Method for preparing paricalcitol
A paricalcitol and chemical reaction technology, applied in the field of 1-α-hydroxy-19-de-methylene vitamin D2 for the treatment of osteoporosis, can solve the uneconomical and complicated operation of paricalcitol , many steps and other problems, to achieve the effect of increasing yield, simplifying process flow and reducing manufacturing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
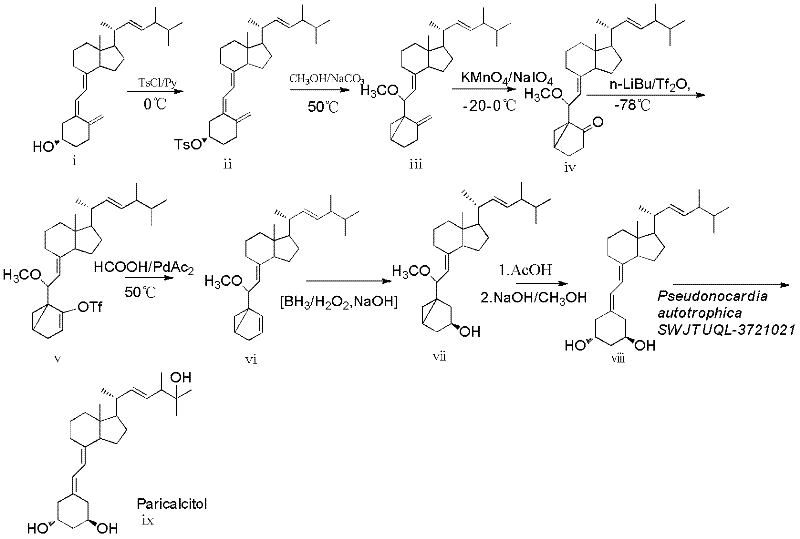
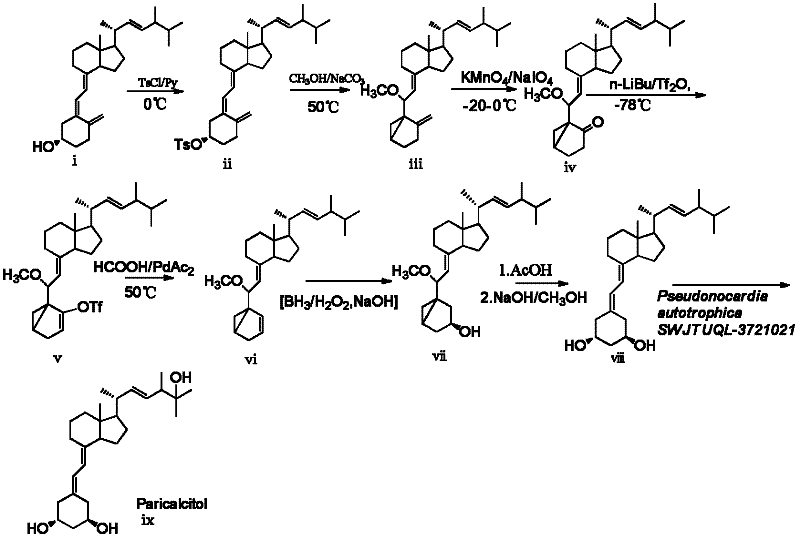
[0034] 1-Hydroxy-19-norvitamin D 2 Preparation of (viii)
[0035] a) 10-oxo-3,5-cyclovitamin D 2 Synthesis of (iv)
[0036] 3,5-cyclovitamin D 2 The synthesis of (iii) adopts the method such as DeLuca (US4195027) vitamin D 2 Converted to 3,5-cyclized vitamin D 2 (iii), add 3.88g, 9.46mmol) 3,5 cyclic vitamin D in the reaction flask 2 , dissolved in 300ml ethanol, cooled to -20°C, and then potassium permanganate solution (KMnO 4 3.33g, H 2 O 100ml), about 20 minutes. Stir at this temperature for 1 h, and then stir for 15 min in a water bath at 40 °C. After filtration, the filtrate was concentrated to dryness under reduced pressure to obtain light yellow oily mixture 4 (3.67 g). The crude product was dissolved in 300 ml of ethanol, 100 ml of saturated sodium periodate was added, and stirred at room temperature for 2.5 h. The reaction solution was extracted with ethyl acetate (2×200ml) and washed with saturated sodium chloride solution (1×200ml). The organic layer was ...
Embodiment 2
[0042] a) 10-oxo-3,5-cyclovitamin D 2 Synthesis of (iv)
[0043] 3,5-cyclovitamin D 2 The synthesis of (iii) adopts the method such as DeLuca (US4195027) vitamin D 2 Converted to 3,5-cyclized vitamin D 2 (iii), 3,5-cycling VD 2 5 g, dissolved in 150 ml of ethanol, placed in a low-temperature bath at -20°C, and added dropwise with 3% KMnO 4(3% aqueous solution) 320 milliliters, dropwise, continue to react for 2 hours, timed TLC detection, 3 hours, raw material consumption is complete, 40 ℃ stirs 15 minutes, solidifies manganese dioxide, diatomaceous earth filter, concentrates to remove alcohol, adds ethyl acetate 100 ml of ester, extracted twice, concentrated to dryness, dissolved the crude product in 300 ml of ethanol, added 100 ml of saturated sodium periodate, and stirred at room temperature for 2.5 h. The reaction solution was extracted with ethyl acetate (2×200ml) and washed with saturated sodium chloride solution (1×200ml). The organic layer was dehydrated with anhy...
Embodiment 3
[0049] 100ml culture medium in a 500m shaker flask, 1.5% glucose, 1.5% soybean cake powder, 0.5% corn steep liquor, 0.5% NaCl, 0.2% CaCO 3 , pH 7.0. The medium was sterilized at 121°C for 20 minutes. Insert 2 ml of spore suspension of strain CGMCC 5098, rotate at 27°C at 240rpm, culture for 4 days, 50mg of 1-hydroxy-19-norvitamin D 2 (viii) and 10 mg of β-cyclodextrin and 10 mg of Tween-80 were dissolved in 2 mL of ethanol, and the mixture was added to the culture medium, continued to cultivate for 3 days, filtered, and the filtrate was extracted with 2×50 mL of ethyl acetate, and the extract Concentrate and separate with a silica gel column [column chromatography silica gel, ethyl acetate:petroleum ether (1:2)] to obtain 31 mg of the 25-hydroxylation product Paricalcitol with a yield of 60%. 1 H NMR (400MHz, CD 3 OD) δ6.21(d, 1H, J=11Hz, H6), 5.88(d, 1H, J=11Hz, H7), 5.35(dd, 1H, J=8 and 15Hz, H23), 5.27(dd, 1H , J=8 and 15Hz, H22), 4.04(m, 1H, H1), 3.98(m, 1H, H3), 2.84(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com