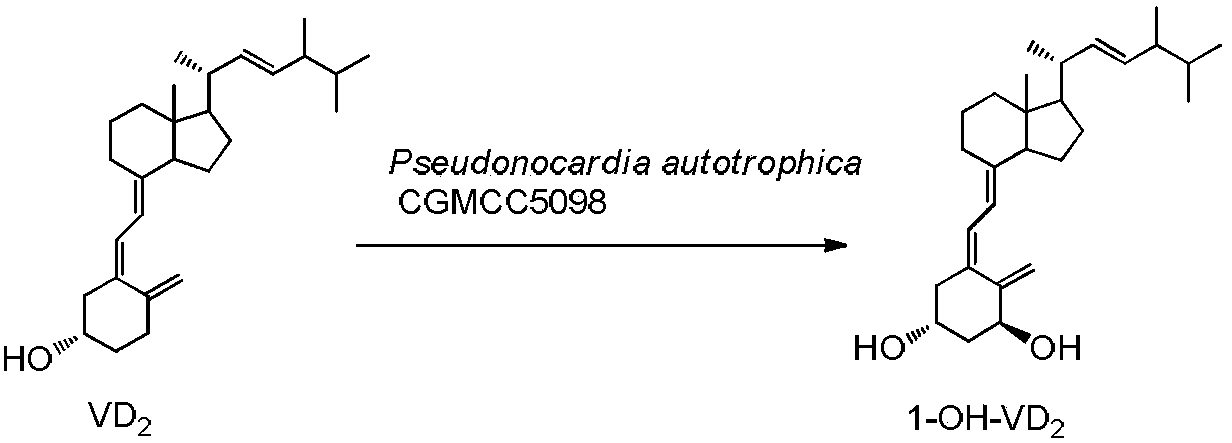
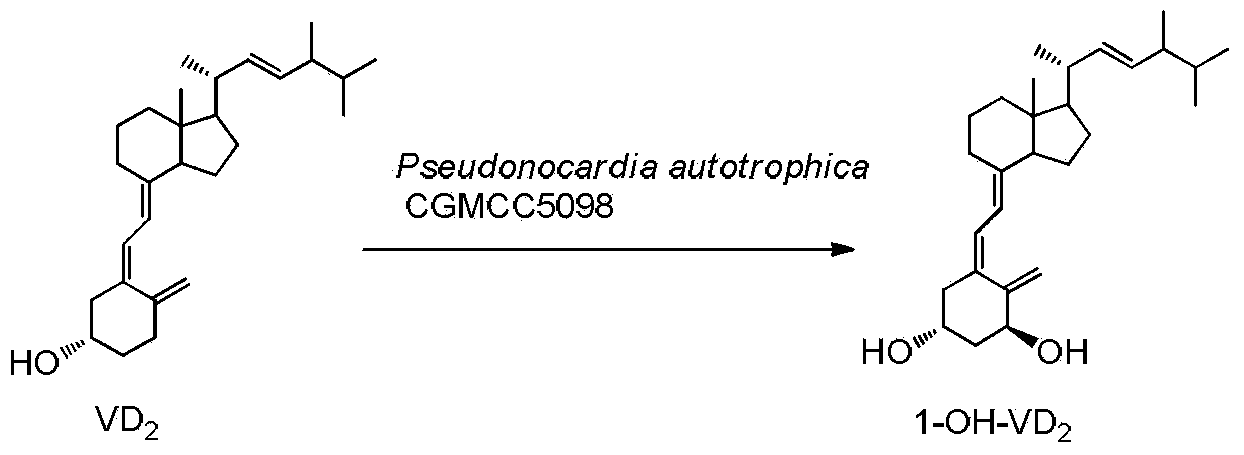
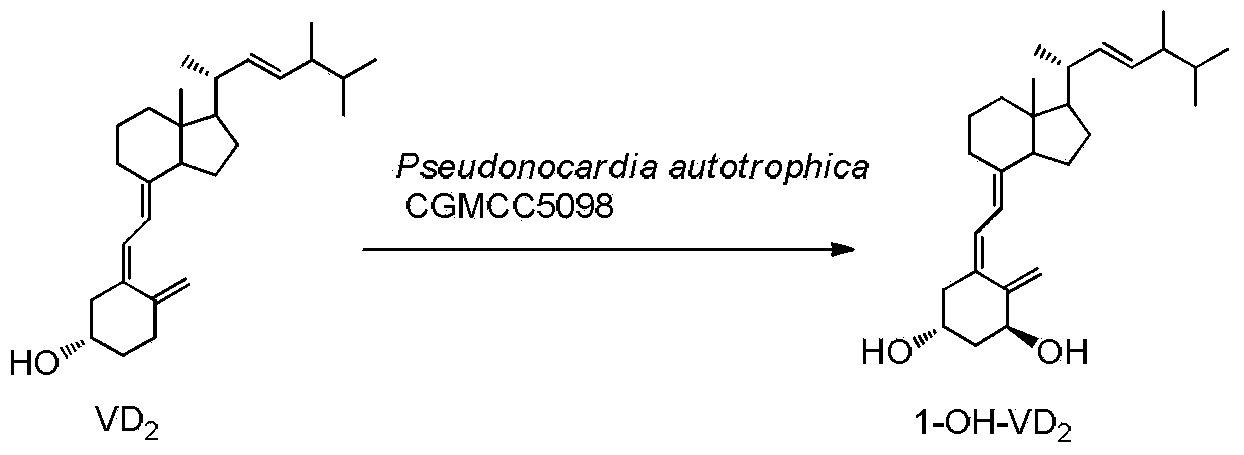
Method for preparing 1alpha-hydroxy vitamin D through microbial conversion
A technology for the transformation of hydroxyvitamin and microorganisms, which is applied in microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of complex separation and purification, complicated operations, and numerous steps, and achieves high yield, simple operation, and low cost. The effect of manufacturing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 100ml of culture medium in a 500ml shake flask, 1.5% glucose, 1.5% soybean cake powder, 0.5% corn steep liquor, 0.5% NaCl, 0.2% CaCO3, pH 7.0. The culture medium was sterilized at 121°C for 30 minutes. Insert 2 ml of the spore suspension of the strain CGMCC No.5098, culture with rotary shaking at 27°C at 240 rpm, culture for 4 days, 50 mg of vitamin D 3 Dissolve 10 mg of β-cyclodextrin and 10 mg Triton X-100 in 2 mL of ethanol, add this mixture into the culture medium, continue to cultivate for 3 days, filter, extract the filtrate with ethyl acetate 2×50 mL, concentrate the extract, and use silica gel Column [column chromatography silica gel, ethyl acetate:petroleum ether (1:2)] separation to obtain 25 mg of alfacalcidol, a hydroxylated product at the 1α position, with a yield of 50%.
[0024] U V: λmax=265nm, λmin=211nm, 1 H NMR (400MHz, DMSO) 6.38(1H,d), 6.02(1H,d), 5.33(1H,s), 5.01(1H,s), 4.444(1H,m), 4.23(1H,m), 2.82 (1H,m), 2.60(1H,m), 2.32(1H,m), 0.92(3H,d), 0....
Embodiment 2
