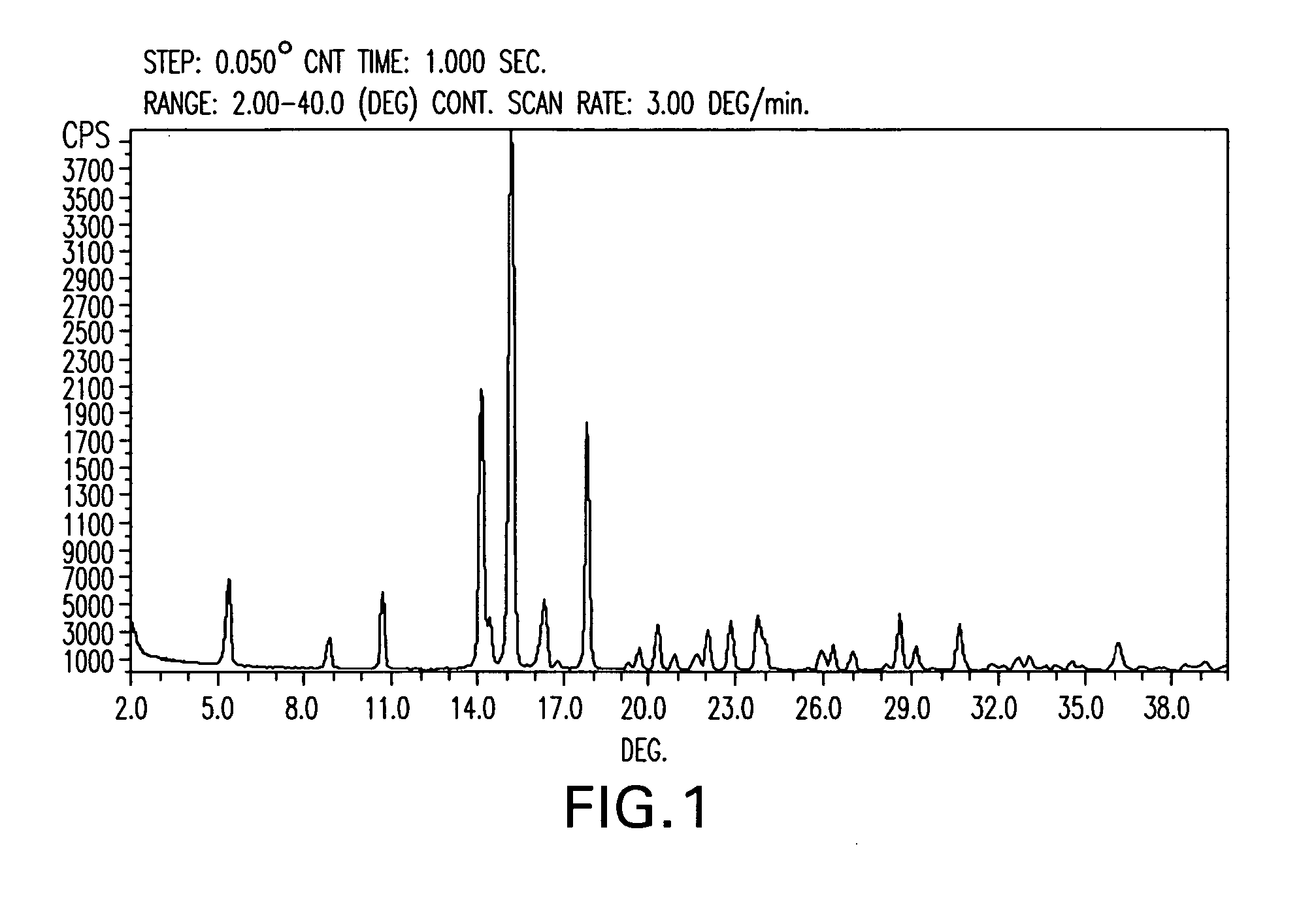
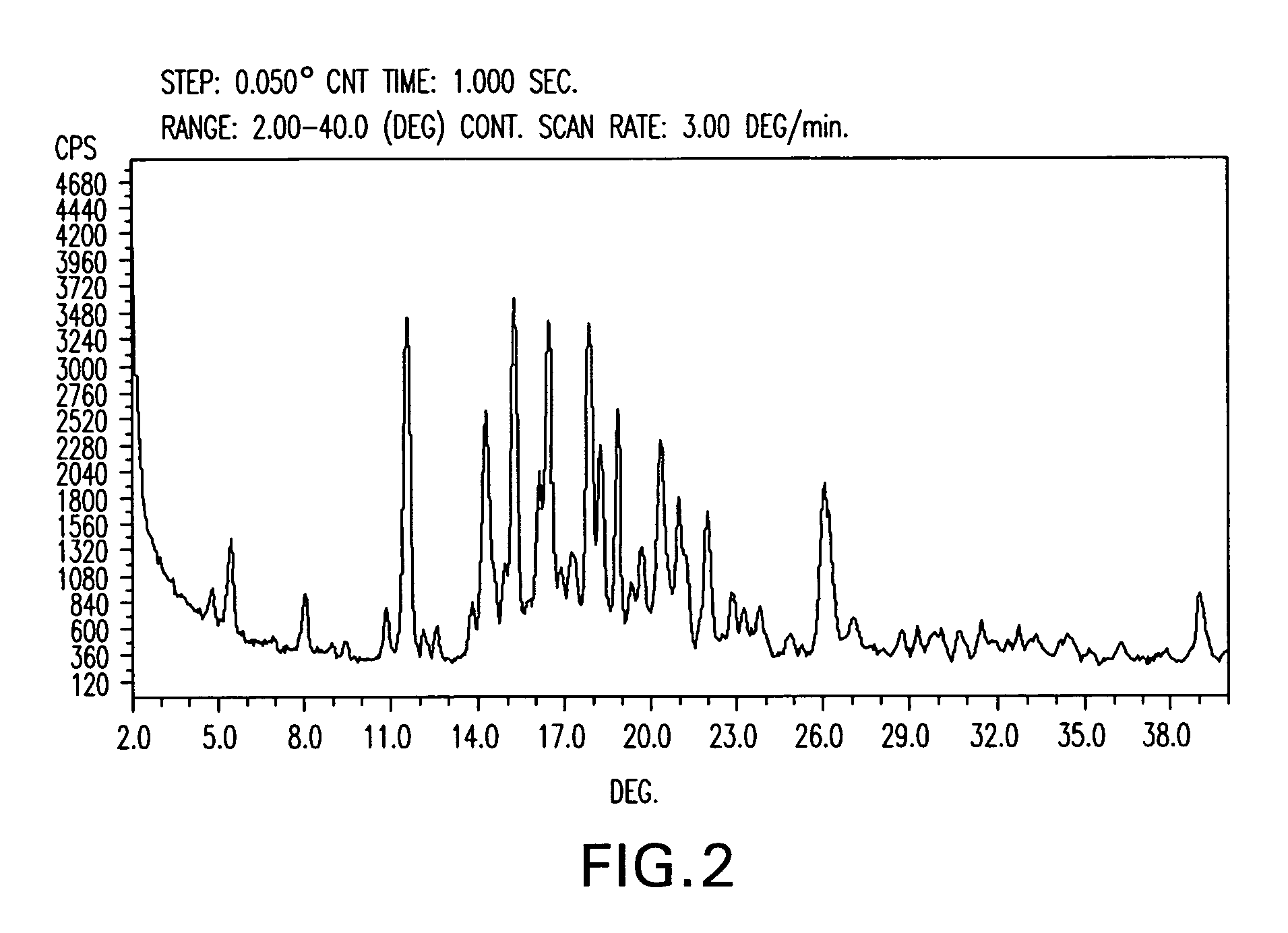
Paricalcitol, a synthetic vitamin D analog, is purified to a purity greater than 99.7% by crystallization from solution in isopropyl acetate solvent, followed by filtration and vacuum drying. Isopropyl acetate appears to be unique among commonly available and pharmaceutically acceptable solvents in its ability to precipitate paricalcitol in this high purity, essentially free of isomers thereof.
