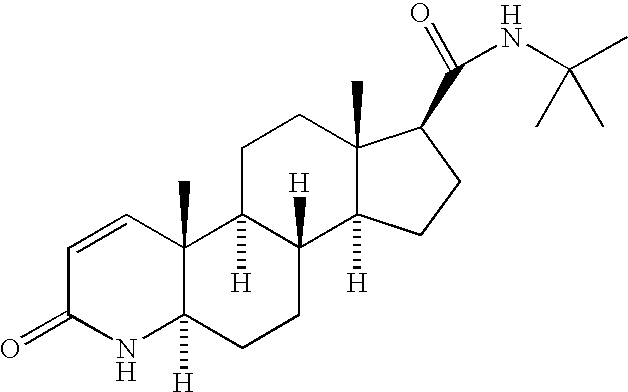
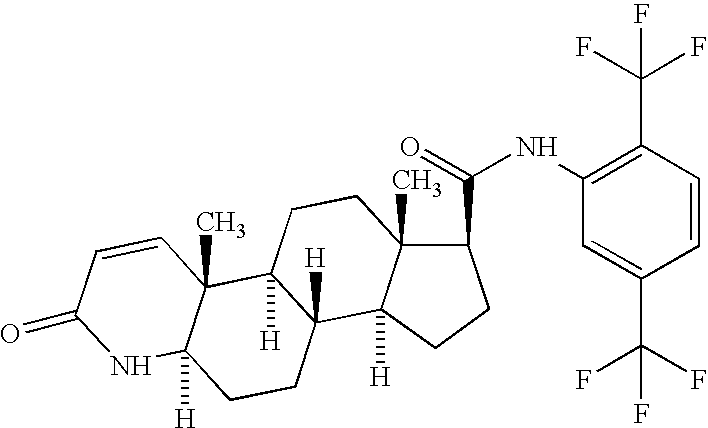
