Patents
Literature
106 results about "Dutasteride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used in men to treat the symptoms of an enlarged prostate (benign prostatic hyperplasia-BPH).
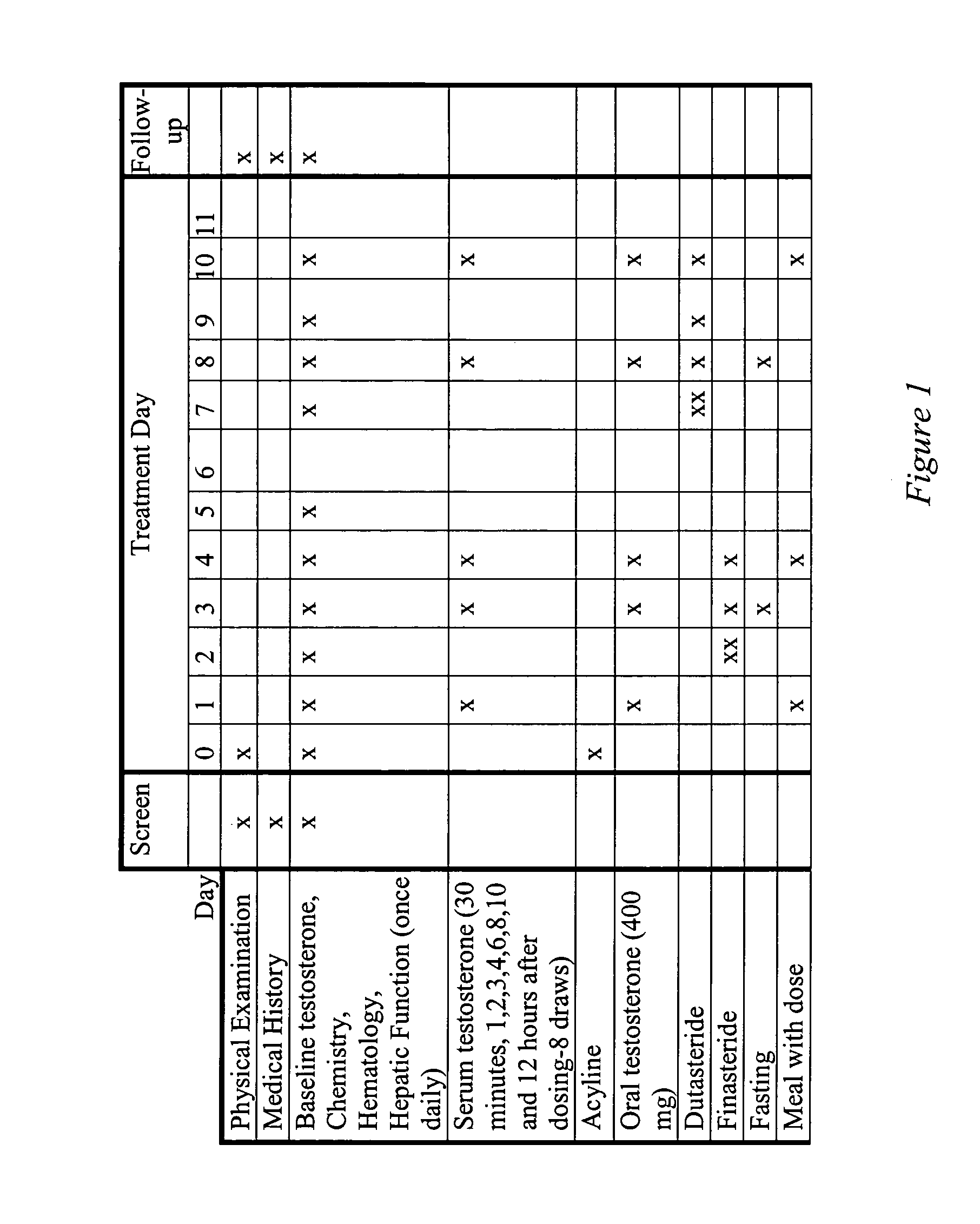
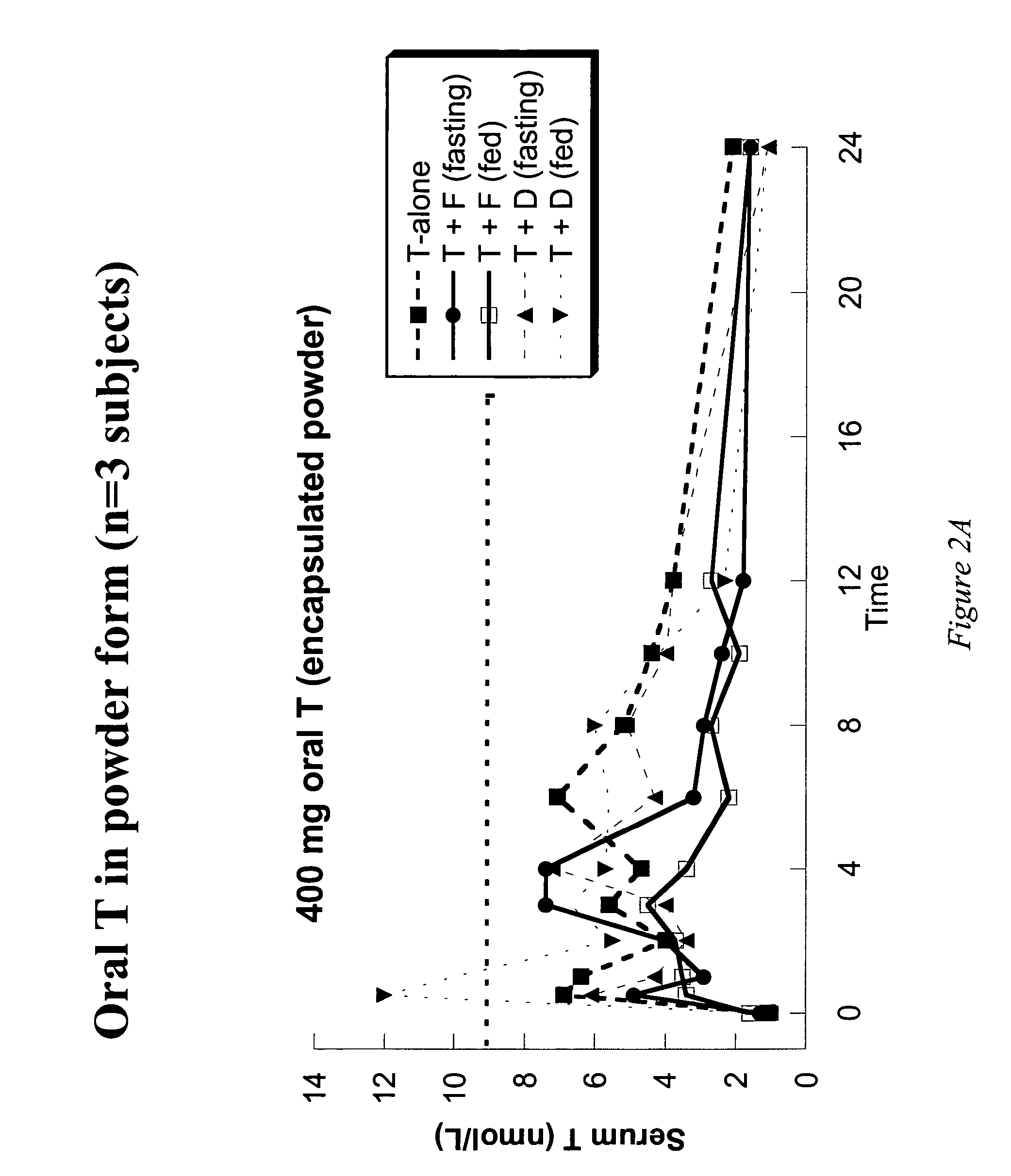
Oral androgen therapy using modulators of testosterone bioavailability
InactiveUS20050176692A1Increase heightImprove bioavailabilityBiocideAnimal repellantsBioavailabilityFinasteride
This invention provides methods of treating a mammalian subject in need of androgen therapy by orally administering to the subject testosterone, a testosterone ester, or a testosterone precursor in an oil vehicle and by administering to the subject a modulator such as finasteride or dutasteride which increases testosterone bioavailability in the subject.
Owner:UNIV OF WASHINGTON
Formulations of a nanoparticulate finasteride, dutasteride or tamsulosin hydrochloride, and mixtures thereof