Owner:SANDOZ INC
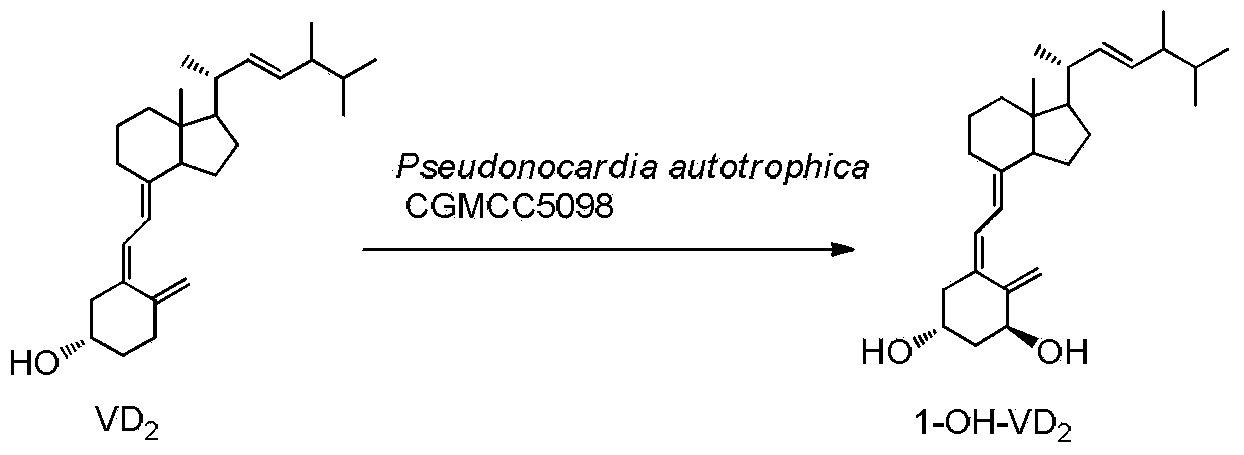
Method for preparing 1alpha-hydroxy vitamin D through microbial conversion
InactiveCN103451242AMild transformation conditionsRegionally selectiveMicroorganism based processesFermentationManufacturing cost reductionChemical reaction
The invention discloses a new industrial preparation method of a medicament 1-alpha-hydroxy vitamin D2 or D3, namely paricalcitol and doxercalciferol, for treating osteoporosis. According to the preparation method, pseudonocardia CGMCC No.5098 is adopted to perform microbial conversion on the first site of vitamin D to directly obtain the 1-alpha-hydroxy vitamin D through only one step. Compared with a chemical method, the method disclosed by the invention is mild, protection is not needed, toxic selenium dioxide is not needed to perform oxidation, the method has regioselectivity and does not need radical protection, the biological conversion yield is up to 40-50%, the method is environment-friendly, the process route is greatly shortened through a one-step conversion reaction, the yield is improved, the manufacturing cost is reduced, the known chemical reaction with about 6 steps for synthesizing paricalcitol and doxercalciferol is shortened to have one step, and the total yield is improved to 40-50%.
Owner:SOUTHWEST JIAOTONG UNIV
Preparation of Paricalcitol
This invention relates to a method for purifying Paricalcitol by reverse phase chromatography. This invention also relates to a purified Paricalcitol prepared by said method. This invention further relates to a method for purifying Paricalcitol by crystallization.
Owner:FORMOSA LAB
Preparation method of paricalcitol
InactiveCN101880253BEasy to operateShort routeOrganic chemistryBulk chemical productionSide chainDouble bond
The invention relates to a preparation method of paricalcitol, which is characterized in that after hydroxyl in the vitamin D2 is protected by p-toluenesulfonates, in the presence of alkali, intramolecular cyclization reaction happens in methanol to generate a compound 5; the compound 5 undergoes allylic oxidation and hydroxyl is protected to obtain a key intermediate 7; in the presence of ozone,the side chains and exocyclic terminal double bonds of the key intermediate 7 are cut off to obtain a compound 8; the primary hydroxyl in the compound 8 is selectively protected, a three-membered ring is opened in the presence of acid and then hydroxyl is protected to obtain a key intermediate 11; after the secondary hydroxyl in the key intermediate 11 is protected by sulphonate, a compound 12 isobtained through reduction by LiAlH4; a compound 13 is obtained after the compound 12 is subjected to Swern oxidation and carries out Wittig reaction with the compound 12 to obtain a compound 14; andthe target compound can be obtained by removing the protective group in the compound 14. The reagents used in the method are simple and are convenient to operate, the reactions concerning regioselectivity and stereoselectivity are few, the route is shorter and 12 steps of reactions are carried out.