[0026] Liquid medium formula: 1.5% glucose, 1.5% soybean cake powder, 0.5% corn steep liquor, 0.7% yeast extract, 0.5% NaCl, 0.2% CaCO3, natural pH. The culture medium was sterilized at 121°C for 30 minutes. Inoculate the bacteria in 100mL of the above-mentioned liquid medium, culture at 28°C, 200rpm, on a shaker for 60h, and the substrate VD 2 Dissolve 340 mg in 5 ml of absolute ethanol, add 1 gram of methylated β-cyclodextrin, 100 mg Triton X-100 and 5 mlw sterile water, dissolve and mix well, and add to the fermentation broth. Continue to cultivate for 72h. Extraction of fermentation broth: Add 30ml of acetone to 100mL of fermentation broth, add 50mL of ethyl acetate, shake well, let it stand, take the supernatant, concentrate, and perform chromatography, ethyl acetate / petroleum ether=1 / 2, get 1α-hydroxylation product calciferol 140mg, the yield is about 40%.
[0027] U V: λmax=265nm, λmin=211nm, MS(EI)(m / z): 412(M + ), 1 H NMR (400MHz, CDCl 3)δ0.57(s,3H),0.76-0.91(m,...
Embodiment 3
[0029] 250ml shake flask with 50ml culture medium, 1.5% glucose, 1.5% soybean cake powder, 0.5% corn steep liquor, 0.5% NaCl, 0.2% CaCO3, pH 7.0. The culture medium was sterilized at 121°C for 30 minutes. Insert the colony of strain CGMCC No.5098, culture with rotary shaking at 27°C, rotation speed 200rpm, culture for 3 days, 30mg vitamin D 2 Dissolve 10 mg of β-cyclodextrin, 5 mg of γ-cyclodextrin, and 5 mg of Triton X-100 in a mixed solvent of 2 mL of ethanol and 1 m of sterile water, add this mixture to the culture medium, continue to cultivate for 3 days, and filter , the filtrate was extracted with ethyl acetate 2×50mL, the extract was concentrated, and separated with a silica gel column [column chromatography silica gel, ethyl acetate:petroleum ether (1:2)] to obtain 1α hydroxylated product calciferol 10mg, Yield 33%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com