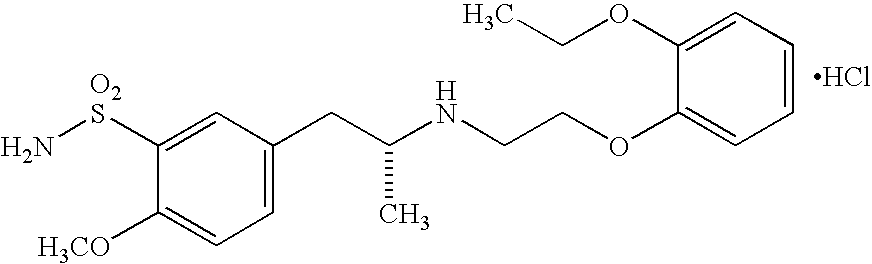
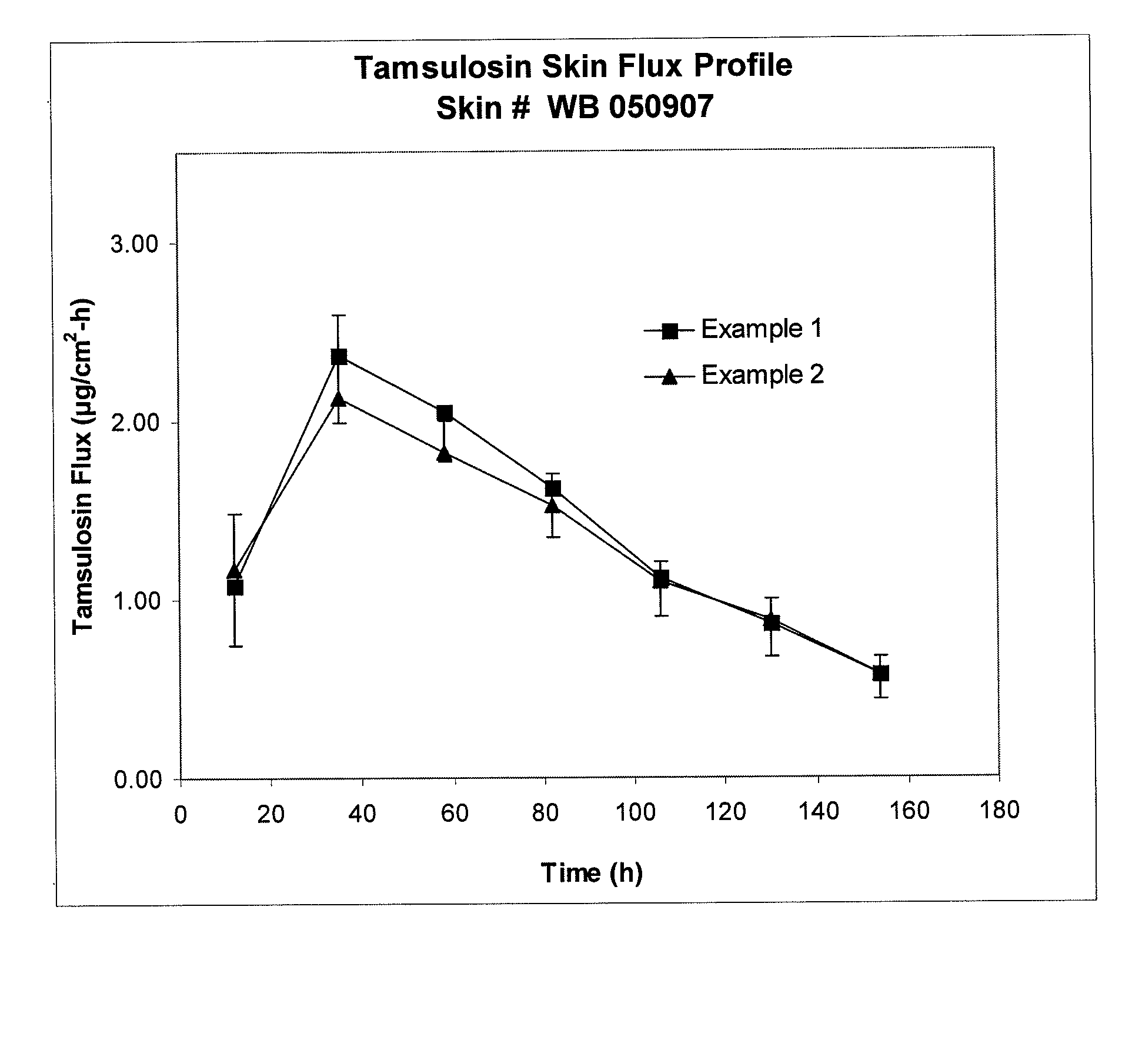