Described are nanoparticulate compositions of finasteride, dutasteride, tamsulosin hydrochloride, or a combination thereof. The formulations exhibit unexpectedly prolonged release and can be maintained in a depot for release to a patient for a period of up to six months.
Owner:ELAN PHRMA INT LTD
Oral androgen therapy using modulators of testosterone bioavailability
InactiveUS7138389B2Improve bioavailabilityRestore levelBiocideAnimal repellantsBioavailabilityFinasteride
This invention provides methods of treating a mammalian subject in need of androgen therapy by orally administering to the subject testosterone, a testosterone ester, or a testosterone precursor in an oil vehicle and by administering to the subject a modulator such as finasteride or dutasteride which increases testosterone bioavailability in the subject.
Owner:UNIV OF WASHINGTON
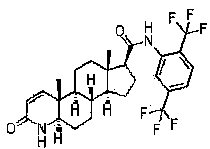
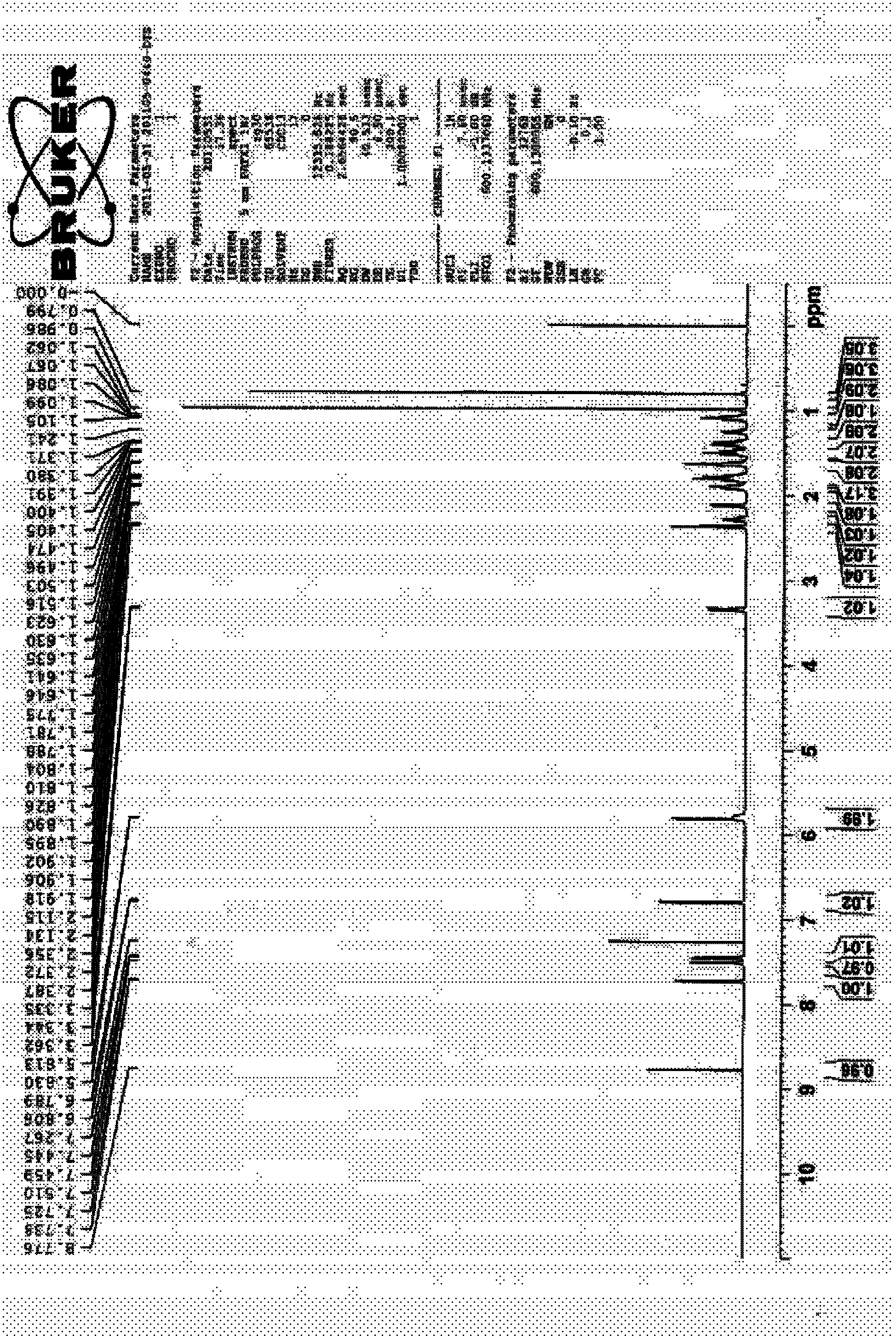
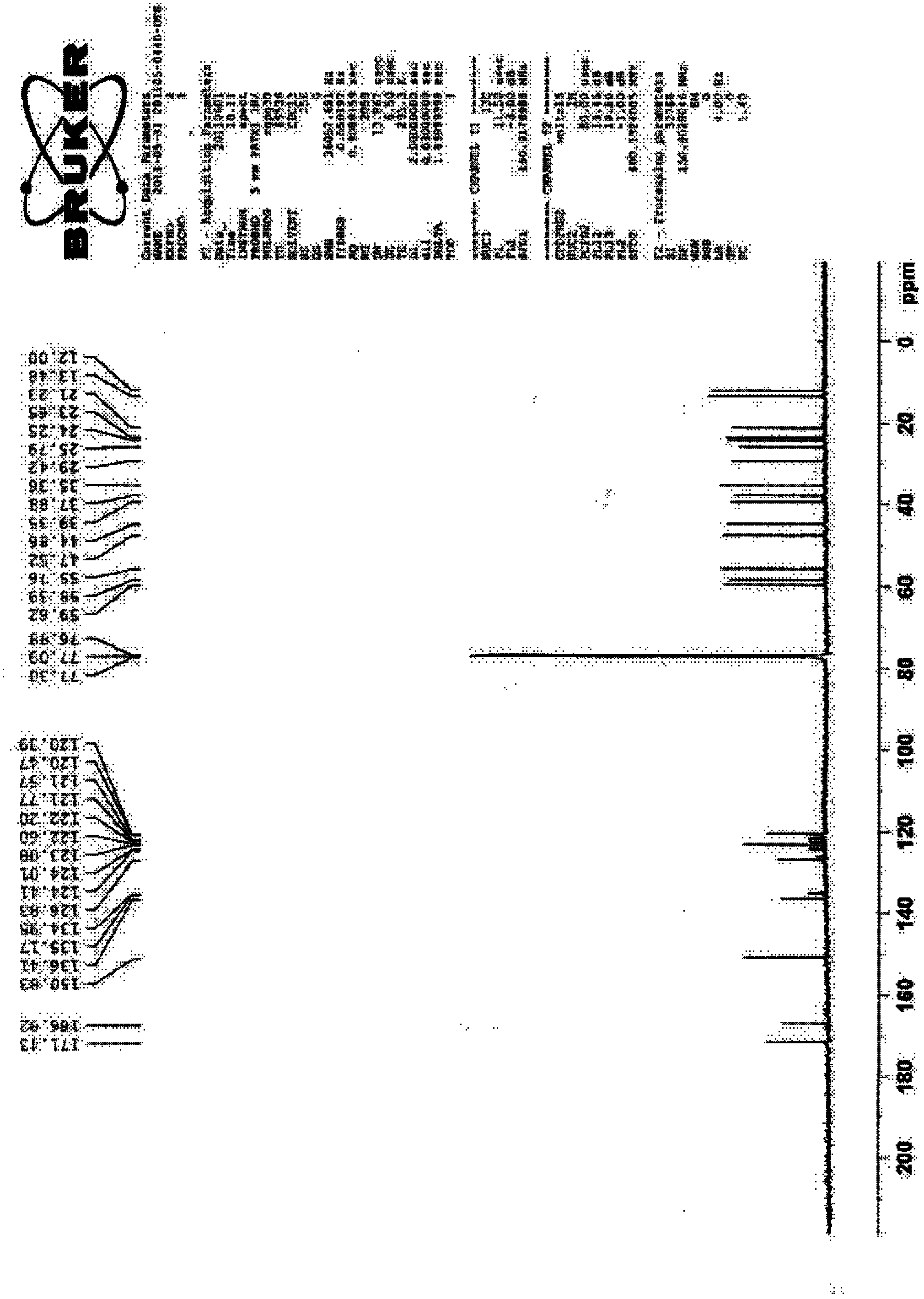
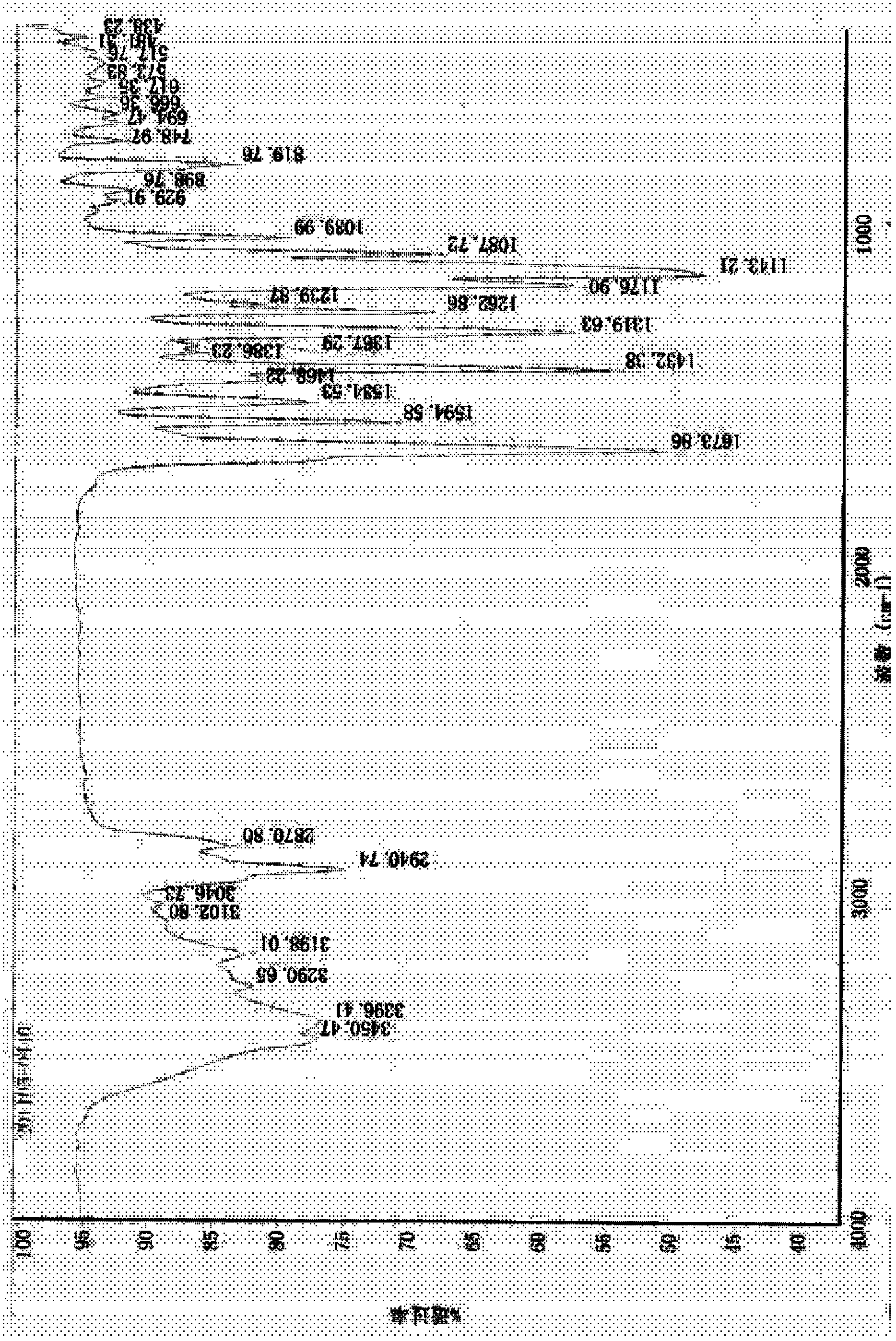
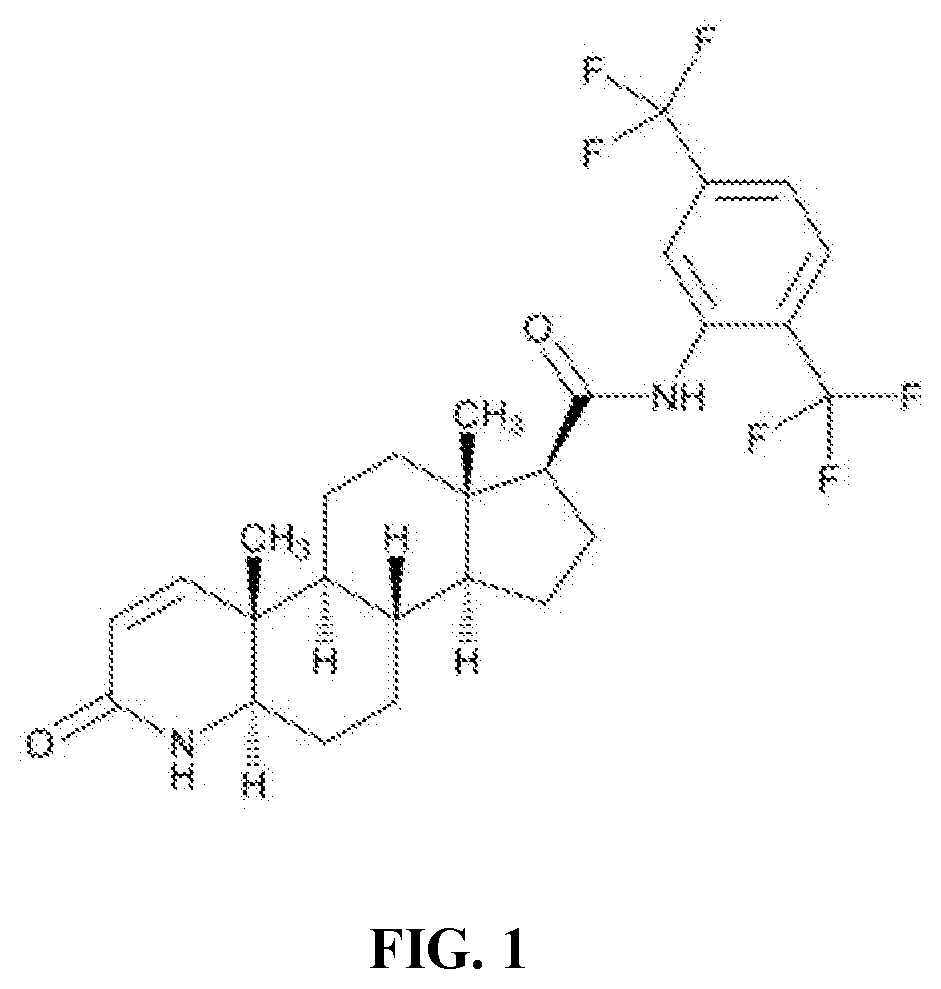
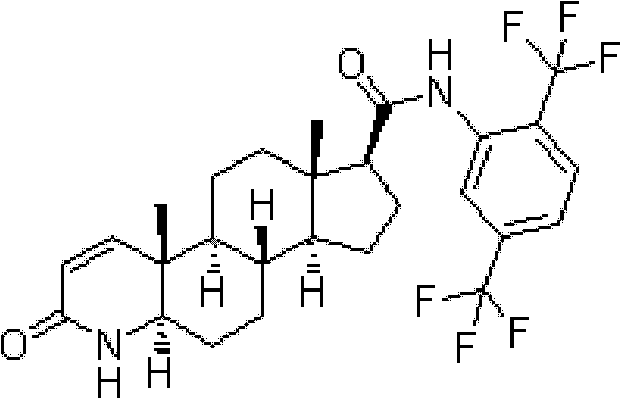
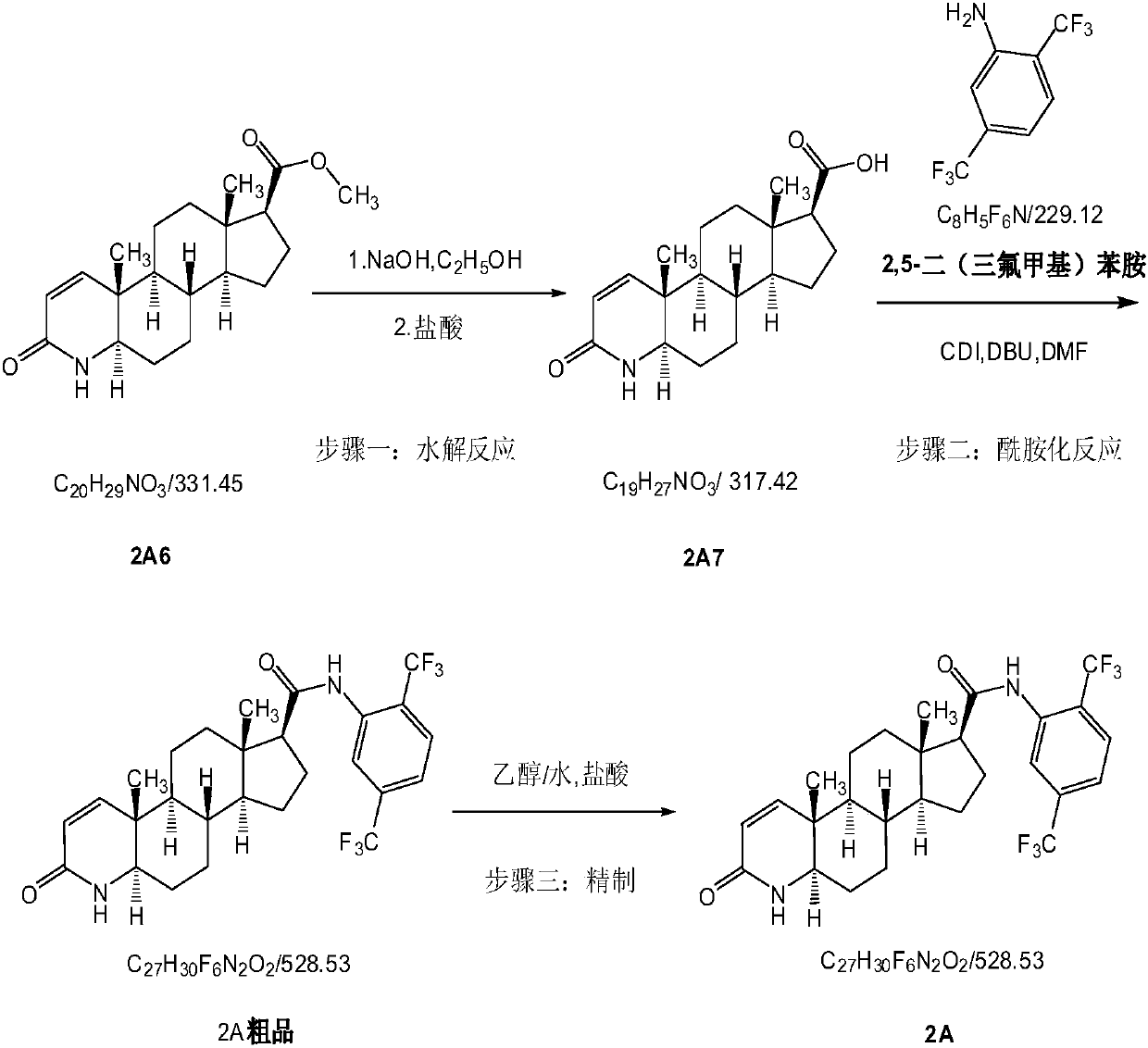
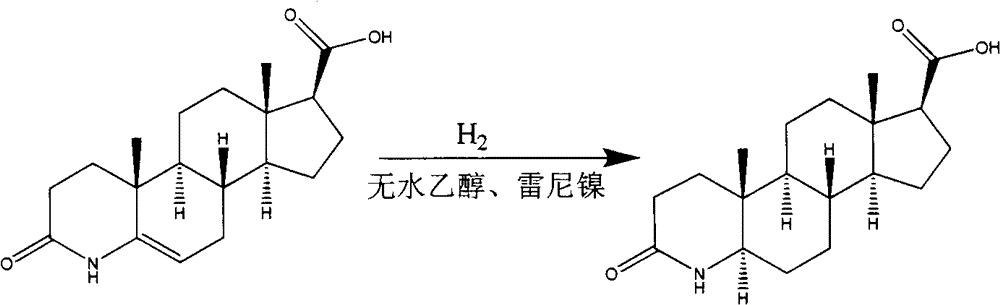
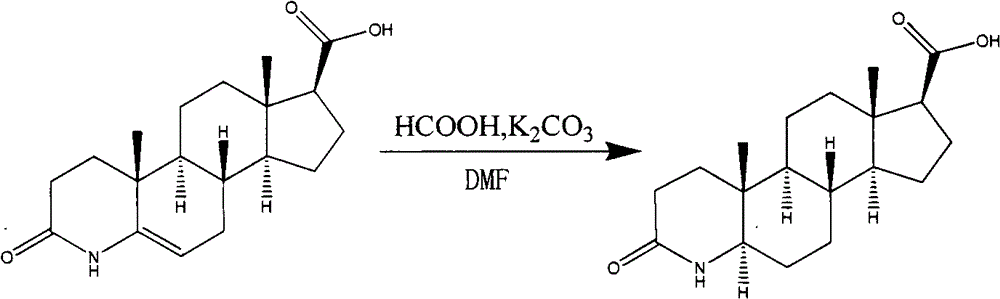
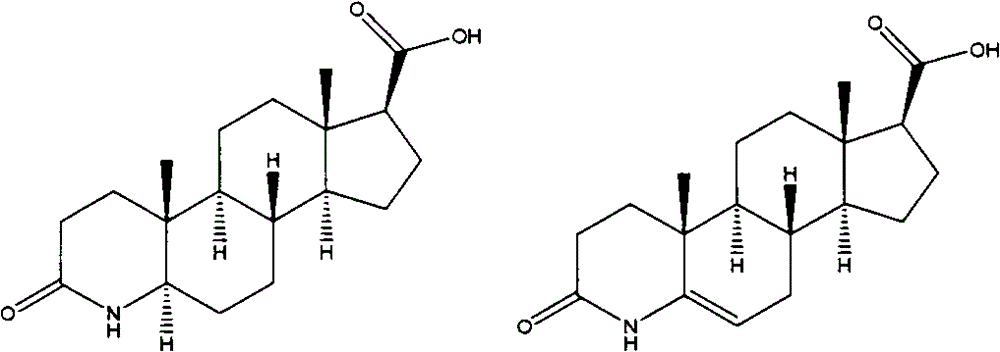
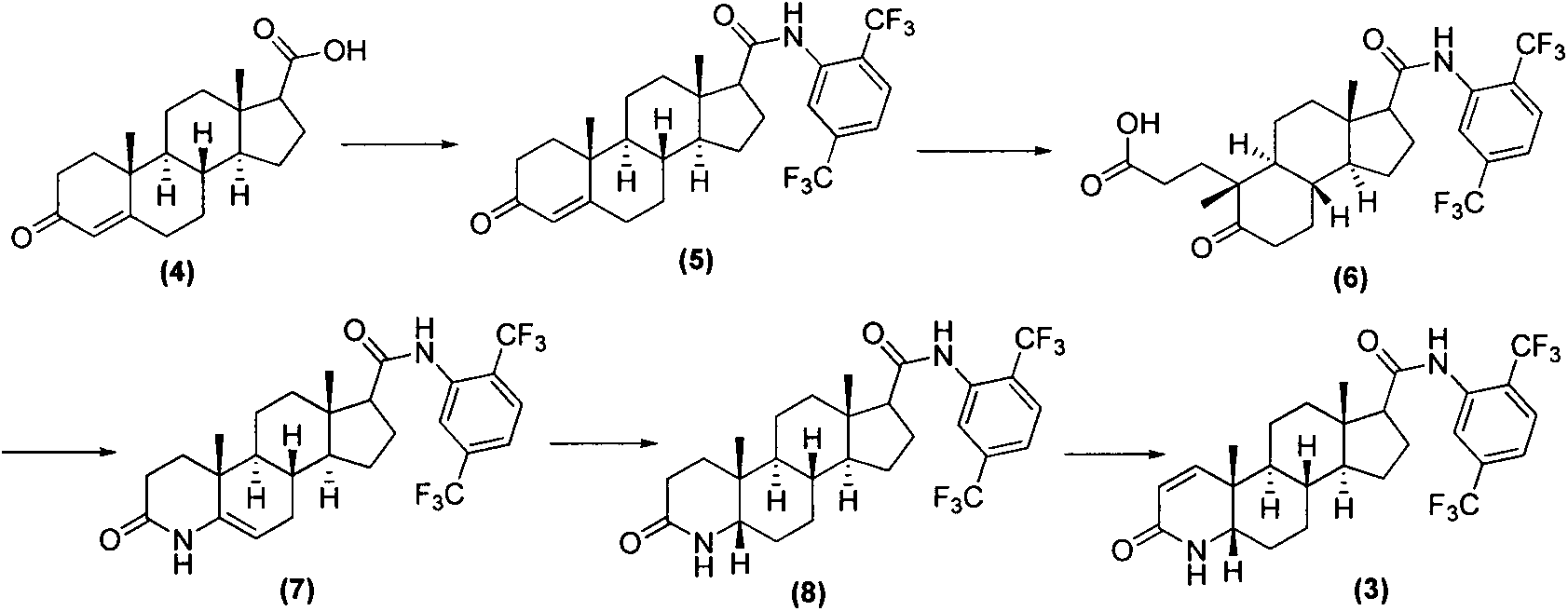
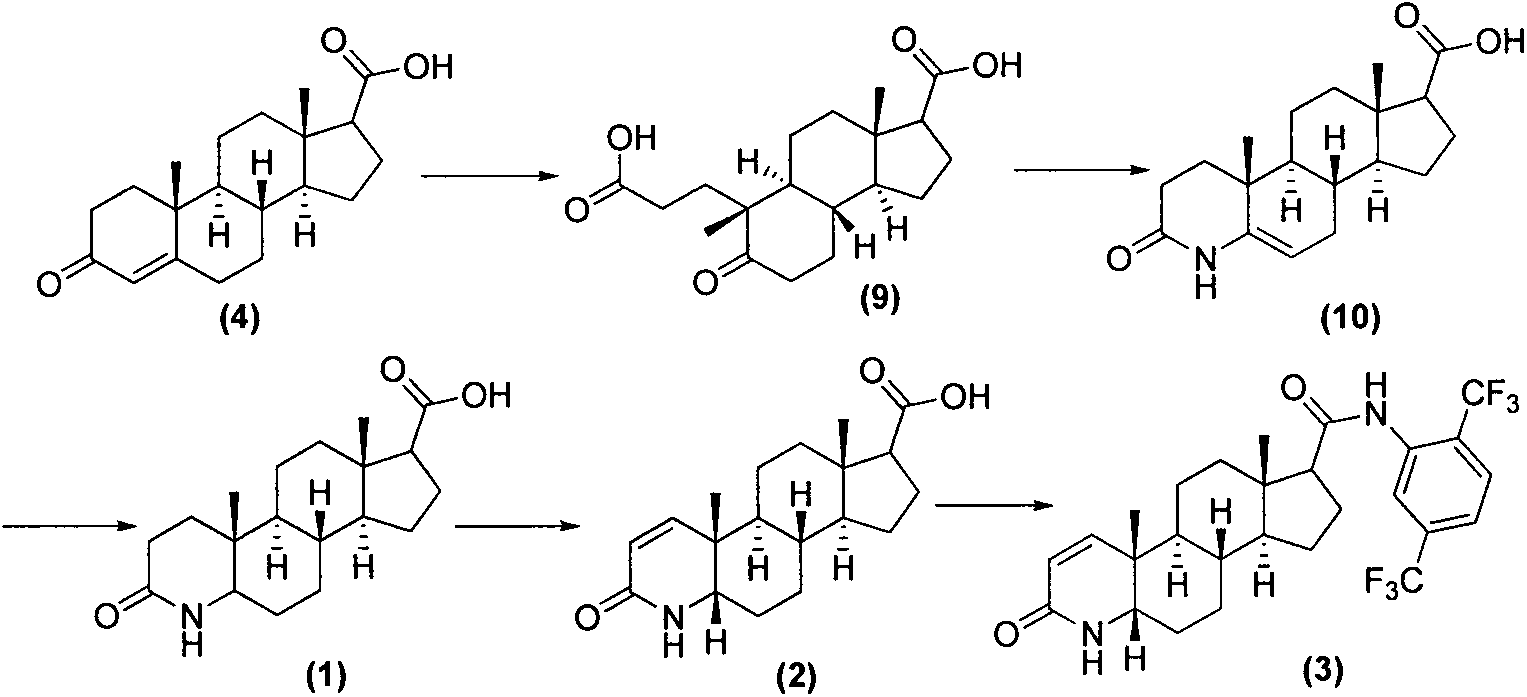
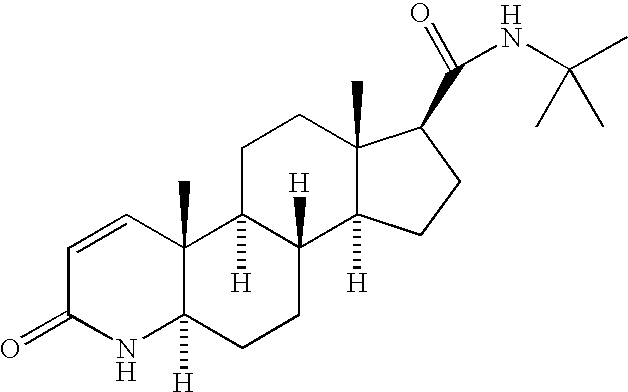
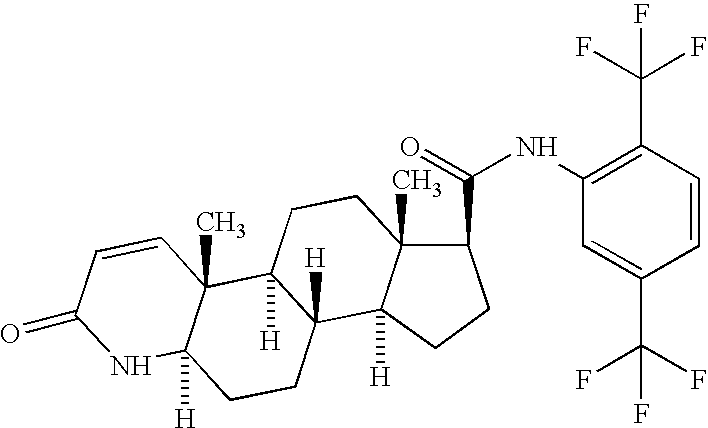
Process for the preparation of 17beta-N-[2,5-bis(trifluoromethyl)phenyl] carbamoyl-4-aza-5-alpha-androst-1-en-3-one
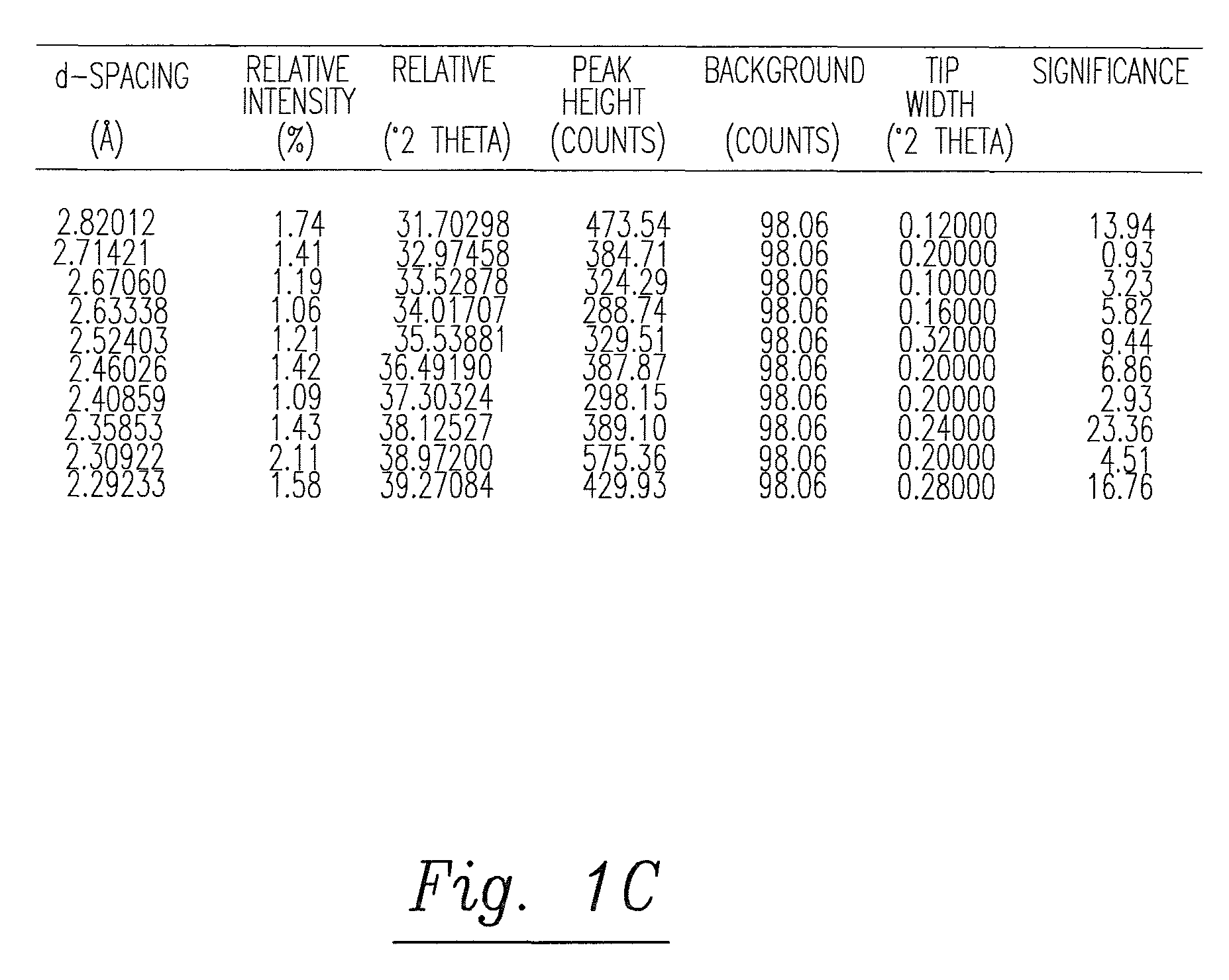
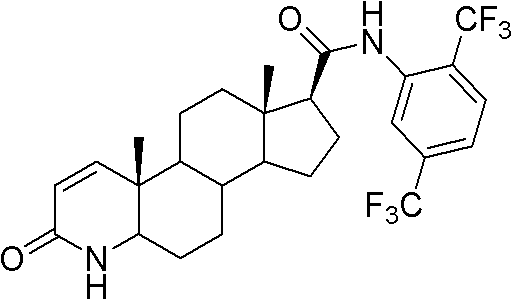
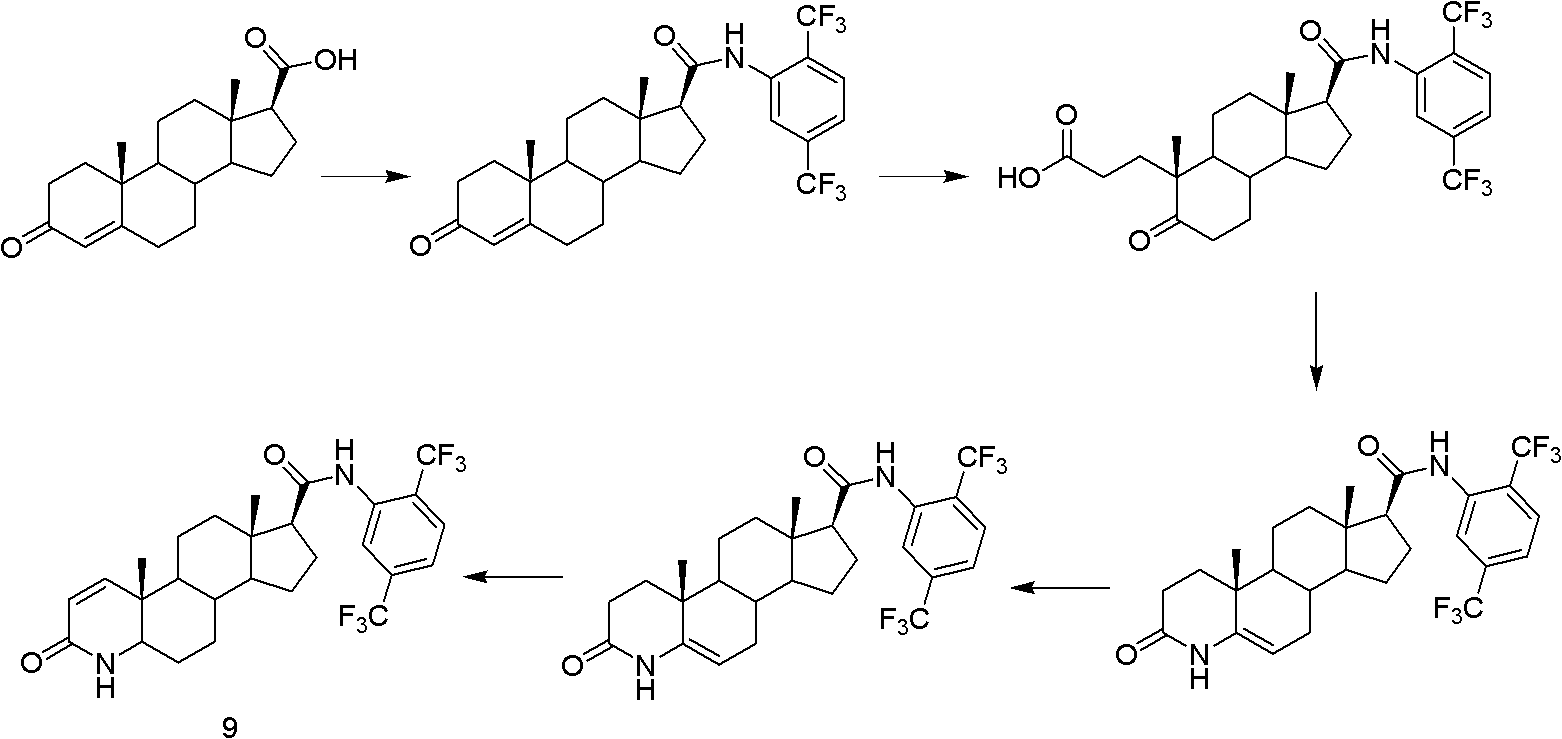
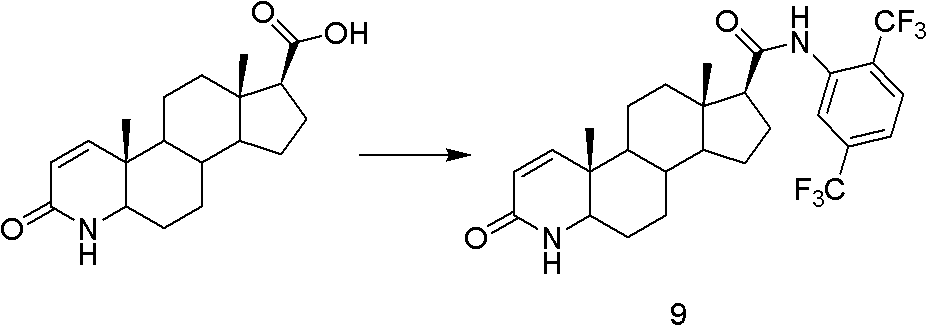
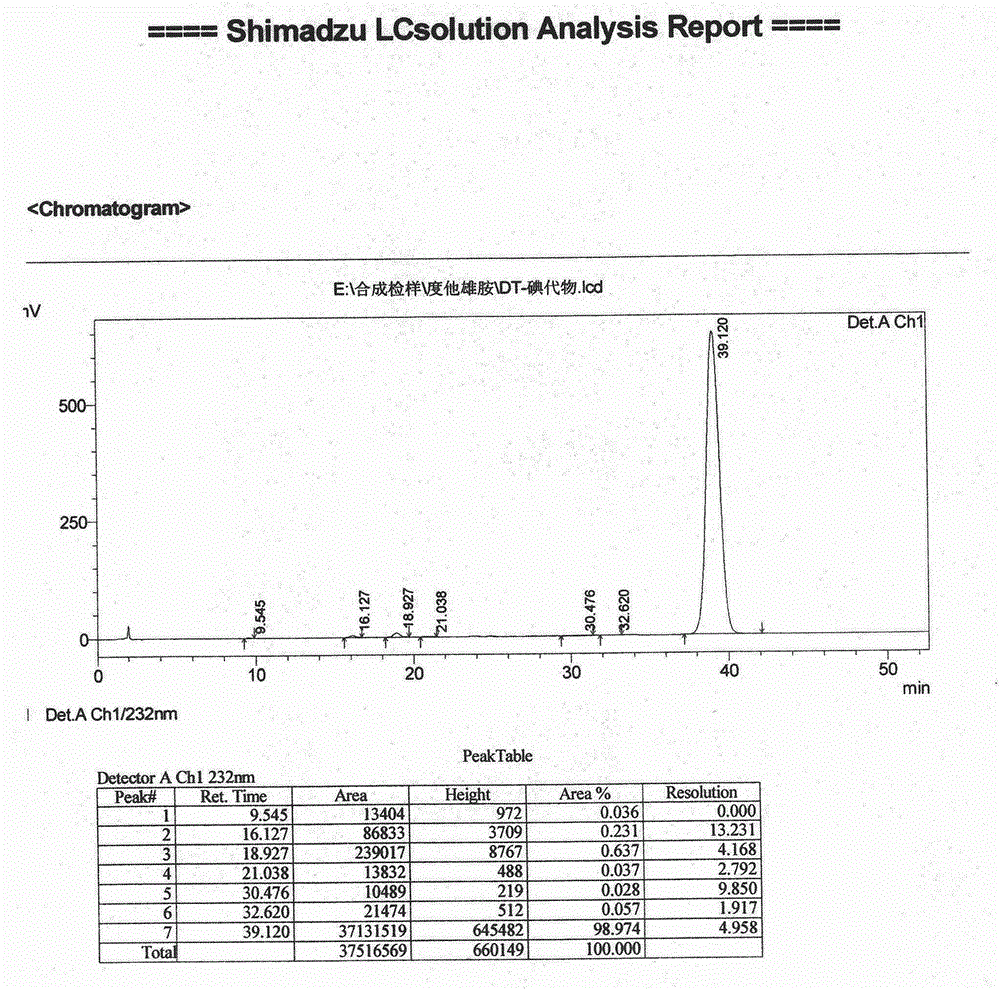
