High performance liquid chromatography method for simultaneously separating and analyzing paricalcitol and isomer impurities in paricalcitol injection
A high-performance liquid chromatography and paricalcitol technology, which is applied in the field of analytical chemistry, can solve the problems of no separation and analysis method for detecting isomer impurities, no literature report, etc., and achieves the effect of high accuracy and avoiding interference.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
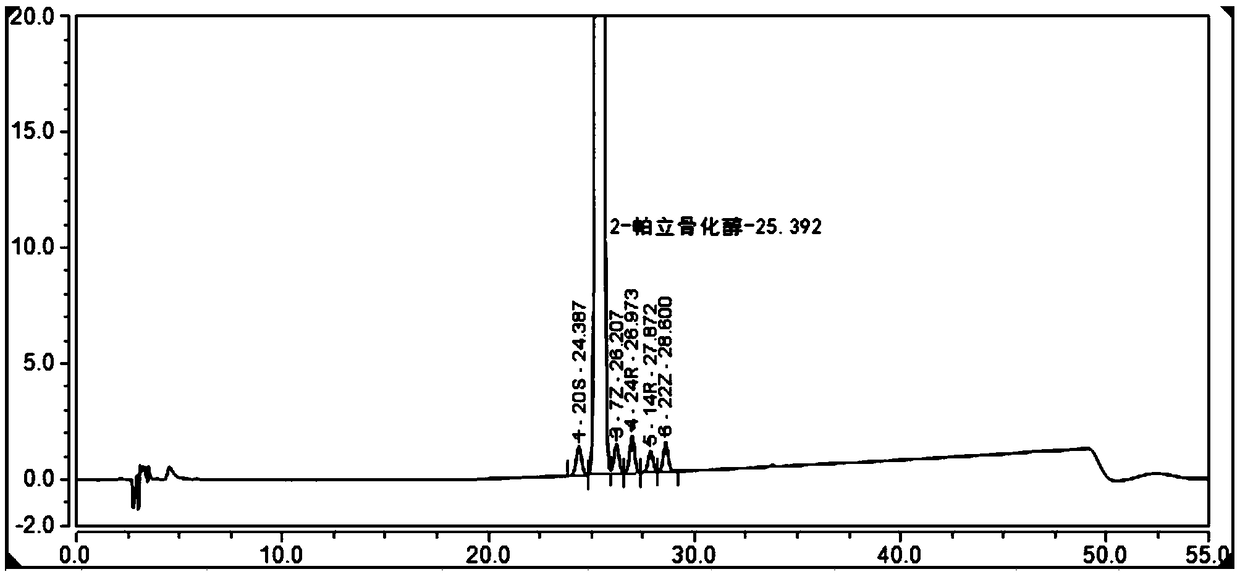
[0066] Example 1 Simultaneous separation and analysis of Paricalcitol and isomer impurities in Paricalcitol Injection
[0067] 1. Reference substance solution: keep away from light. Get this product Paricalcitol solution, as need testing solution; Accurately measure and take the Paricalcitol reference substance stock solution in right amount, make about containing Paricalcitol 50ng in every 1ml with 50% ethanol quantitative dilution solution, as a contrast solution;
[0068] 2. Sensitivity solution: Accurately measure an appropriate amount of the reference substance solution, and quantitatively dilute it with 50% ethanol to make a solution containing about 5 ng of paricalcitol per 1 ml, as the sensitivity solution;
[0069] 3. System suitability solution: Accurately measure the appropriate amount of paricalcitol reference substance stock solution and impurity 20S, 7Z, 24R, 14R, 22Z reference substance stock solution respectively, and dilute with 50% ethanol to make each 1ml c...