Owner:CHONGQING TAIHAO PHARM CO LTD
New synthones for preparation of 19-nor vitamin d derivatives
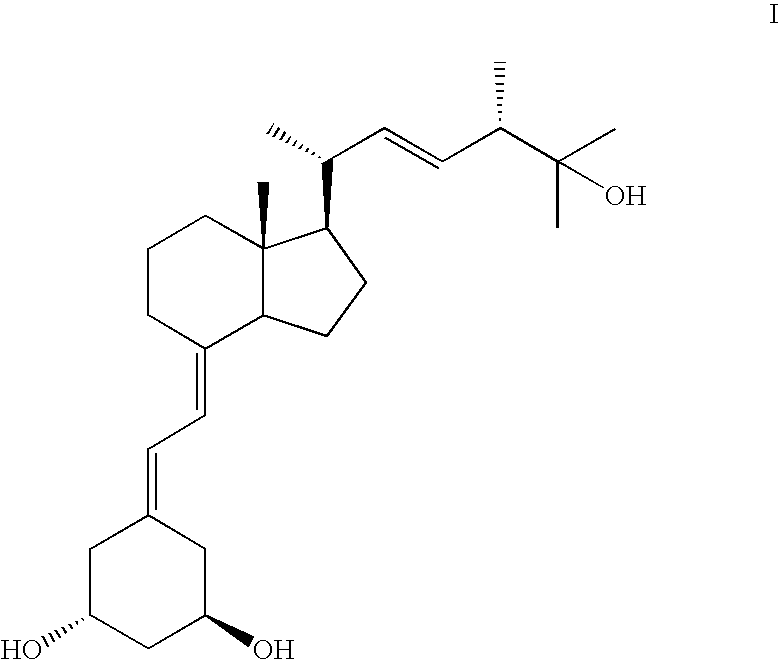
InactiveUS20130006003A1Group 4/14 element organic compoundsOrganic compound preparationHydrogen atomDouble bond
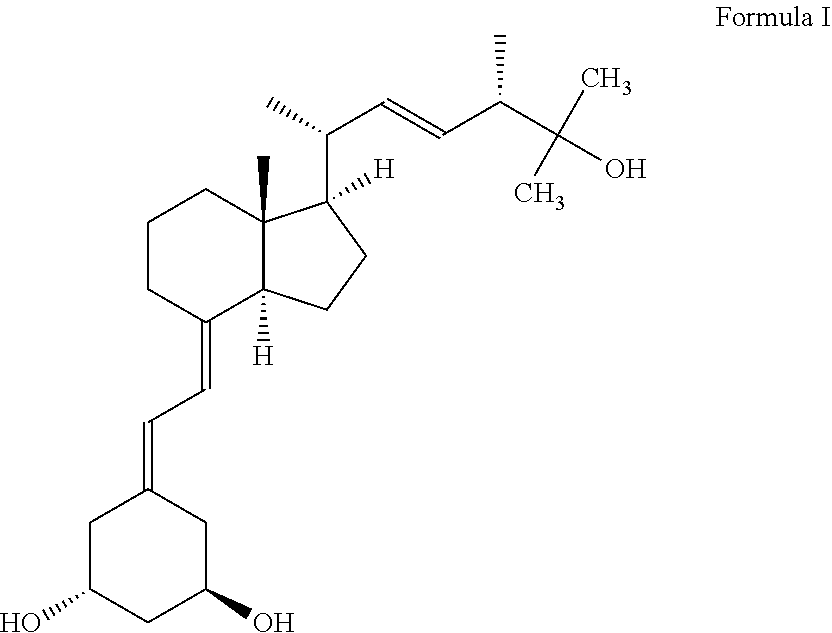
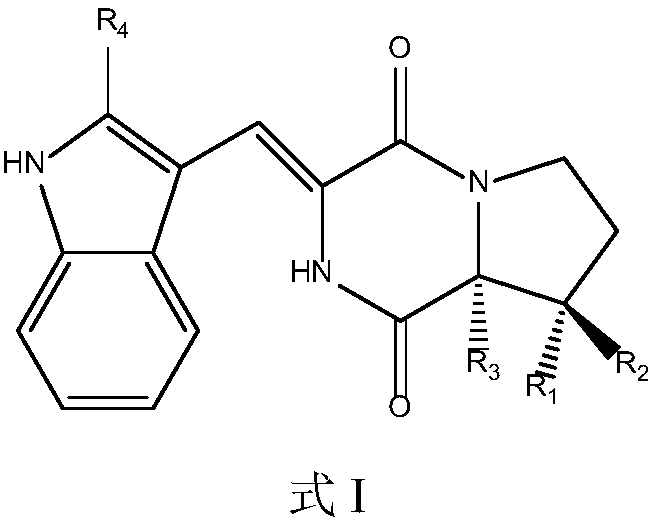
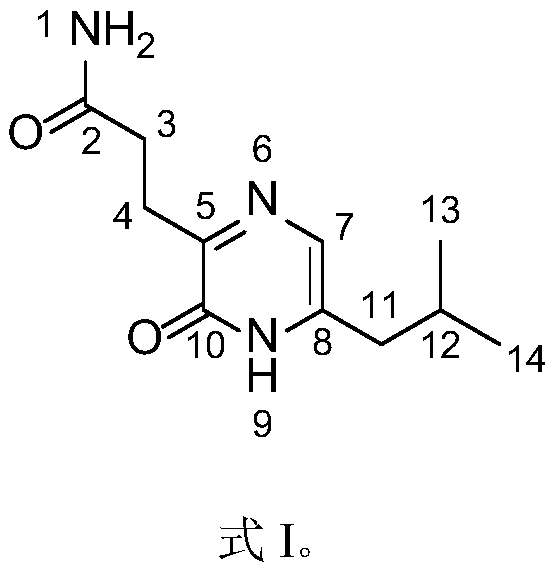
The present invention discloses the synthone of Formula (I), wherein R1 and R2 are the same or different and represent independently hydrogen atom or hydroxyl protecting group, and its use for preparation of 19-nor vitamin D derivatives of general Formula (IV), wherein represents single or double bond, p represents an integer 0 to 3, R1 and R2 represent independently hydrogen atom or hydroxyl protecting group, R3 represents hydrogen atom, CH3 or hydroxyl group, R4, R5 and R6 represent independently hydrogen atom, C1-C3-alkyl or hydroxyl group or two of R4, R5 and R6 substituents altogether form cyclopropyl group, in particular for preparation of paricalcitol.
Owner:INSTITUT FARMACEUTYCZNY
New secondary metabolite LW-4 of Aspergillus terreus SKL-001
InactiveCN109293662AFacilitated releaseHigh extraction rateAntibacterial agentsOrganic chemistryHalogenSecondary metabolite
The invention discloses a new secondary metabolite LW-4 of Aspergillus terreus SKL-001, wherein the new secondary metabolite LW-4 has the following structure defined in the specification, wherein R1 and R2 respectively and independently are H, OH, C1-C4 alkoxy, C1-C4 alkylsulfonyl and C2-C4 alkyl acyl, one of R1 and R2 is H, R3 and R4 respectively and independently are H, OH, halogen, amino, a group defined in the specification, C1-C4 alkyl, C2-C4 alkyl acyl, C1-C4 haloalkyl, C1-C4 alkylsulfonyl and C2-C4 alkenyl, and at least one