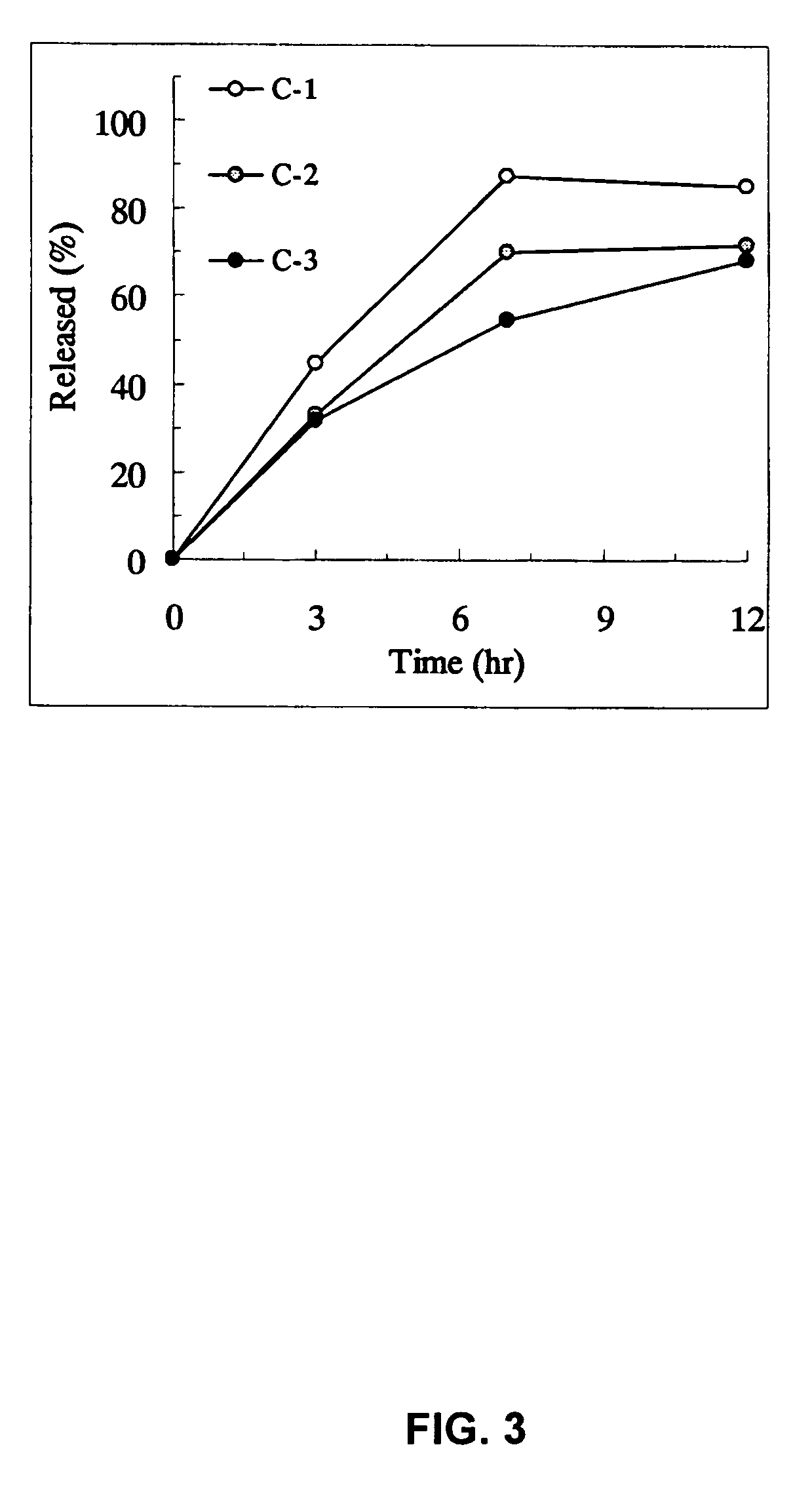
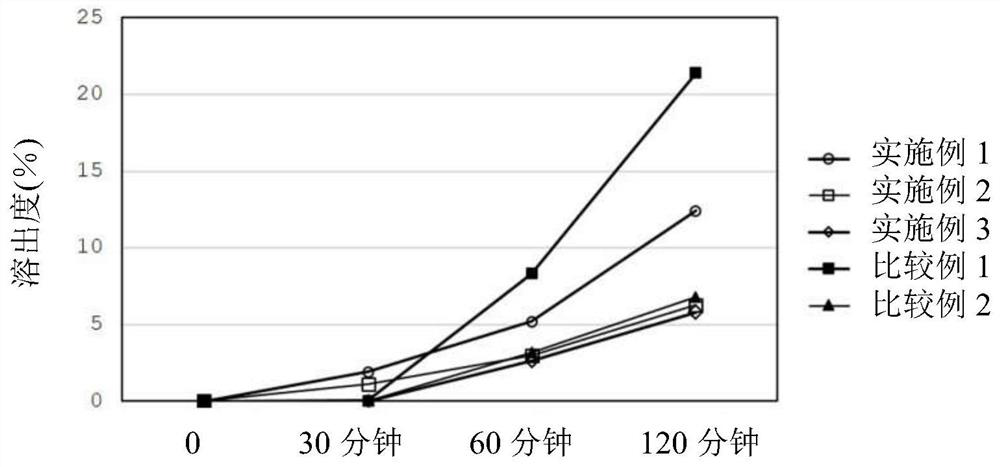
