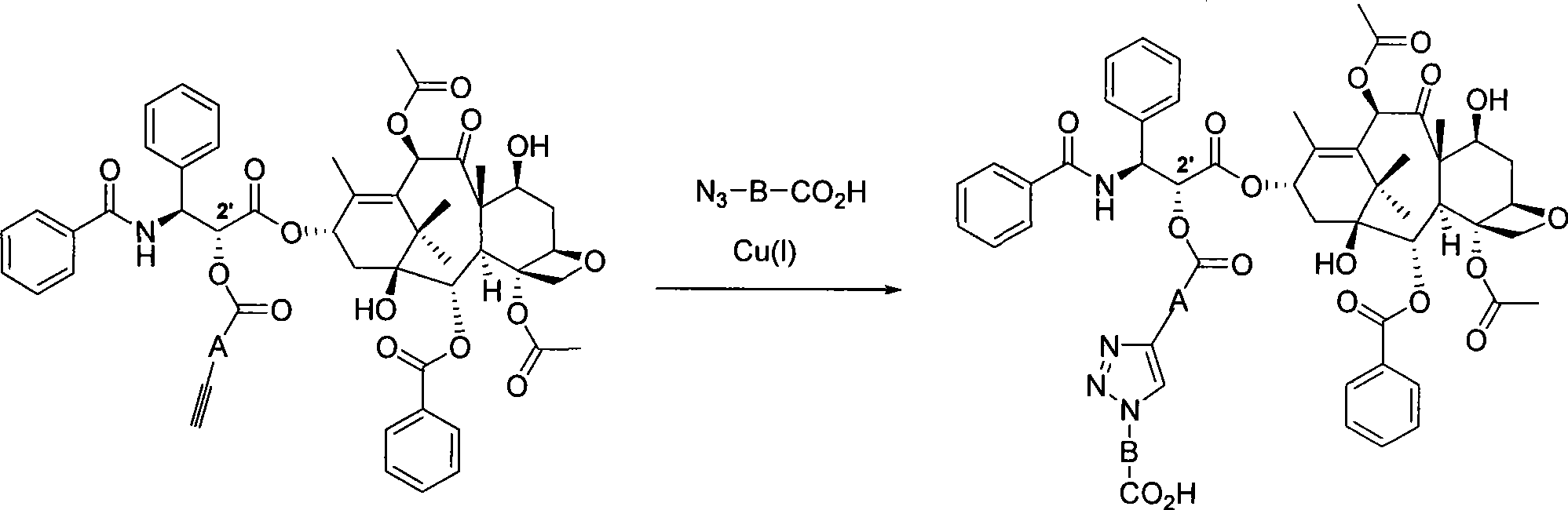Water-soluble paclitaxel ester compounds containing 1,2,3-triazole and preparation method
A technology for paclitaxel esters and compounds, which is applied in the directions of medical preparations containing active ingredients, organic chemistry, pharmaceutical combinations, etc., can solve the problems of complicated separation, easy precipitation, and reduced product yield, and achieves simplified synthesis steps and increased water solubility. , the effect of easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0044] The following examples are used to further illustrate the present invention, but this does not imply any limitation to the present invention.
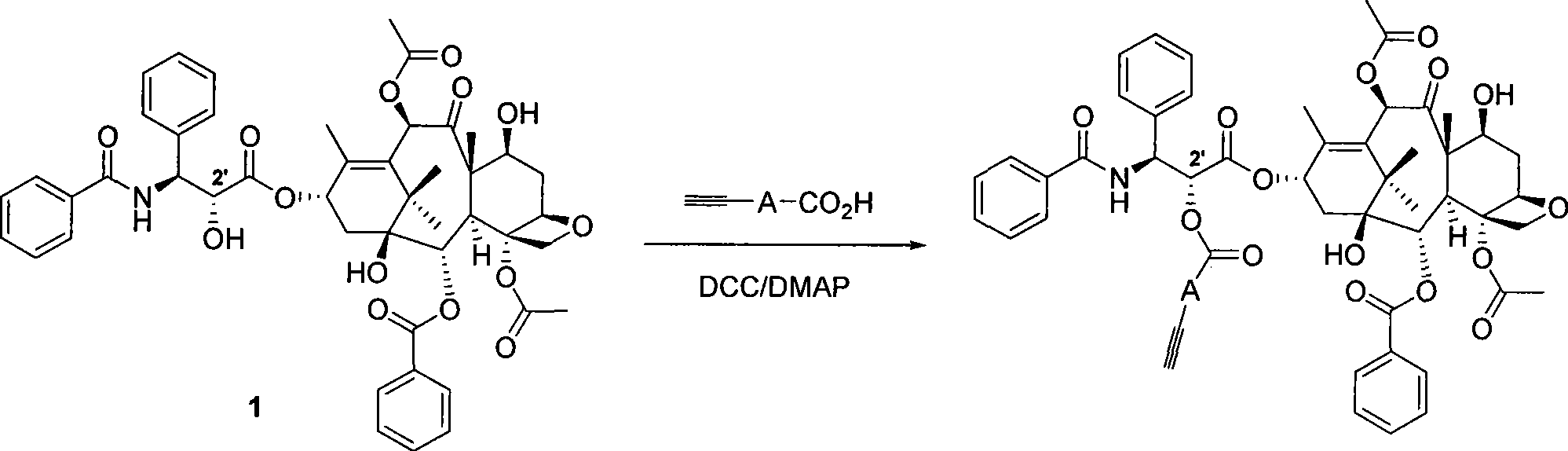
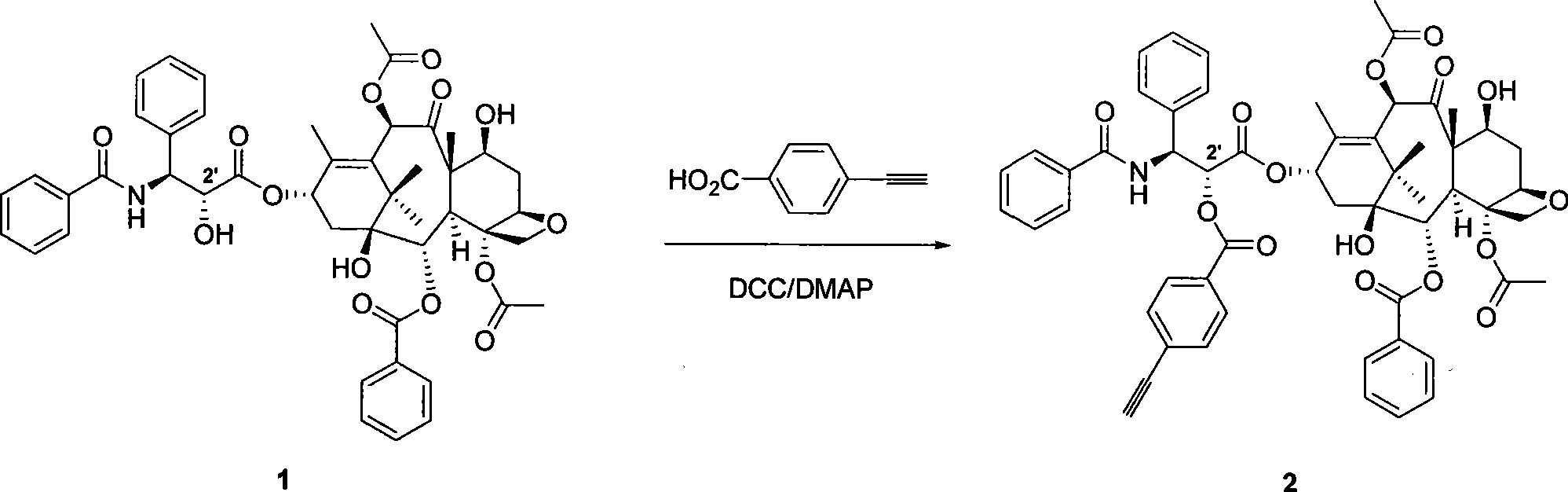
[0045] A) Synthesis of alkyne-containing paclitaxel 2
[0046] Such as image 3 As seen in the reaction formula, paclitaxel 1 (0.03mmol, 25.6mg) (Aladdin's reagent, 99%) was added to a dry reaction bottle, and dissolved in 3mL of benzene. Add p-carboxyphenylacetylene (0.03 mmol, 4.4 mg) and 4-dimethylaminopyridine DMAP (0.04 mmol, 5 mg) to the reaction solution, and stir at room temperature for 15 minutes. Then N, N-dicyclohexylcarbodiimide DCC (0.15 mmol, 31 mg) was added, the reaction was continued to stir at room temperature for 21 hours, and then the reaction was terminated. The solvent was evaporated under reduced pressure, and then separated by column chromatography (eluent: ethyl acetate / n-hexane = 1:2) to obtain a white powdery substance, of which 8% was C-7 substituted acetylene benzoate paclitaxel, and the rest It i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com