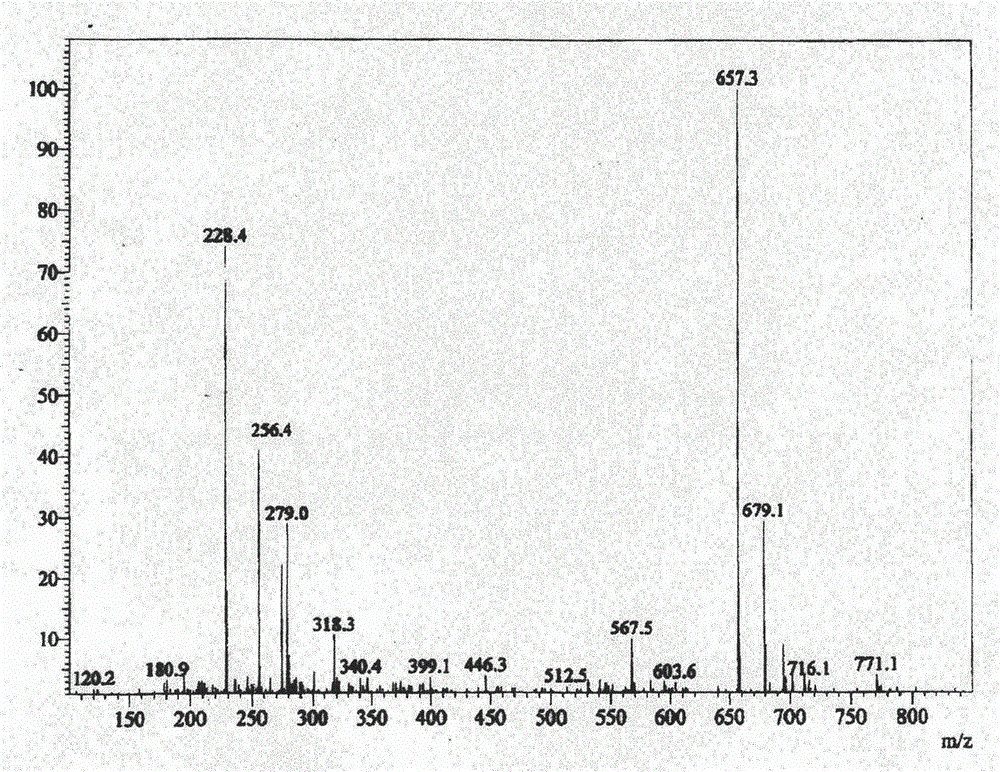
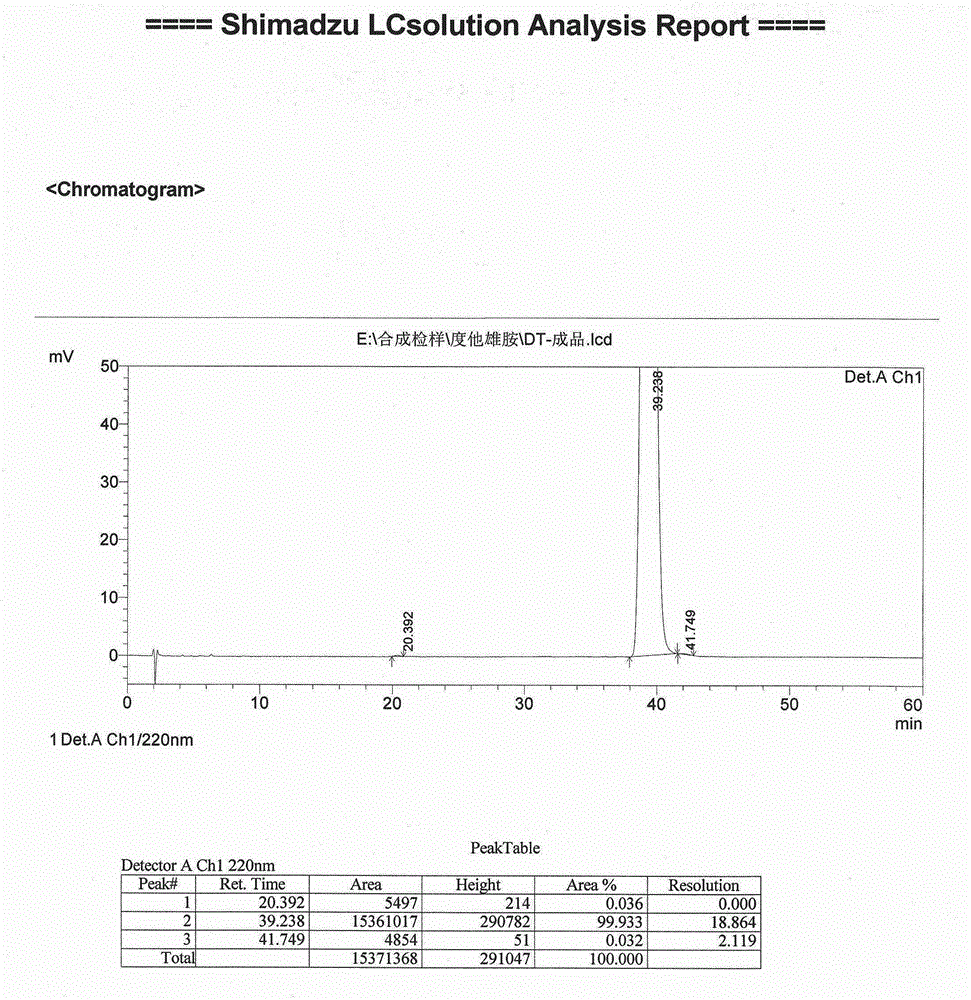
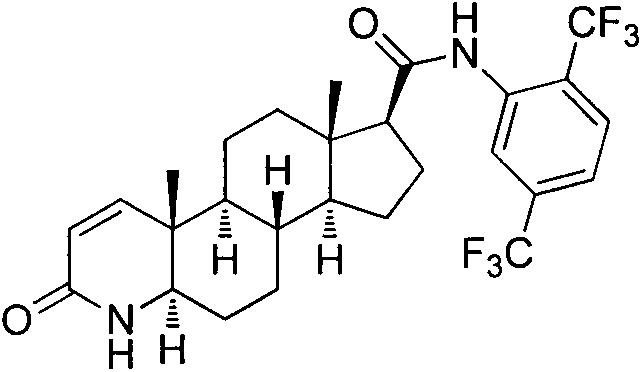
The present invention relates to a process for the preparation of Dutasteride, which is chemically known as 17β-N-[2,5-bis (trifluoromethyl) phenyl] carbamoyl-4-aza-5-α-androst-1-en-3-one and can be represented by Formula (I).
Owner:DR REDDYS LAB LTD +1
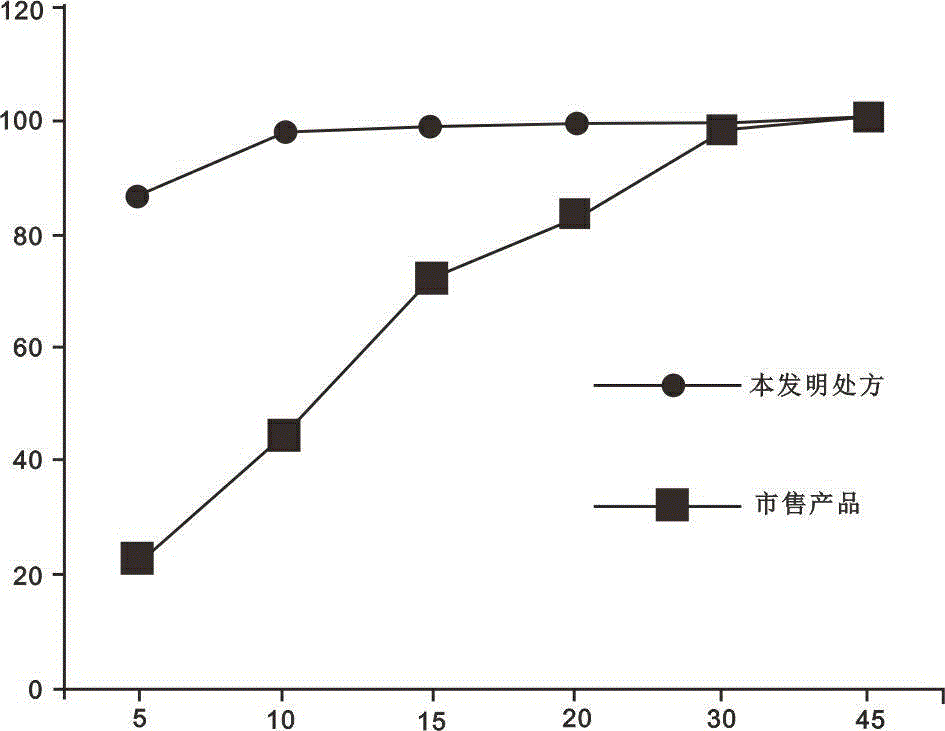
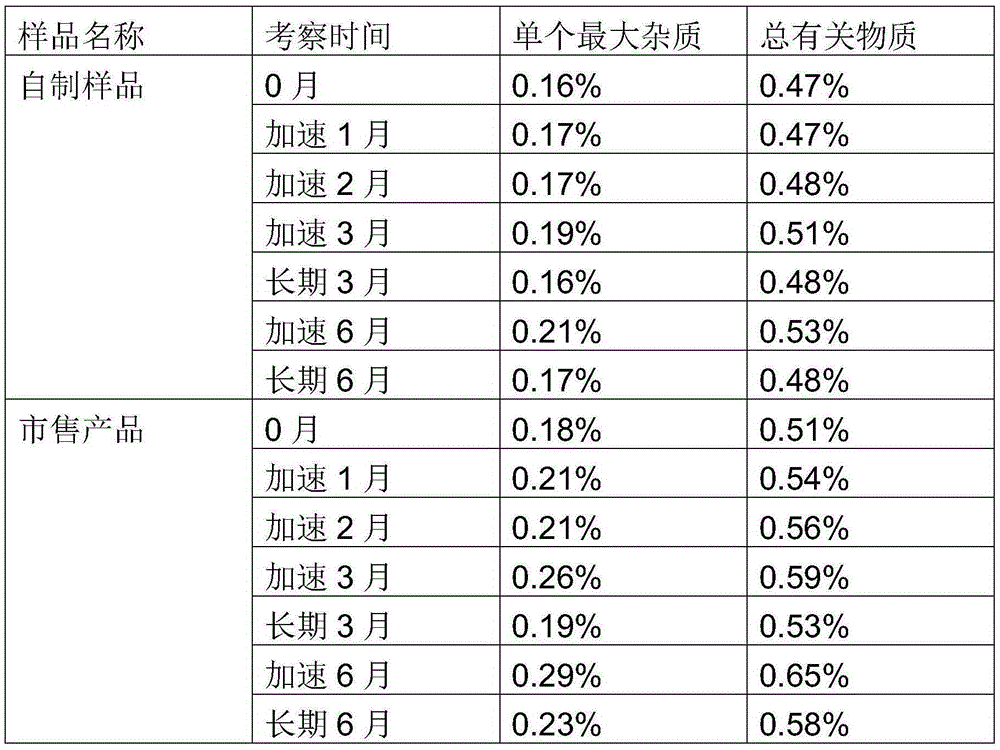
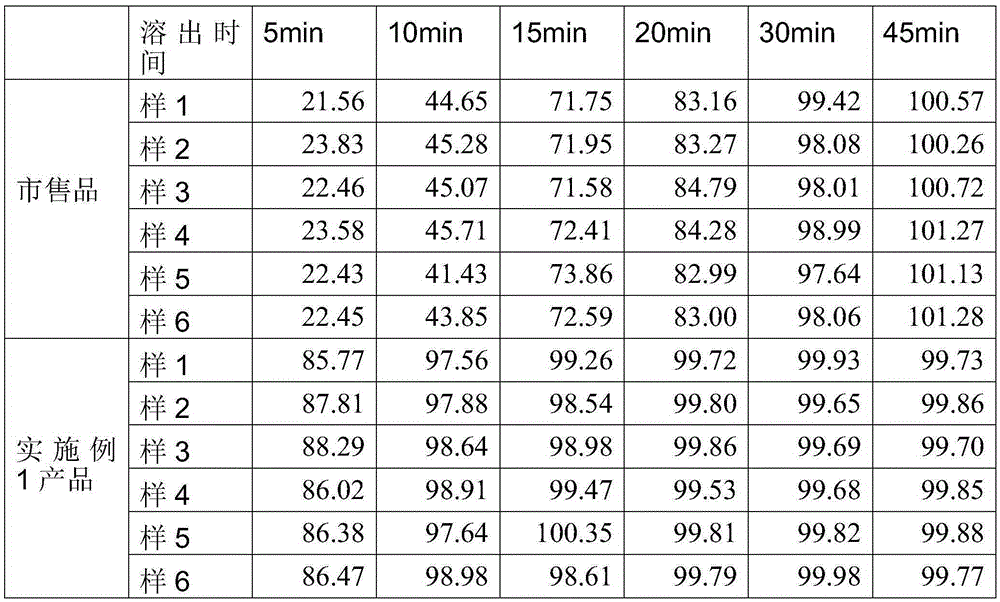
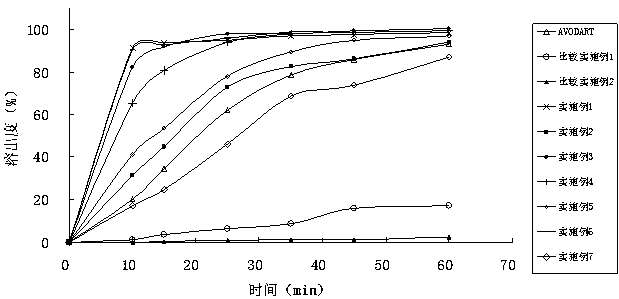
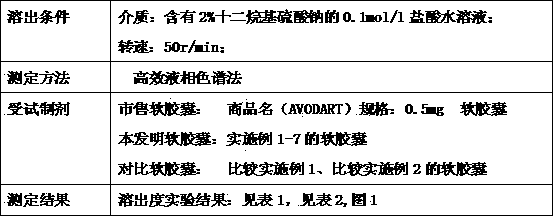
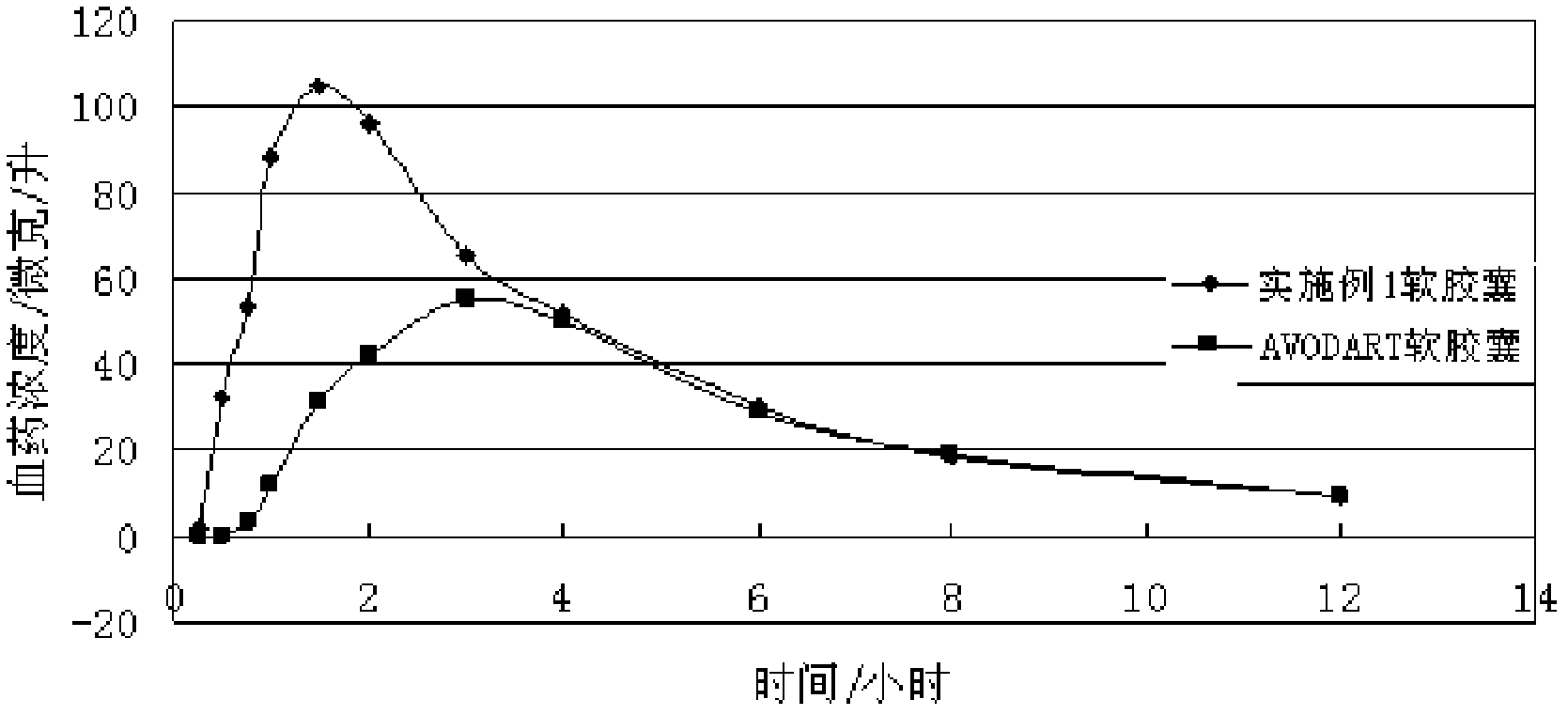
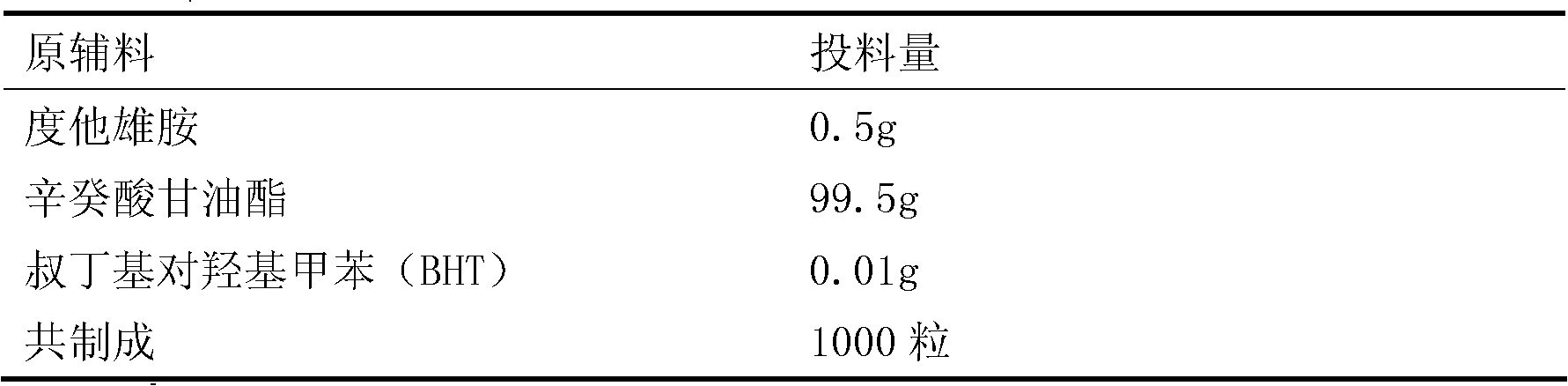
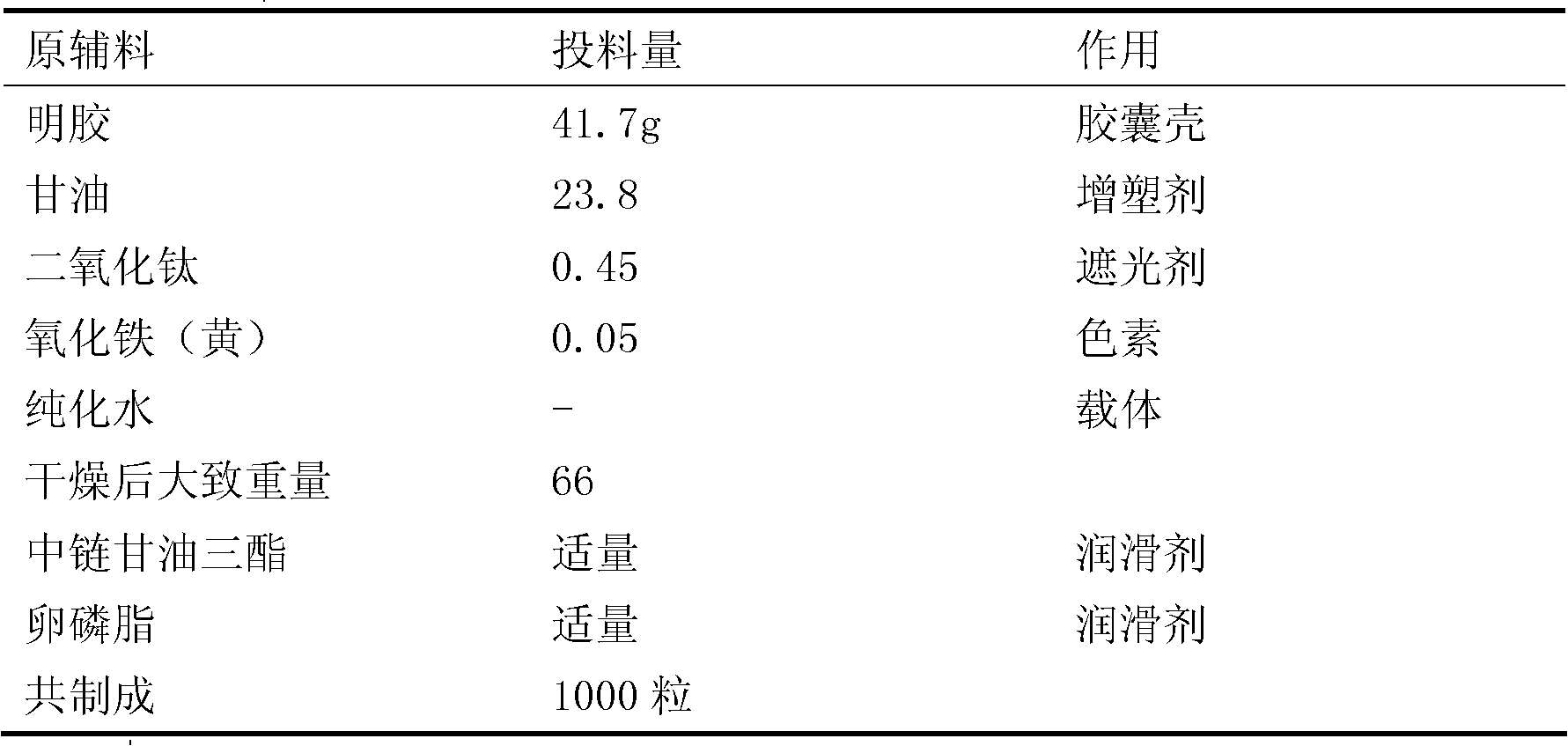
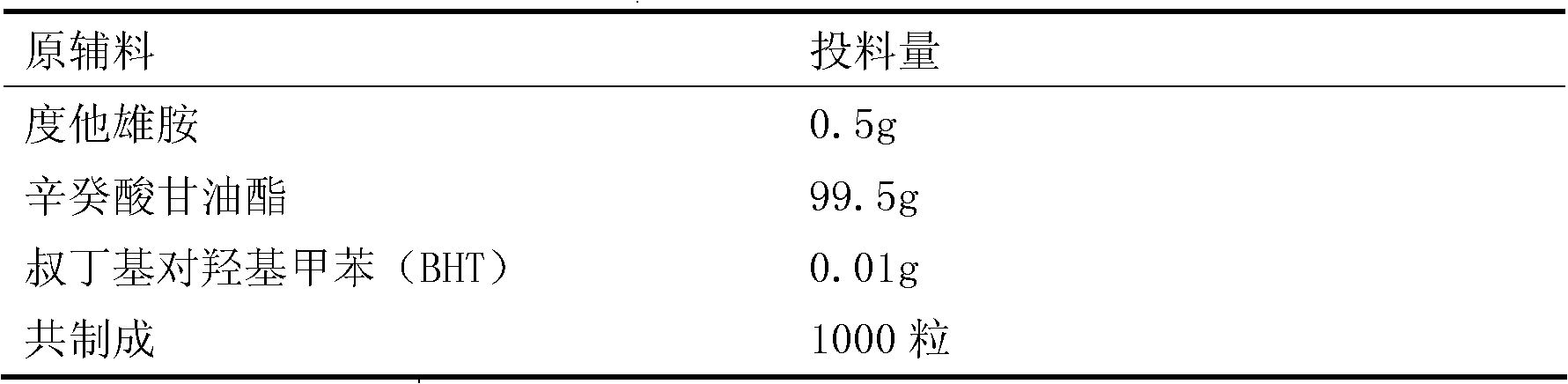
Dutasteride soft capsule preparation and preparation process thereof
ActiveCN105395517AImprove dissolution efficiencyImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsCresolPolyethylene glycol