of R3 and R4 is not H. The preparation method comprises: addingan epigenetic modification regulator SAHA, paricalcitol and triglyceride to a culture medium, and carrying out standing fermentation culture. According to the present invention, Aspergillus terreus SKL-001 is subjected to epigenetic modification by adding the epigenetic modification regulator SAHA to the culture medium to promote the production of the diversified secondary metabolites, such thatthe new secondary metabolite LW-4 is separated; and the new secondary metabolite LW-4 of the Aspergillus terreus SKL-001, and the stereoisomer or pharmaceutically contactable salt thereof have long-acting antibacterial and anti-inflammatory effects.
Owner:ZHEJIANG OCEAN UNIV
A kind of stable paricalcitol pharmaceutical composition and preparation method thereof
The invention discloses a high-stability paricalcitol pharmaceutical composition and a preparation method thereof. The medicinal composition comprises a cyclodextrin inclusion compound containing an active component paricalcitol and other pharmaceutically acceptable carriers, and is prepared by adopting a freeze-drying dry granulation process and strictly controlling temperature and humidity in the preparation process. The paricalcitol is included by cyclodextrin, and the freeze-drying and dry granulating preparation process is adopted, so that the defects that the main drug paricalcitol is extremely easily hygroscopic and extremely poor in stability and cannot be prepared into oral preparations can be greatly overcome, and the disintegration or main drug component dissolution cannot be influenced; and the paricalcitol pharmaceutical composition is not a coated tablet, so that the defect of slow dissolution of a conventional coated tablet can be avoided.