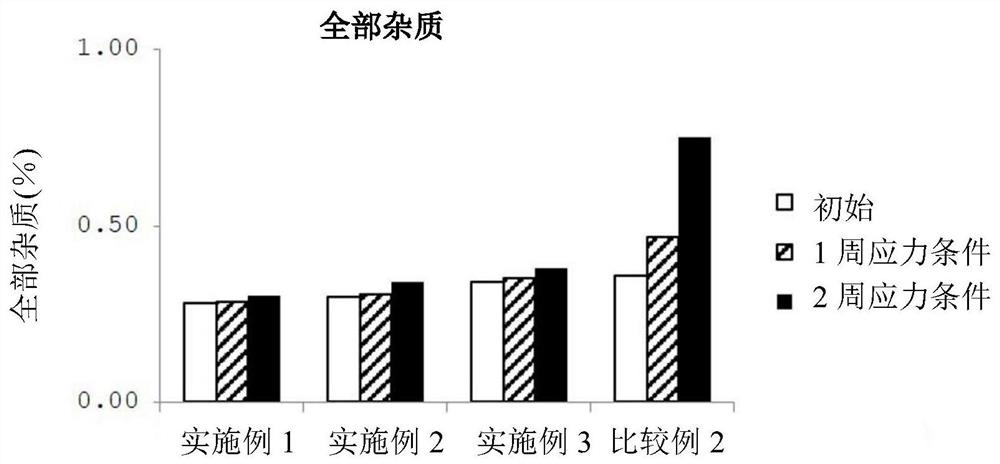
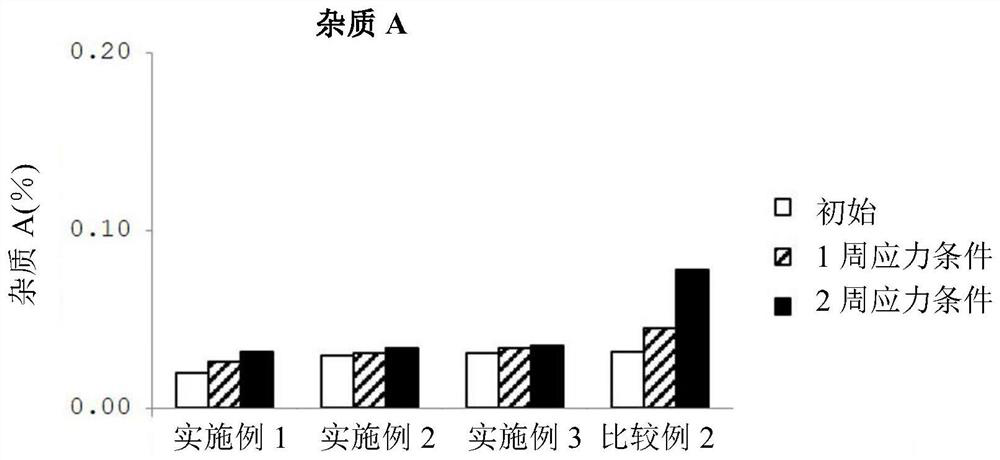