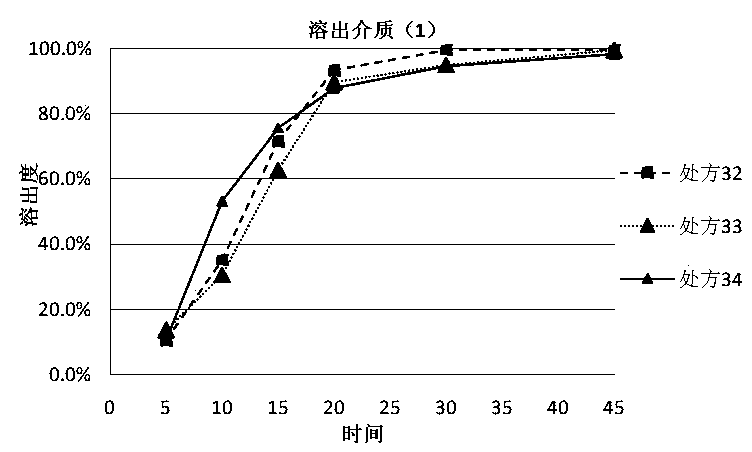
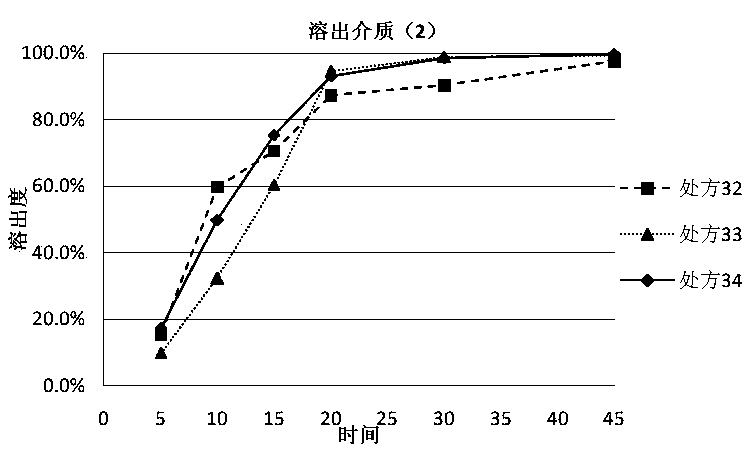
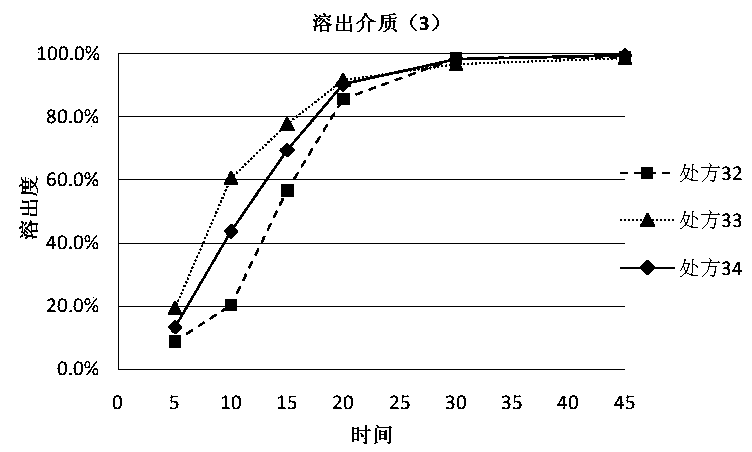
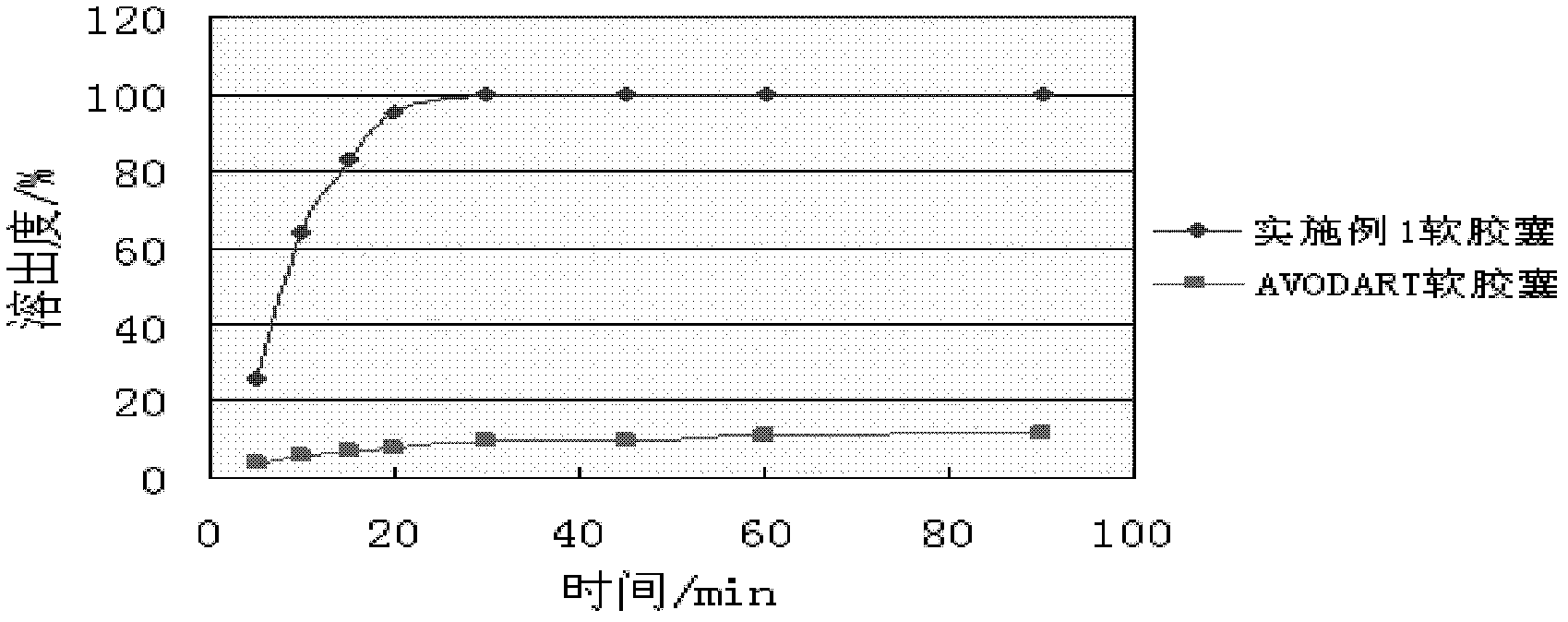
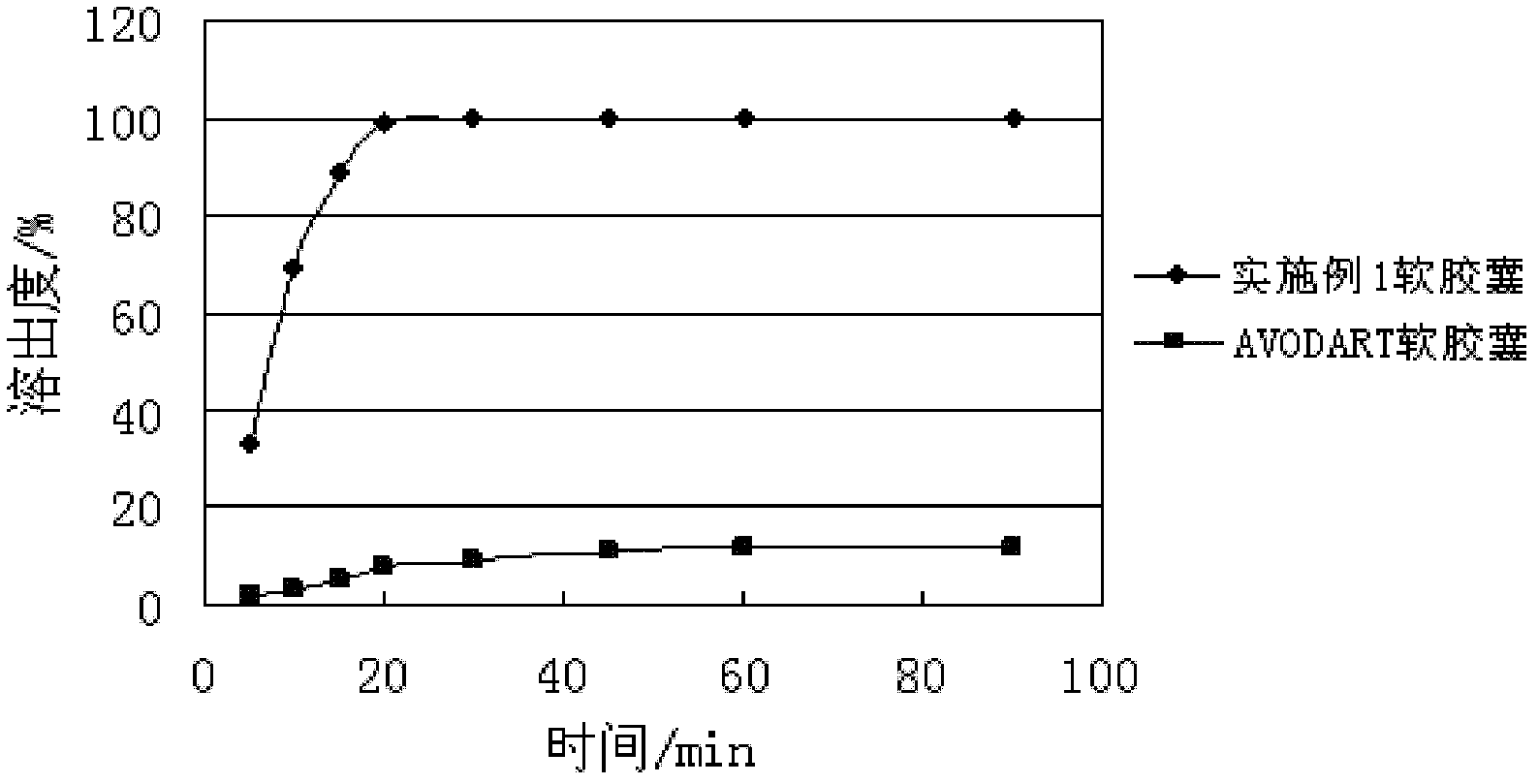
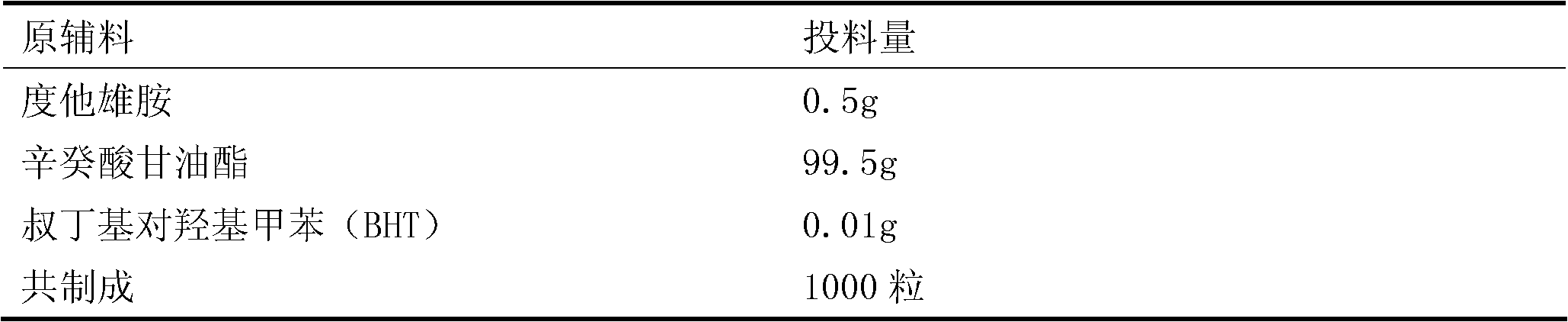
The invention discloses a dutasteride soft capsule preparation, comprising a soft capsule and contents. A prescription for the contents comprises 0.5 g of a dutasteride raw material, 200 to 250 g of labrasol, 30 to 60 g of polyglycerol oleate, 50 to 100 g of medium-chain triglyceride and 0.02 to 0.05 g of 2,6-di-t-butyl p-cresol. The dutasteride soft capsule prepared in the invention is substantially improved in accumulated dissolution in 5 minutes of dissolving-out and enhanced in the dissolving-out efficiency of a main drug in a preceding time period and has good stability and good appearance quality.