Owner:BEIJING TIDE PHARMA
Application of novel secondary metabolite LW-4 of aspergillus terreus SKL-001
InactiveCN109125326AHigh extraction rateLong-lasting antibacterial and anti-inflammatory effectsAntibacterial agentsOrganic active ingredientsEscherichia coliSecondary metabolite
The invention discloses application of a novel secondary metabolite LW-4 of aspergillus terreus SKL-001. The novel secondary metabolite LW-4 is prepared by steps: adding an epigenetic modification regulating agent SAHA, paricalcitol and triglyceride into a fungal culture medium, performing fermentation culture, adopting ethyl acetate and 80% acetone-water for extracting fermentation liquor and mycelia respectively, and subjecting fermentation concentrate to column chromatography and thin-layer chromatography separation and purification to obtain the novel secondary metabolite LW-4. The epigenetic modification regulating agent SAHA is adopted for epigenetic modification of aspergillus terreus SKL-001 to obtain the novel secondary metabolite LW-4 through separation. The secondary metaboliteLW-4 of aspergillus terreus SKL-001 has great escherichia coli inhibiting activity, has a minimum inhibition concentration of 15microgram / mL and can be potentially developed for preparation of pharmaceutical preparations resistant to Gram-negative bacteria.
Owner:ZHEJIANG OCEAN UNIV
Use of vitamin Ds to down regulate the renin-angiotensin-aldosterone system
InactiveUS20050074488A1Preventing and treating and delaying progression of diseasePrevent ACE/aldosterone escapeOrganic active ingredientsBiocidePhysiologyBiological activation