Embodiment 2
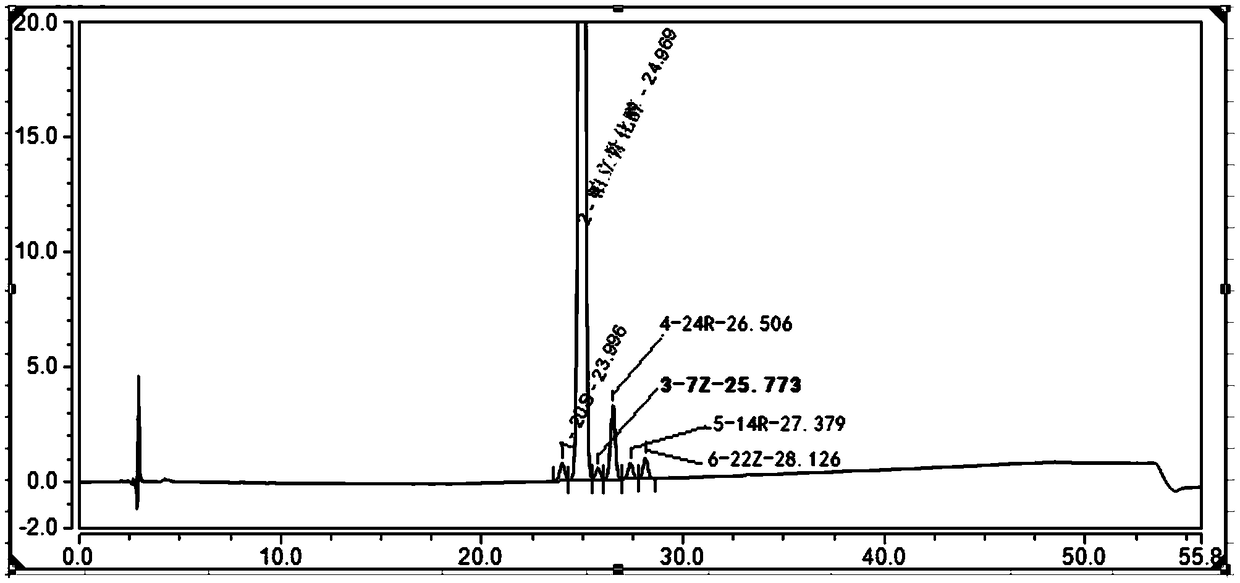
[0076] Example 2 Simultaneous separation and analysis of paricalcitol and isomer impurities in paricalcitol injection
[0077] The preparation of the reference solution, the sensitivity solution and the system suitability solution is the same as the steps 1-3 of Example 1, and the separation, analysis and detection are carried out according to the chromatographic conditions in Table 3. The chromatogram results are as figure 2 As shown, the separation degree of each impurity is greater than 1.5.
[0078] Table 3. Chromatographic conditions
[0079]
Embodiment 3
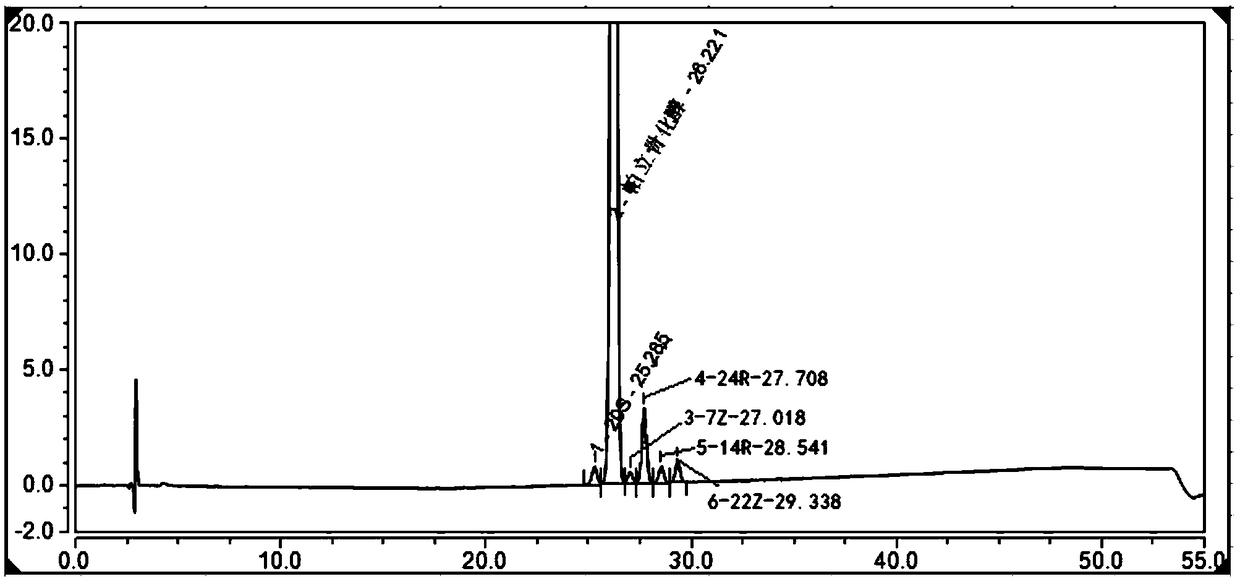
[0080] Example 3 Simultaneous Separation and Analysis of Paricalcitol and Isomer Impurities in Paricalcitol Injection
[0081] The preparation of the reference solution, the sensitivity solution and the system suitability solution is the same as the steps 1-3 of Example 1, and the separation, analysis and detection are carried out according to the chromatographic conditions in Table 4. The chromatogram results are as image 3 As shown, the separation degree of each impurity is greater than 1.5.
[0082] Table 4. Chromatographic conditions
[0083]
[0084]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com