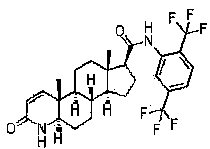
Dutasteride liquid soft capsules
A technique for dutasteride and soft capsules, which is applied in the field of dutasteride liquid soft capsules, can solve problems such as the meaninglessness of dutasteride solubility, and achieve the effects of stable and controllable quality, simple preparation process, and improved dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
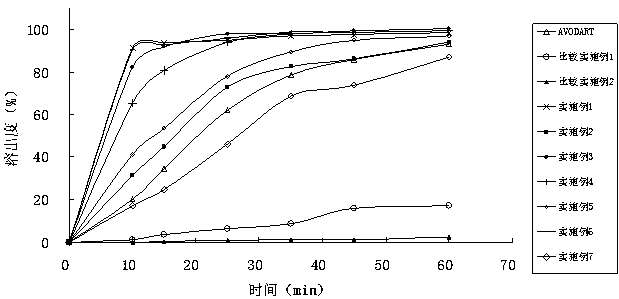
Image
Examples
Embodiment 1
[0021] prescription:
[0022] Dutasteride 0.153g
[0023] Medium Chain Triglycerides 19.885g
[0024] Glyceryl monocaprylate 80g
[0025] BHT0.005g
[0026] Preparation method: Weigh the prescribed amount of dutasteride and BHT, dissolve it in the mixture of monocaprylic glycerin and medium-chain triglyceride heated to 70°C, stir evenly, and fill it into soft capsules to obtain dutasteride Soft capsules, made 306 soft capsules altogether.
Embodiment 2
[0028] prescription:
[0029] Dutasteride 0.153g
[0030] Medium Chain Triglycerides 94.647g
[0031] Glyceryl Monocaprylate 5g
[0032] BHT 0.2g
[0033] Preparation method: Weigh the prescribed amount of dutasteride and BHT, dissolve it in the mixture of monocaprylic glycerin and medium-chain triglyceride heated to 35°C, stir evenly, and fill it into soft capsules to obtain dutasteride Soft capsules, made 306 soft capsules altogether.
Embodiment 3
[0035] prescription:
[0036] Dutasteride 0.05g
[0037] Medium Chain Triglycerides 49.94g
[0038] Glyceryl monocaprylate 50g
[0039] BHT0.01g
[0040] Preparation method: Weigh the prescribed amount of dutasteride and BHT, dissolve it in the mixture of monocaprylic glycerin and medium-chain triglyceride heated to 60°C, stir evenly, and fill it into soft capsules to obtain dutasteride Soft capsules, made 100 soft capsules altogether.
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com