The present invention relates to the use of Vitamin D, preferably paricalcitol, to treat, prevent and delay disease progression of diseases associated with over activation of the renin-angiotensin aldosterone system.
Owner:UNIVERSITY OF CHICAGO
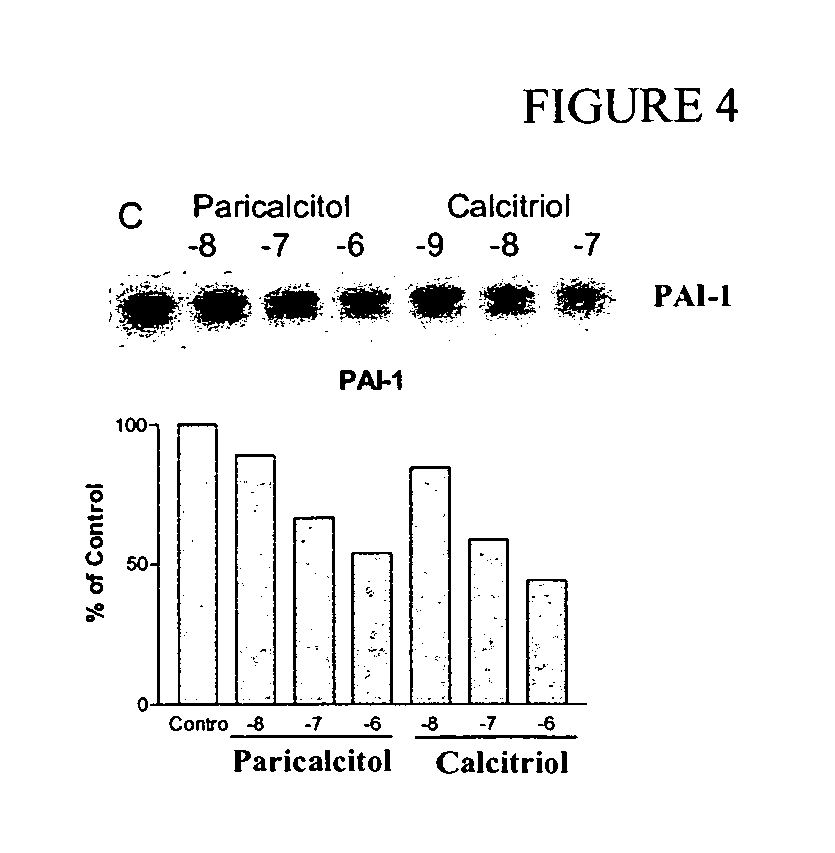
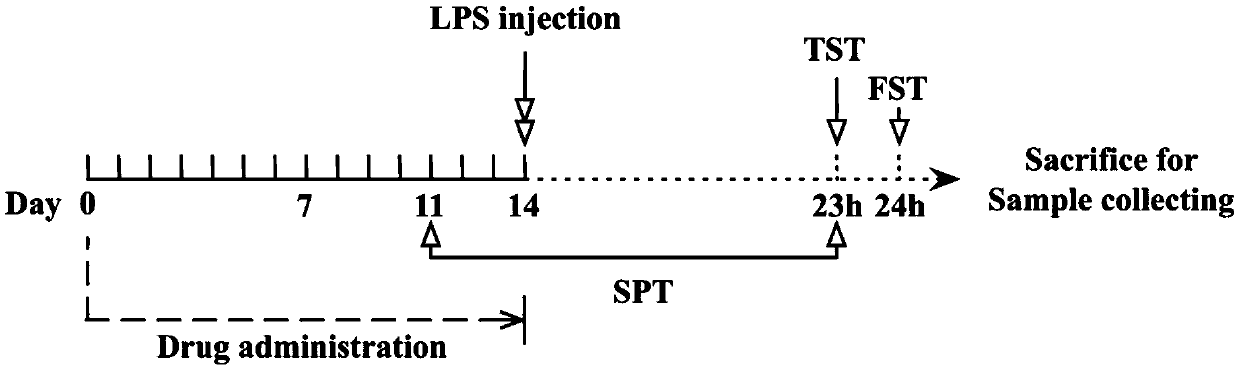
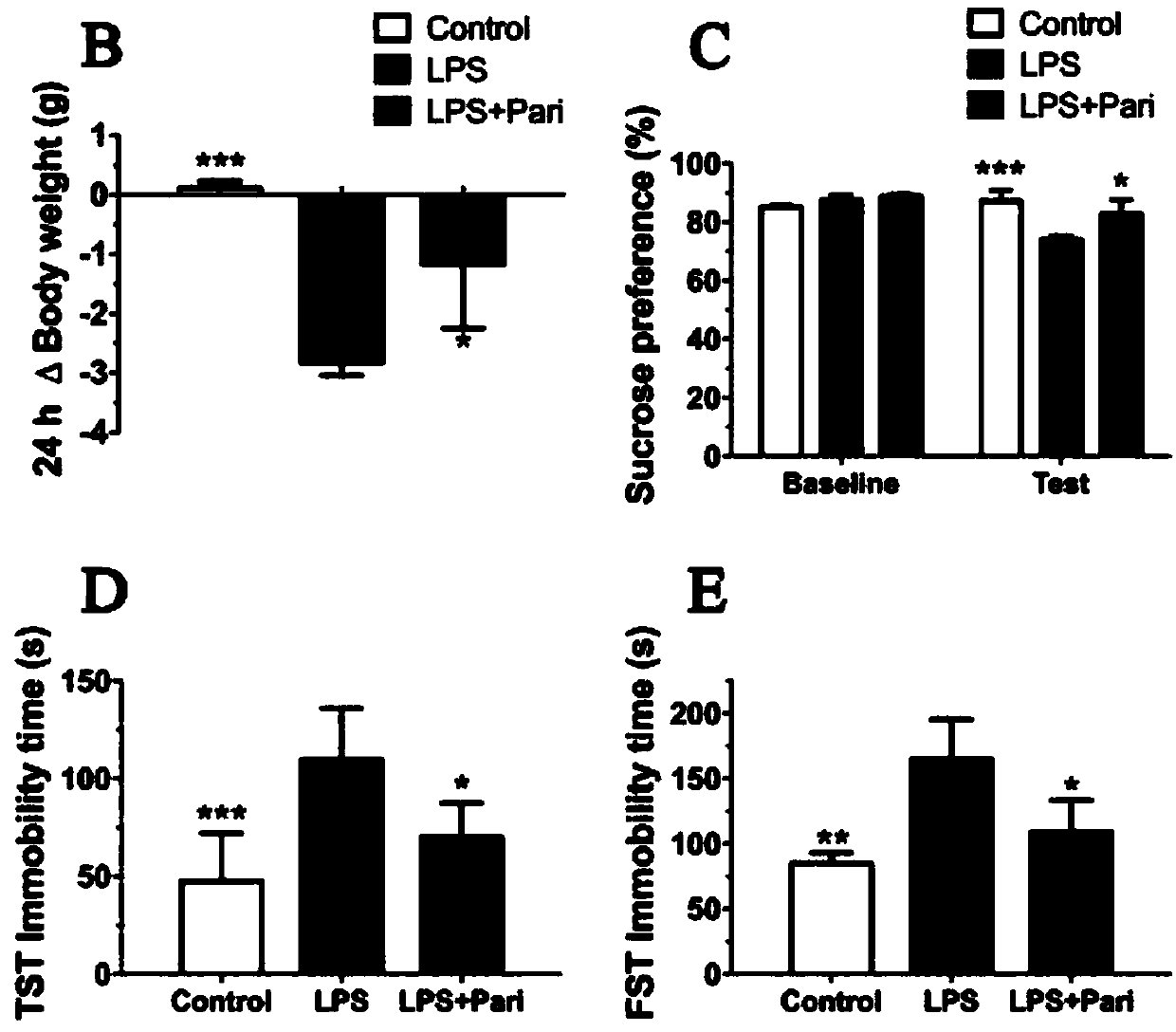
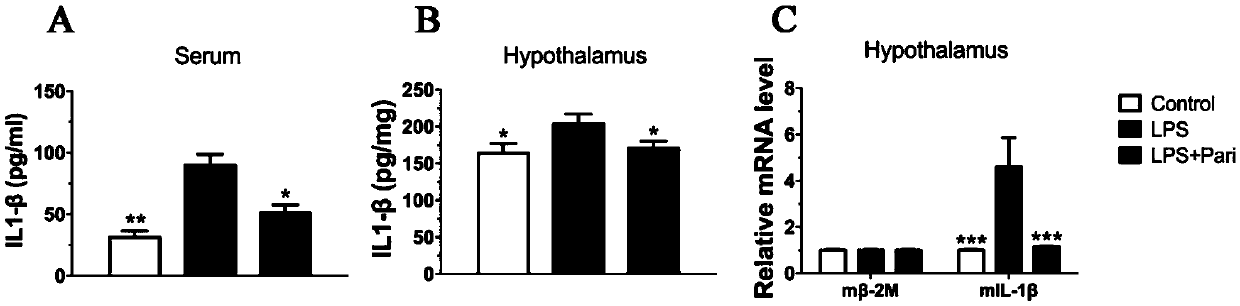
Application of paricalcitol in preparation of medicine for preventing and treating depression
Owner:LONGHUA HOSPITAL SHANGHAI UNIV OF TRADITIONAL CHINESE MEDICINE
Method for preparing paricalcitol
InactiveCN102392053ARegionally selectiveSimple processMicroorganism based processesFermentationChemical reactionKetone
The invention discloses a method for preparing drug paricalcitol for treating osteoporosis, which comprises the following steps of: taking vitamin D2 as raw materials and obtaining a target object through the steps of chemical reaction and biological reaction; omitting the use of an expensive toxic dihydroxylation reagent and bond-breaking dihydroxylation adopting cheap effective reagent oxide potassium permanganate solution into ketone; isomerizing into olefin under an alkaline reagent, carrying out hydroboration reaction by applying a borohydride reagent 9-BBN, borane and other reagents; introducing 1-hydroxyl, ring-opening to obtain 19-demethyl vitamin D2, taking pseudonocardiaautotrophica SEJTUQL-3701021 (Autotrophic false Nocardia CGMCC 5098) as hydroxylation bacteria, carrying out 25-direct hydroxylation by using a biotransformation method and obtaining the paricalcitol through extraction and separation. Through a chemical-biological transformation combination method, the reaction steps of the paricalcitol are shortened from known 27 to 9, so that the total yield is improved, and the process route is shortened. The invention provides a new process route for solving the industrial preparation of the paricalcitol.