Owner:成都华宇制药有限公司
Dutasteride liquid soft capsules
InactiveCN103830201APromote absorption in the bodyImprove the dissolution rate of dutasterideOrganic active ingredientsOrganic non-active ingredientsSoftgelGlycerol
The present invention relates to dutasteride liquid soft capsules and a preparation method thereof. The soft capsules comprise a capsule shell and contents, wherein the contents mainly comprise, by weight, 0.05-0.3% of dutasteride, 19.8-94.8% of medium chain triglyceride, and 5-80% of mono-caprylin glycerate. The soft capsules have characteristics of rapid dissolution, simple preparation process and the like.
Owner:CHONGQING PHARMA RES INST +1
Solid and crystalline dutasteride and processes for preparation thereof
The solid state chemistry of 17β-N-[2,5-bis(trifluoromethyl)phenyl]carbamoyl-4-aza-5-α-androst-1-en-3-one of which the international nonproprietary name is Dutasteride (the active ingredient in products marketed as Avodart, Avidart, Avolve, Duagen, Dutas, Dutagen, Duprost) and its process for preparing. The synthetic process comprises formation of the mixed anhydride, its subsequent reaction with 2,5-bis(trifluoromethyl)phenylamine in the presence of an appropriate Lewis catalyst and its isolation, purification and crystallization from acetonitrile / water.
Owner:GADOR
Composition and Methods for Treating Hair Loss
InactiveUS20120258972A1Accelerated and robust effectPromotes hair growthCosmetic preparationsBiocideCream basePhysiology
A method for treating hair loss caused by androgenic alopecia and / or male pattern baldness. The method, which not only slows hair loss but causes hair re-growth, includes approximately daily application to a subject's scalp of a novel composition comprising finasteride (Propecia® or Proscar®), dutasteride (Avodart®), and minoxidil (Rogaine®) as active ingredients in a hypoallergenic cream-based vehicle, preferably coupled with daily ingestion of 1 mg per day of finasteride (Propecia® or Proscar®), application to the scalp of 5% minoxidil (Rogaine®) foam approximately once per day, and use of a ketoconazole-containing shampoo (e.g., Nizoral®) approximately 2-3 times per week. The method described herein also resolves scalp dermatitis in atopic subjects suffering therefrom. A method for making the novel composition is also provided.
Owner:RAFI ASIF +1
Method for preparing dutasteride
The invention discloses a method for preparing dutasteride, belonging to the field of chemical synthesis of drugs. In the invention, with androstendione as a raw material, the dutasteride is prepared by eight reaction steps of ring opening, ring closing, Grignard reaction, dehydration, reduction, oxidation, dehydrogenation and condensation. In the method disclosed by the invention, double bonds at sites 5 and 6 and double bonds at sites 16 and 27 are simultaneously hydrogenated and reduced; and amidation of a site 17 and condensation of a site 20 are continuously carried out so that the steps are saved; potassium cyanide under an acidic condition is avoided and the process safety is improved; and the expensive 4-di(trifluoromethyl)-2-iodobenzene is replaced with cheap 2,5-di(trifluoromethyl) aniline so that the cost is largely reduced.
Owner:湖南尔文水电建材有限公司
Dutasteride-containing soft capsule
The invention relates to a soft capsule with contents containing dutasteride, a diluent and a surfactant, wherein the diluent is selected from one of a group consisting of soybean oil, peanut oil, corn oil and a medium-chain triglyceride, and the surfactant is selected from one or two of a group consisting of span 80 and span 85. Preferably, the weight percentage content of dutasteride accounts for 0.042%-0.143% of that of the contents, the weight percentage content of the diluent accounts for 79.87%-98.46% of that of the contents, and the weight percentage content of the surfactant accounts for 1.498%-19.99% of that of the contents. The dutasteride-containing soft capsule prepared by the prescription provided by the invention has wide raw material sources, strong stability, good safety, high dissolution rate and good bioavailability.
Owner:SICHUAN GOWELL PHARMA
Preparation method of dutasteride
The invention discloses a novel feeding sequence for preparing dutasteride by taking 3-ketone-4-aza-5alpha-aetioallocholane-1-olefin-17beta-carboxylic acid, pyridine, thionyl chloride and 2,5-(trifluoromethyl)phenylamine as raw materials, as well as concrete operation steps and embodiments. By using the method, the problems of low yield and high cost of the process and operation for synthesizing a crude product of the dutasteride by using the 3-ketone-4-aza-5alpha-aetioallocholane-1-olefin-17beta-carboxylic acid as a raw material at current are solved, the yield of the crude product of the dutasteride synthesized by using the 3-ketone-4-aza-5alpha-aetioallocholane-1-olefin-17beta-carboxylic acid as the raw material is greatly increased, the industrial cost is reduced, and the process is simple, is simple and convenient to operate and is more suitable for the industrial production of the dutasteride.
Owner:CHENGDU GUOHONG PHARMA
Preparation method of dutasteride
The invention relates to a preparation method of dutasteride. The method comprises the following steps: preparing acyl chloride from a compound shown in the formula (1), performing an amidation reaction with 2,5-bis(trifluoromethyl)aniline to obtain a compound shown in the formula (2), and performing 1,2-dehydrogenation to obtain dutasteride shown in the formula (3). The preparation method provided by the invention has the following advantages: the reaction conditions are mild, the intermediate of each step can be purified easily, and the quality is stable and controllable; the yield is higher, and the cost is low; and the method is suitable for industrial production. The formula (1) is shown in the description.
Owner:CHINA RESOURCES SAIKE PHARMA
Preparation method of dutasteride
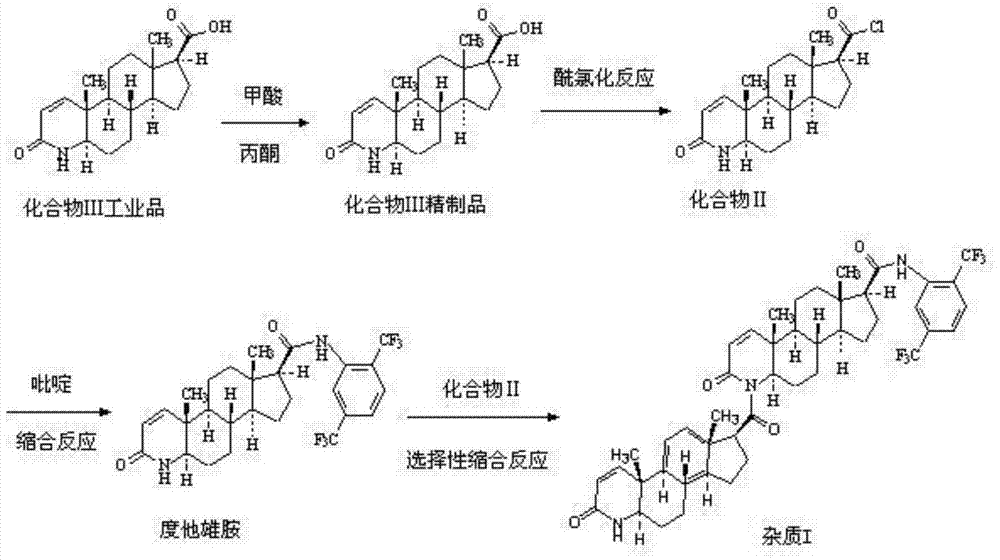
The invention belongs to the technical field of medicine, and provides a preparation method of dutasteride. The preparation method comprises the following steps: A) an industrial-grade compound III is washed by acetone after beaten and refined by formic acid, and a refined compound III product is obtained; B) the refined compound III product has a chlorination reaction to generate a compound II; C) the compound II has a condensation reaction with 2,5-bis (trifluoromethyl) phenylamine, a product is cooled and filtered, a filtrate is collected, a hydrochloric acid solution is added for washing, an organic phase is separated, pressure is reduced to evaporate s solvent, and a crude dutasteride product is obtained; and D) the crude dutasteride product is dissolved by an organic solvent and decolorized, an anti-solvent is added for crystallization, and high-purity dutasteride is obtained. Side reactions are reduced, high-purity dutasteride can be obtained through preparation, and the productive cost is reduced.
Owner:GUANGDONG XIANQIANG PHARMA +1
Dutasteride preparation used for increasing bioavailability and preparation method
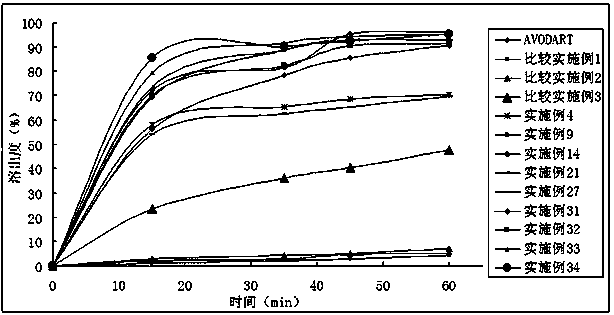
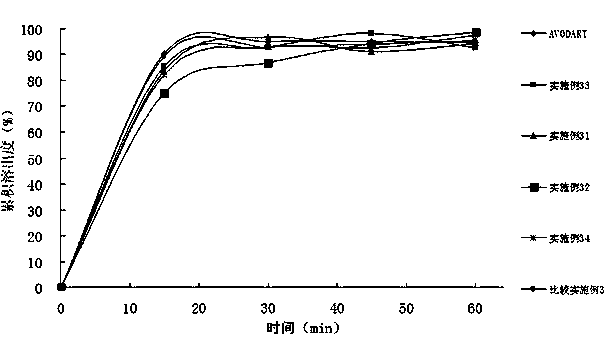
ActiveCN103169712AImprove external dissolutionImprove in vitro dissolutionOrganic active ingredientsUrinary disorderDissolutionSURFACTANT BLEND
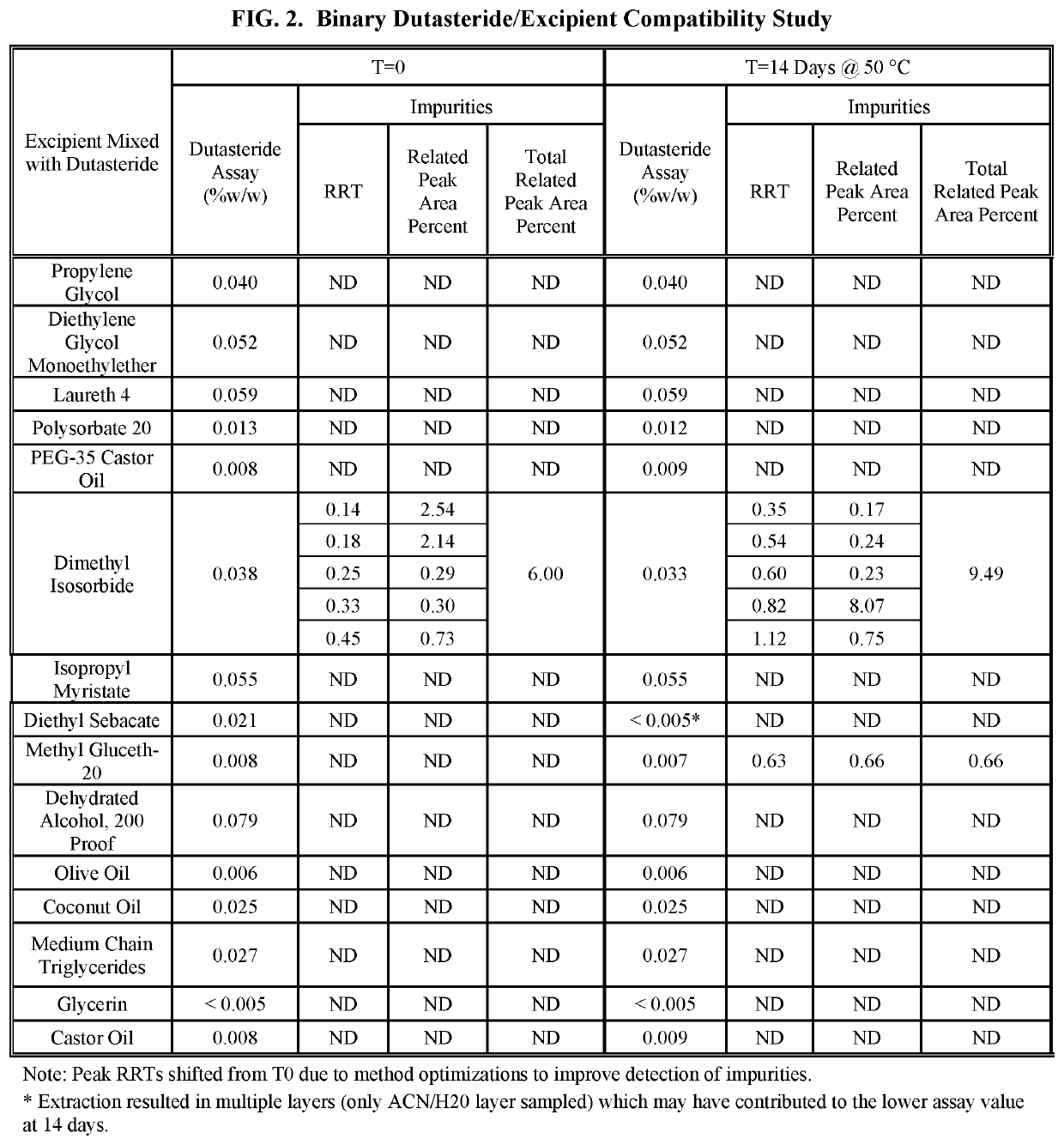
The invention provides a pharmaceutical composition containing dutasteride used for increasing bioavailability and a preparation method, the pharmaceutical composition containing dutasteride comprises 0.02-0.5% (W / W) of dutasteride, 30-70% (W / W) of oily medium and 25-66% (W / W) of surfactant; wherein the surfactant is one or more selected from span, tween, lecithin, soyabean lecithin or cholesterin. Compared with the prior art, the preparation prepared by the composition can obviously increase the dissolution in vitro of dutasteride, and the bioavailability can reach 144%.
Owner:CHONGQING HUAPONT PHARMA
Synthesis technology of dutasteride
The invention discloses a synthesis technology of dutasteride, comprising the following steps of: using the acidity of DT4 carboxyl to directly react with ammonia to generate DT4-ammonium salt; dehydrating the obtained ammonium salt into DT4-amide, and performing an amino exchange reaction between amide and BTFMA in the presence of a catalyst so as to prepare dutasteride. With DT4 as a starting material, dutasteride is synthesized by three reactions of salt formation, dehydration and amino exchange. During the reactions, thionyl chloride, oxalyl chloride, pivaloyl chloride or methylsulfonyl chloride which is sensitive to the environment is avoided, special expensive catalysts such as DBU, copper powder and the like are not needed, and harmful and poisonous chemical materials are avoided. The product has good product yield and high purity, and is easy to refine. The synthesis technology has advantages of low cost and few ''three wastes'', is easy and simple to operate, conforms to green chemical synthesis requirements, and lays a good industrial foundation for realizing large-scale green clean production of high-yield and high-purity dutasteride.
