Stable paricalcitol pharmaceutical composition and preparation method thereof
A technology for paricalcitol and a composition is applied in the field of paricalcitol pharmaceutical composition and preparation thereof, and can solve the problems of unstable hygroscopicity of paricalcitol, inability to prepare oral preparations and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
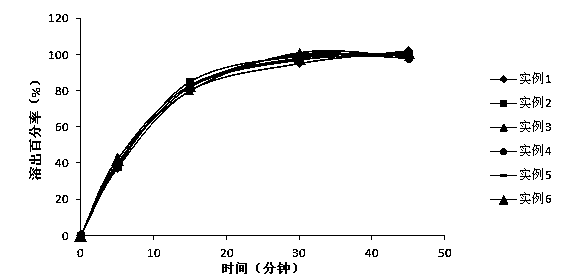
Examples
Embodiment 1
[0035] Prescription Composition of Paricalcitol Tablets
[0036] Paricalcitol 0.002g
[0037] Hydroxypropyl-α-CD inclusion complex 0.168g
[0038]Trehalose 2.5g
[0040] Povidone 4.50g
[0041] Lactose 70.23g
[0043] Made into tablets 85mg / tablet, made into 1000 pieces
[0044] Specific preparation method:
[0045] a. Weigh 0.168g hydroxypropyl-α-CD cyclodextrin and dissolve it in pure water, weigh 0.002g paricalcitol and add it to the cyclodextrin solution, stir until the solution is clear and transparent, forming a paricalcitol ring Dextrin inclusion compound;
[0046] b. Weigh 2.5g of trehalose and dissolve in the above clathrate, freeze-dry, pre-freeze at -40°C, vacuumize at 85 Pa, -20°C, dry at 100 Pa, 20°C to obtain freeze-dried mixture Ⅰ ;
[0047] c. Crush the freeze-dried mixture I, pass through a 100-mesh sieve, add 7.00g of starch slurry, 4.50g of povidone, and 70.23g of lactose according to the pres...
Embodiment 2
[0059] Prescription Composition of Paricalcitol Tablets
[0060] Paricalcitol 0.002g
[0061] Hydroxypropyl-γ-CD inclusion complex 0.004g
[0062] Dextran 1.40g
[0063] Pectin 4.50g
[0064] Sodium carboxymethyl starch 8.00g
[0065] Maltose 70.10g
[0066] Magnesium Stearate 1.00g
[0067] Made into tablets 85mg / tablet, made into 1000 pieces
[0068] Specific preparation method:
[0069] a. Weigh 0.004g hydroxypropyl-γ-CD cyclodextrin and dissolve it in pure water, weigh 0.002g paricalcitol and add it to the cyclodextrin solution, stir until the solution is clear and transparent, forming a paricalcitol ring Dextrin inclusion compound;
[0070] b. Weigh 1.40g of dextran and dissolve it in the above clathrate solution, freeze-dry, pre-freeze at -30°C, vacuum at 75 Pa, -20°C, and dry at 90 Pa, 20°C to obtain freeze-dried mixture Ⅰ ;
[0071] c. Crush the freeze-dried mixture I, pass through a 100-mesh sieve, add 4.50 g of pectin, 8.00 g of sodium carboxymethyl starch,...
Embodiment 3
[0080] Prescription Composition of Paricalcitol Tablets
[0081] Paricalcitol 0.002g
[0082] Hydroxypropyl-α-CD inclusion complex 0.248g
[0083] Mannitol 1.00g
[0084] Hypromellose 10.00g
[0085] Low-substituted hydroxypropyl cellulose 10.00g
[0086] Sorbitol 63.25g
[0087] Micronized silica gel 0.50g
[0088] Made into tablets 85mg / tablet, made into 1000 pieces
[0089] Specific preparation method:
[0090] a. Weigh 0.248g hydroxypropyl-α-CD cyclodextrin and dissolve it in pure water, weigh 0.002g paricalcitol and add it to the cyclodextrin solution, stir until the solution is clear and transparent, forming a paricalcitol ring Dextrin inclusion compound;
[0091] b. Weigh 1.00 g of mannitol and dissolve it in the clathrate solution, freeze-dry, pre-freeze at -30°C, vacuum at 75 Pa at -20°C, and dry at 90 Pa at 20°C to obtain a freeze-dried mixture I;
[0092] c. Crush the freeze-dried mixture I, pass through a 100-mesh sieve, add 10.00g of hydroxypropylmethylc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com