Owner:SOUTHWEST JIAOTONG UNIV
Method for separating and extracting new compound LW-1 from secondary metabolites of aspergillus terreus
InactiveCN109053601AHigh extraction rateFacilitated releaseOrganic chemistryAntipyreticMetaboliteSecondary metabolite
The invention discloses a method for separating and extracting a new compound LW-1 from secondary metabolites of aspergillus terreus. The method comprises the steps of spore preparation, strain fermentation, secondary metabolite extraction as well as separation and purification, wherein the strain fermentation comprises the following steps: adding an epigenetic modification regulator SAHA, paricalcitol and triglyceride into a fungus culture medium, stirring uniformly, standing, and carrying out fermentation culture. The method provided by the invention has the beneficial effects that the paricalcitol and the triglyceride are added into the culture medium, and realize collaborative gain with the epigenetic modification regulator SAHA, so that epigenetic modification is performed on the aspergillus terreus, the aspergillus terreus is promoted to generate the diverse secondary metabolites, and the new secondary metabolite LW-1 is accordingly obtained by means of separation; the new secondary metabolite LW-1, obtained by using the method, of the aspergillus terreus as well as stereoisomers or pharmaceutically acceptable salts of the new secondary metabolite LW-1 have long-acting antibacterial and anti-inflammatory effects.
Owner:ZHEJIANG OCEAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com