Owner:HUBEI TIANSHENG PHARMA
Compound preparation and preparation method thereof
InactiveCN102247379AReduced risk of concurrent acute urinary retentionUrinary disorderAmide active ingredientsActive componentHard Capsule
The invention discloses a compound preparation, and a preparation method thereof. The compound preparation comprises active components of tamsulosin and dutasteride, wherein tamsulosin is enteric pellets, particles or tablets, and dutasteride is soft capsules. tamsulosin and dutasteride are filled in one hard capsule. The invention also discloses the preparation method of the compound preparation.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
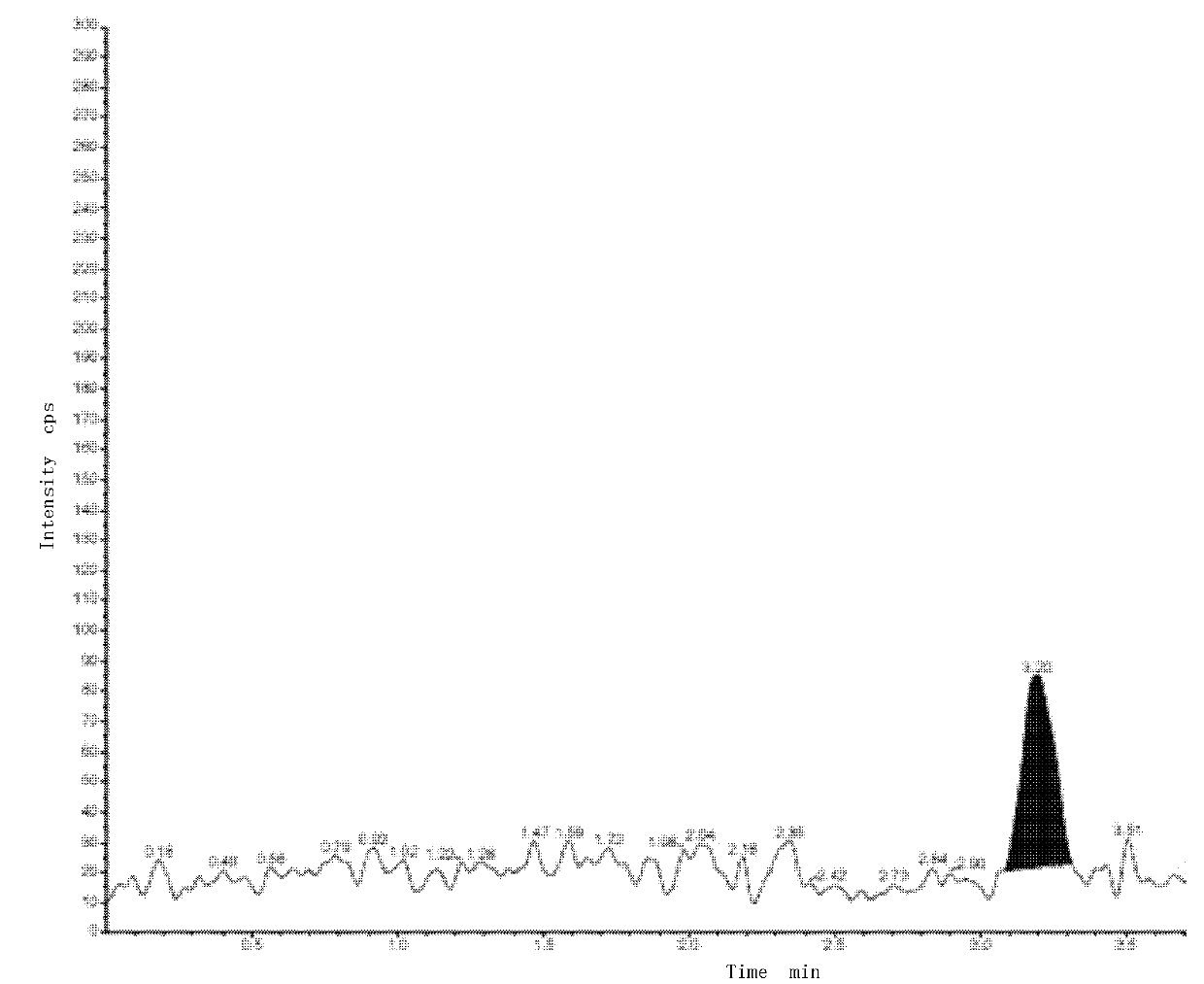
Method of determining low-content paricalcitol through high performance liquid chromatography-tandem mass spectrometry method and application thereof
InactiveCN105372340AStrong specificityHigh sensitivityComponent separationInternal standardDissolution
The invention belongs to the technical field of analytical chemistry and particularly relates to a method of determining low-content paricalcitol through a high performance liquid chromatography-tandem mass spectrometry method and application thereof. According to the method, the high performance liquid chromatography-tandem mass spectrometry method is adopted for determination, an internal standard method is also adopted, and dutasteride is used as an internal standard substance. The method is high in specificity, sensitivity and accuracy, the detection limit of the method can reach 40 pg / ml, and the quantization limit can reach 80 pg / ml. The method can be widely applied to testing of the content and dissolution rate of a low-content paricalcitol preparation, and particularly testing of the dissolution rate of a paricalcitol soft capsule.
Owner:CHONGQING HUAPONT PHARMA
Dutasteride self-microemulsion composition and preparation method thereof
InactiveCN103655470AImprove the dissolution rate of dutasterideSimple preparation processOrganic active ingredientsUrinary disorderSolubilityEmulsion
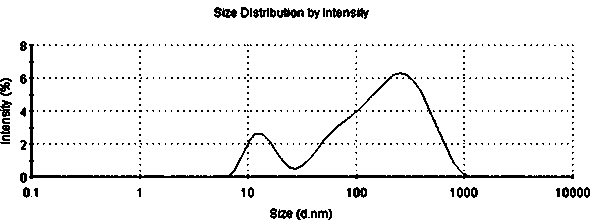
The invention relates to a self-microemulsion dutasteride orally-taken preparation and a preparation method thereof. The self-microemulsion composition comprises the following components in percent by weight: 0.05%-0.3% of dutasteride, 5%-75% of oil, 20%-60% of an emulsifier and 4%-50% of a co-emulsifier. After being cured, the self-microemulsion auxiliary materials can be prepared into preparations such as tablets or capsules; after being quickly disintegrated in a stomach, the preparation can be form emulsion drops with average grain size of 10 nm-100 nm in a gastrointestinal tract after being self-microemulsified, so that solubility and dissolution rate of the dutasteride can be improved, and absorption of the dutasteride in the gastrointestinal tract can be promoted.
Owner:CHONGQING PHARMA RES INST
Topical formulations of 5-alpha-reductase inhibitors and uses thereof
ActiveUS20200147071A1Increase in hair countExpand coverageCosmetic preparationsOrganic active ingredientsSide effectBULK ACTIVE INGREDIENT
Disclosed herein are topical compositions of 5-α reductase inhibitors, such as dutasteride or finasteride, or a pharmaceutically acceptable salt, ester, or derivative thereof and the use of the compositions for the treatment of hair loss secondary to endocrine therapy in patients with breast cancer (Endocrine Therapy-Induced Alopecia or ETIA), androgenetic alopecia (AGA), alopecia areata, and hirsutism. The topical composition is advantageous over the existing oral compositions of 5-α reductase inhibitors because the topical composition is safer and more effective. The topical formulation may allow for a slow release of the active ingredient dutasteride, better penetration at the therapeutically effective amount of dutasteride with an improved safety profile because it does not need to travel through the bloodstream to be efficacious, thereby minimizing the risk of systemic side effects.
Owner:VARSONA THERAPEUTICS INC
Composite preparation and preparing method thereof
InactiveCN102309495AReduced risk of concurrent acute urinary retentionUrinary disorderAmide active ingredientsHard CapsuleTamsulosin
The invention relates to a composite preparation, and preparing method and use thereof. The composite preparation uses Tamsulosin and Dutasteride as active ingredients, wherein Tamsulosin is in the form of enteric pellet, granule or tablet, Dutasteride is in the form of soft capsule, and both of them are filled in a hard capsule. The invention discloses a preparing method of the composite preparation.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Dutasteride liquid hard capsule and preparation method thereof
ActiveCN102319228AImprove solubilityHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsHard CapsuleMedicine
The invention discloses a dutasteride liquid hard capsule which comprises a hard capsule shell and contents, and the contents comprise dutasteride, a diluent and an anti-oxidant. The invention also discloses a preparation method of the dutasteride liquid hard capsule, which comprises the following steps: weighing dutasteride, the diluent and the anti-oxidant according to the weight composition of the contents, dissolving by stirring, well mixing to obtain a mixture which is the contents, filling the mixture into a hard capsule shell, sealing to obtain the dutasteride liquid hard capsule. The dutasteride liquid hard capsule of the invention has the characteristics of high disintegration and dissolution rate, and good stability, and the preparation method has the characteristics of simple operation, low production cost, and controllable quality.
Owner:FERGUSON WUHAN BIOTECH
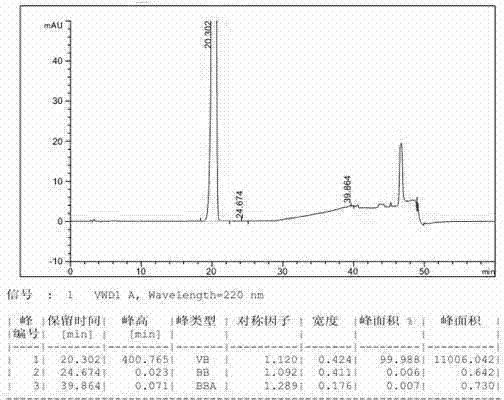
Production process of high-purity dutasteride
The invention discloses a purification production process of high-purity dutasteride. The problems to be solved are that the purity of the dutasteride is improved while the production cost is reduced.According to the method, after a dutasteride crude product is obtained, twice crystallization is carried out, so that the dutasteride with high yield and high purity can be obtained. The production process provided by the invention has the advantages of high efficiency and clean production, and the operability is high. An intermediate is refined, so that the quality of the dutasteride finished product is more easily controlled, the purity of the obtained dutasteride product is not lower than 99.5%, and any single impurity in the product is not higher than 0. 1%.
Owner:JIANGXI GUOYAO PHARMA LLC +1
Preparation method of dutasteride intermediate
InactiveCN103059097AAvoid pressurizationEfficient separationSteroidsHydrogen pressureCarboxylic acid
The invention relates to a preparation method of dutasteride intermediate, and specifically relates to a preparation method of 3-carbonyl-4-aza-5[alpha]-androstane-17[beta]-carboxylic acid. According to the preparation method, for hydrogenation, a carboxylic acid derivative is used as a hydrogen donor to perform transfer hydrogenation, so that hydrogen pressure reduction is prevented and expensive catalysts such as PtO2, Pd / C, raneys nickel, zinc powder and the like are not needed; and for purification, a specific alcoholic solvent is used to perform crystallization, so that 5-beta isomer produced by reduction can be separated effectively. The obtained product has high purity, operations are simplified and the method is suitable for industrialized production.
Owner:SHANGHAI INST OF PHARMA IND +1
Preparation method of dutasteride impurity I
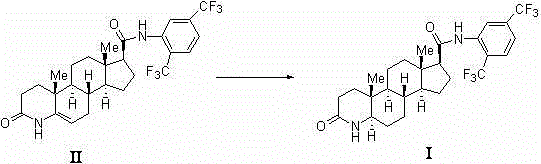
The invention belongs to the technical field of medicines and discloses a preparation method of a dutasteride impurity I. The preparation method of the dutasteride impurity I comprises the following steps: preparing a compound III refined product, carrying out acylating chlorination on the compound III refined product to generate a compound II, carrying out condensation reaction on the compound II and 2,5-bis (trifluoromethyl) phenylamine in the presence of pyridine as an acid binding agent to obtain a dutasteride crude product, carrying out selective condensation reaction on the compound II and the dutasteride crude product in the presence of an appropriate acid binding agent to obtain a dutasteride impurity I crude product, dissolving the dutasteride impurity I crude product by virtue of dichloromethane, and carrying out column chromatography on silica gel by adopting ethyl acetate-petroleum ether, so that the high-purity dutasteride impurity I is obtained.
Owner:GUANGDONG ZHONGSHENG PHARMA
Synthesis method of dutasteride intermediate
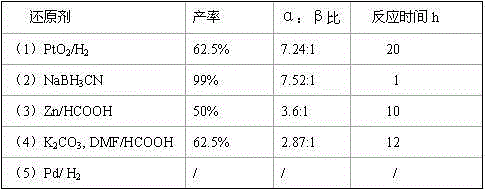
The invention relates to a synthesis method of a dutasteride intermediate, belonging to the field of medicament. The synthesis method comprises the steps of dissolving 3-oxo-4-aza-androst-5-ene-17beta-carboxamide in organic solvent, adjusting pH value, stirring at room temperature, slowly adding reducing agent NaBH3CN, carrying out hydrogenation reaction for 1h, adjusting pH value with 10% NaOH solution, extracting with CH2Cl2, drying, filtering, evaporating filtrate under reduced-pressure to be dry to obtain crude product and recrystallizing the crude product. The synthesis method has the advantages that the ratio of the obtained alpha-isomer is high, the yield is higher, the production cost is simultaneously reduced, and the experiment safety is improved.
Owner:王履诚
Green synthetic method of highly pure dutasteride
The invention discloses a new green industrial preparation method of highly pure dutasteride. The method is realized through a synthetic route represented by a figure shown in the specification. The preparation method is adopted to construct a steroid 1,2-olefinic bond in order to avoid raw materials being harmful to environment and having large toxicity, and use of a severely toxic oxidant DDQ is thoroughly avoided from a reaction principle; and the method has the advantages of high efficiency, high purity, greenization, clean industrialization, strong maneuverability and high yield, and the total yield of a two-step reaction is greater than 80%. An iodo intermediate is purified to easily control the quality of finished dutasteride, the purity of the obtained dutasteride product is not smaller than 99.5%, the content of any single impurity does not exceed 0.1%, and medicinal demands are completely met.
Owner:大道隆达(北京)医药科技发展有限公司
Process for preparing dutasteride
The invention discloses a process for preparing dutasteride, and belongs to the field of pharmaceutical chemical synthesis. The process comprises the following steps: carrying out 1,2-position dehydrogenation on a compound shown in a formula (1) so as to obtain a compound shown in a formula (2); and after the compound shown in the formula (2) is prepared into acyl chloride, carrying out amidation on the acyl chloride and 2,5-bis(trifluoromethyl)aniline so as to obtain dutasteride shown in a formula (3). The process disclosed by the invention is simple in operation, mild in reaction conditions, safe and reliable in production, high in reaction yield, low in cost, and suitable for industrial production.
Owner:青岛富康化工科技有限公司 +2
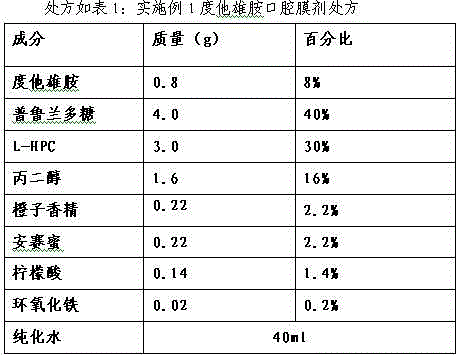
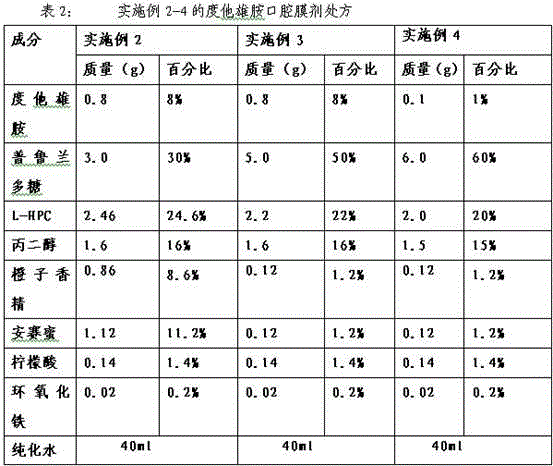
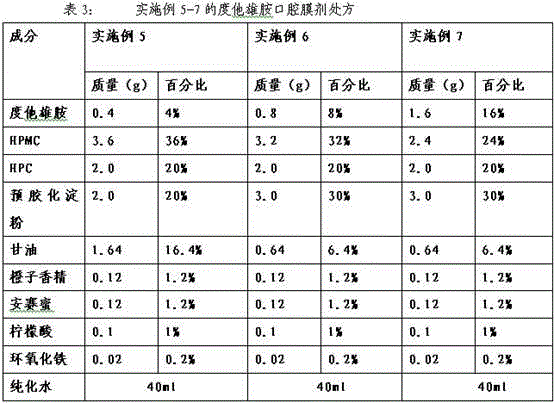
Dutasteride oral cavity film agent and preparation method thereof
InactiveCN103816136AUniform and complete appearanceUniform thicknessOrganic active ingredientsUrinary disorderPlasticizerDentistry
The invention relates to a dutasteride oral cavity film agent and a preparation method thereof. The film agent mainly comprises 1-16% (w / w) of dutasteride, 30-70% (w / w) of water-soluble medicinal high molecular material, 5-18% (w / w) of a plasticizer, 10-40% (w / w) of a filling agent and a proper amount of an additive. The dutasteride oral cavity film agent is uniform in appearance, does not stick a tooth, and is completely disintegrated within 30 s. The film agent is used for treating moderate and severe benign prostatic hyperplasia.
Owner:CHONGQING PHARMA RES INST
Dutasteride soft capsule medicine composition
ActiveCN111759821ASmall sizeImprove complianceOrganic active ingredientsUrinary disorderSodium stearateSoftgel
The invention discloses a dutasteride soft capsule medicine composition. Contents of the dutasteride soft capsule medicine composition comprise dutasteride, an oil phase and an antioxidant. A capsulecase comprises gelatin, glycerin, a surfactant, an opacifier and a pigment, wherein the surfactant is one or two kinds of materials of lauryl sodium sulfate or sodium stearate. The dutasteride soft capsule medicine composition using the recipe provided by the invention has the advantage that the migration of the dutasteride in the contents towards the capsule case can be effectively prevented.
Owner:四川奥邦投资有限公司
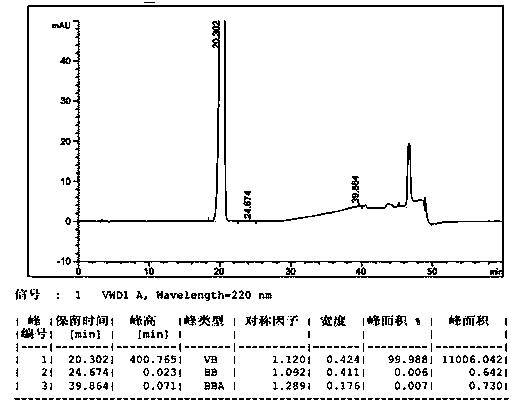
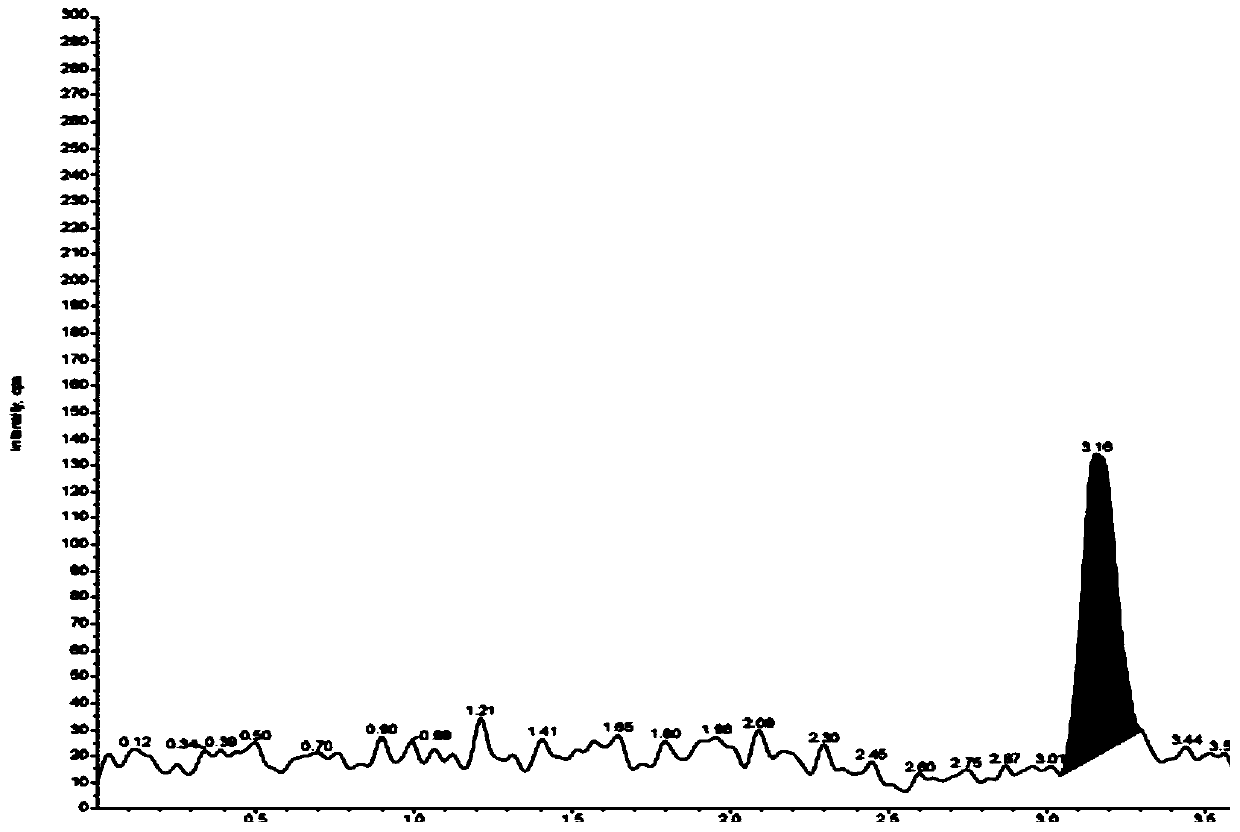
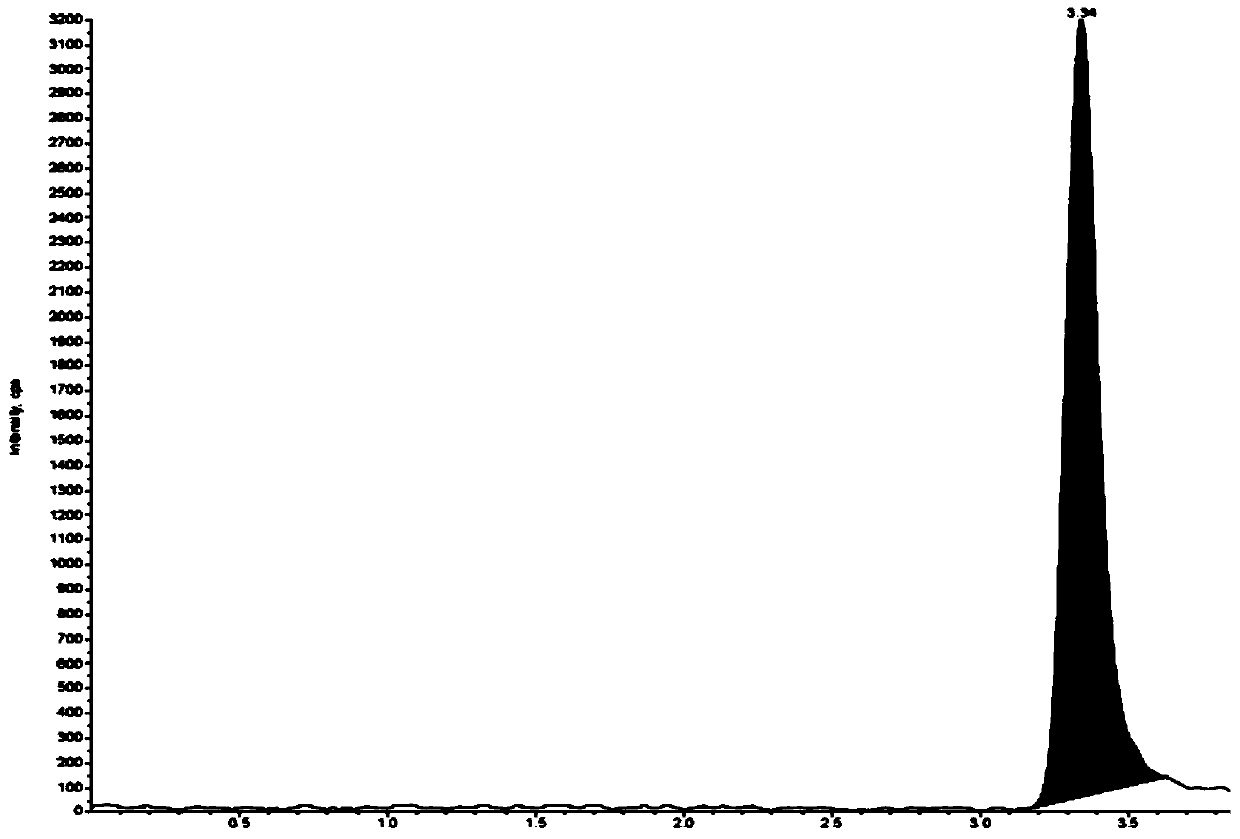
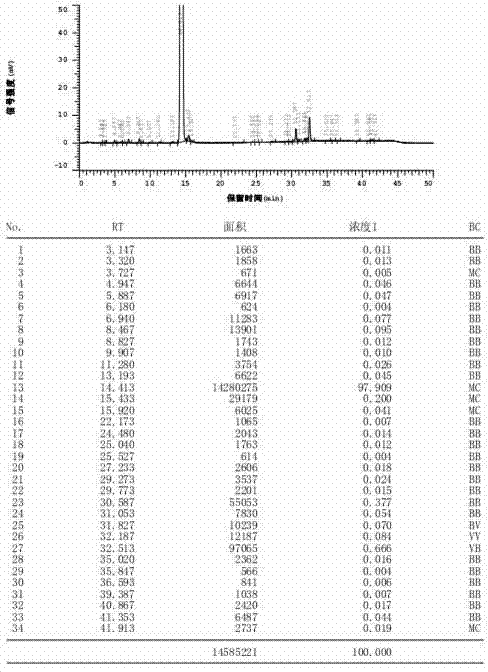
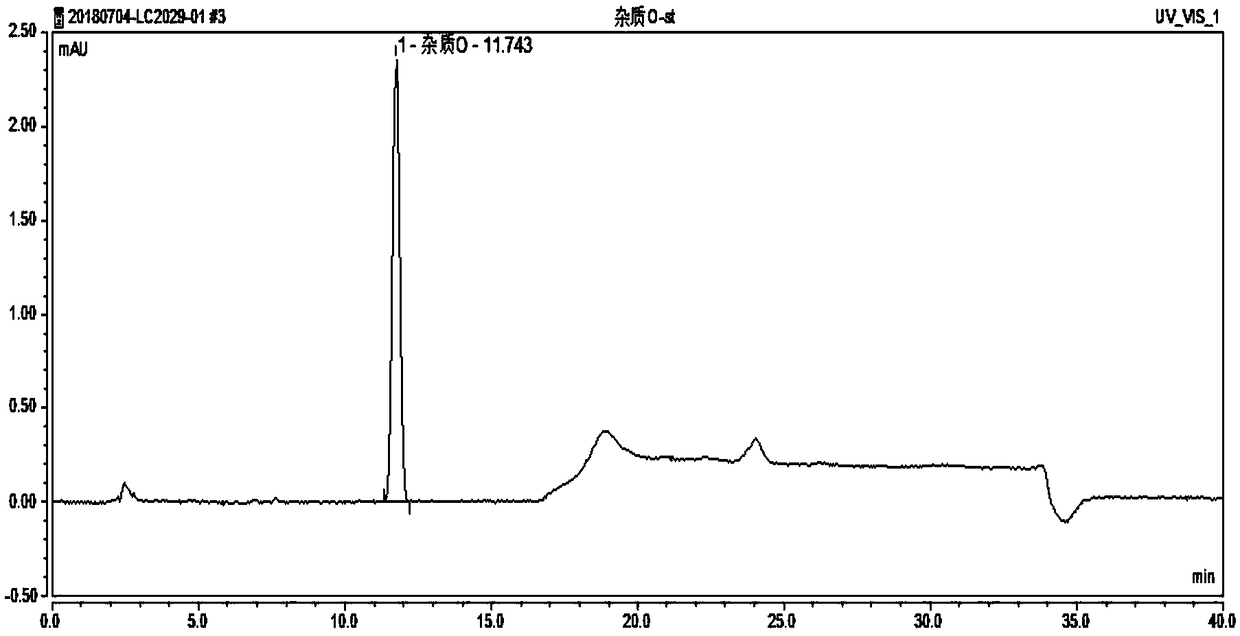
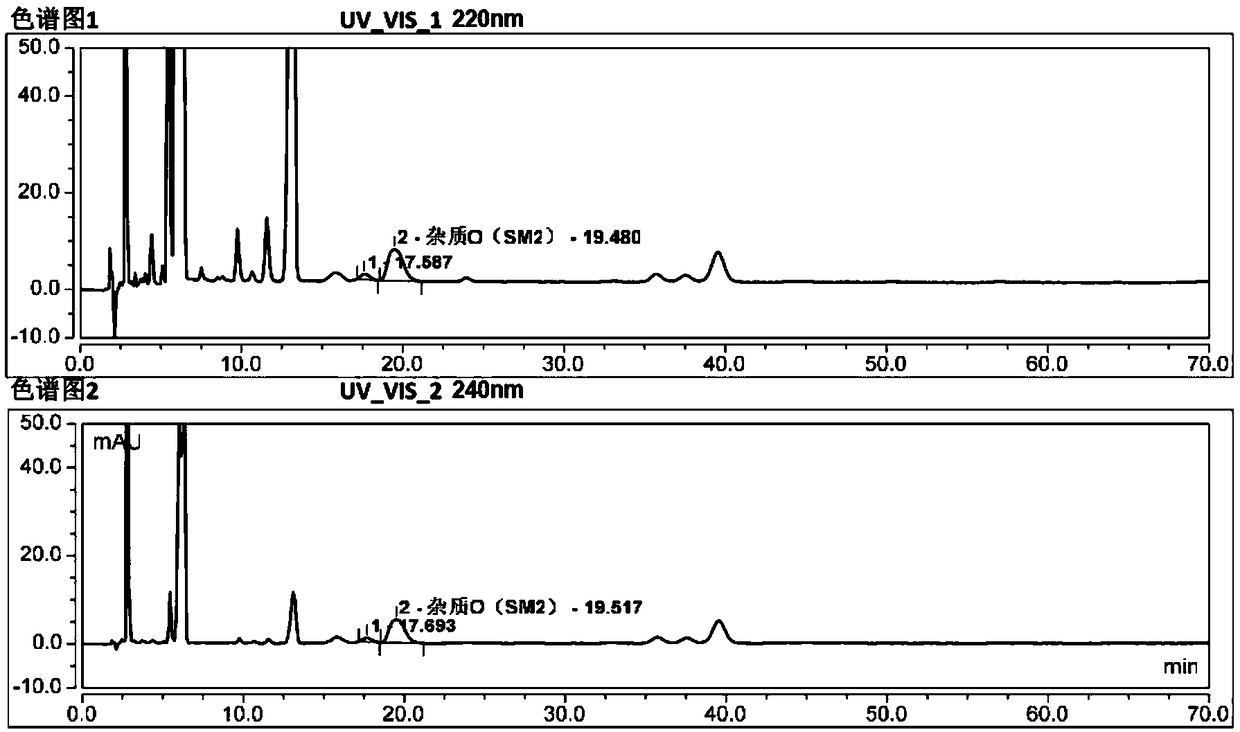
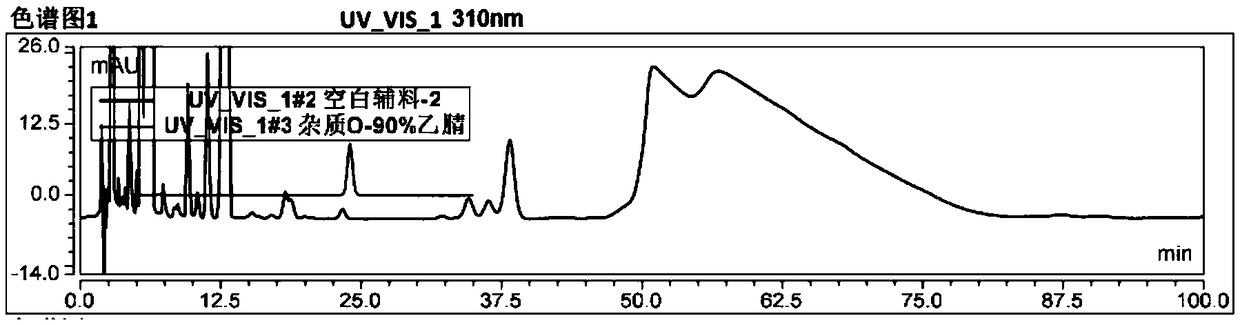
Method for separating and determining degradation impurities in dutasteride raw material drug and preparation by virtue of HPLC
ActiveCN109490444ADetermination does not interfereStrong specificityComponent separationForced degradationSilica gel
Owner:CHONGQING HUAPONT PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Process for the preparation of 17beta-N-[2,5-bis(trifluoromethyl)phenyl] carbamoyl-4-aza-5-alpha-androst-1-en-3-one Process for the preparation of 17beta-N-[2,5-bis(trifluoromethyl)phenyl] carbamoyl-4-aza-5-alpha-androst-1-en-3-one](https://images-eureka.patsnap.com/patent_img/3f96473f-9d28-46e6-8aae-912e88ba51a7/US20050059692A1-20050317-C00001.png)
![Process for the preparation of 17beta-N-[2,5-bis(trifluoromethyl)phenyl] carbamoyl-4-aza-5-alpha-androst-1-en-3-one Process for the preparation of 17beta-N-[2,5-bis(trifluoromethyl)phenyl] carbamoyl-4-aza-5-alpha-androst-1-en-3-one](https://images-eureka.patsnap.com/patent_img/3f96473f-9d28-46e6-8aae-912e88ba51a7/US20050059692A1-20050317-C00002.png)
![Process for the preparation of 17beta-N-[2,5-bis(trifluoromethyl)phenyl] carbamoyl-4-aza-5-alpha-androst-1-en-3-one Process for the preparation of 17beta-N-[2,5-bis(trifluoromethyl)phenyl] carbamoyl-4-aza-5-alpha-androst-1-en-3-one](https://images-eureka.patsnap.com/patent_img/3f96473f-9d28-46e6-8aae-912e88ba51a7/US20050059692A1-20050317-C00003.png)