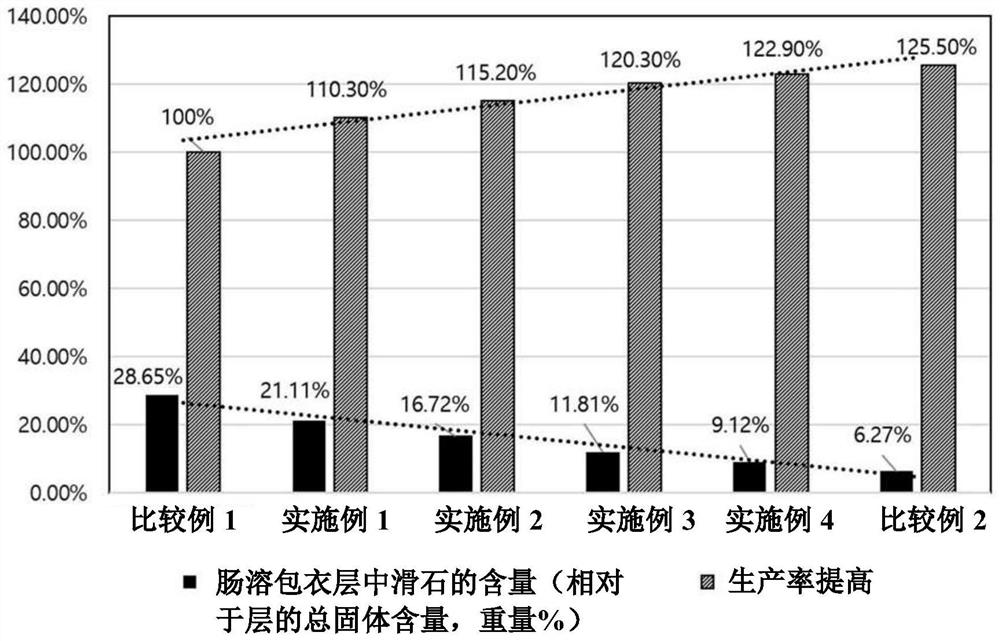
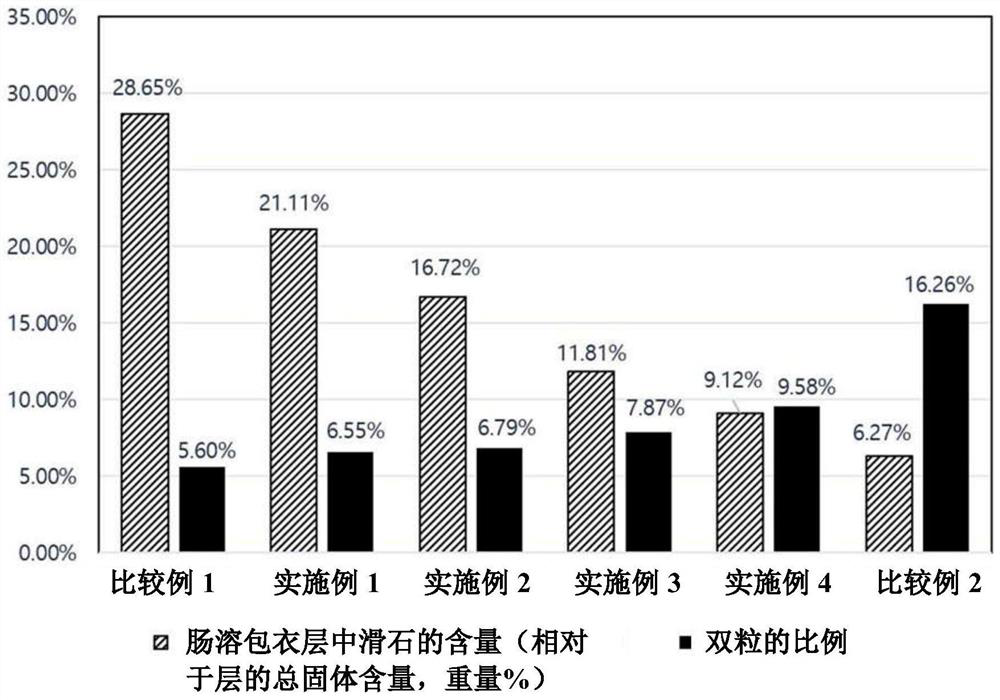
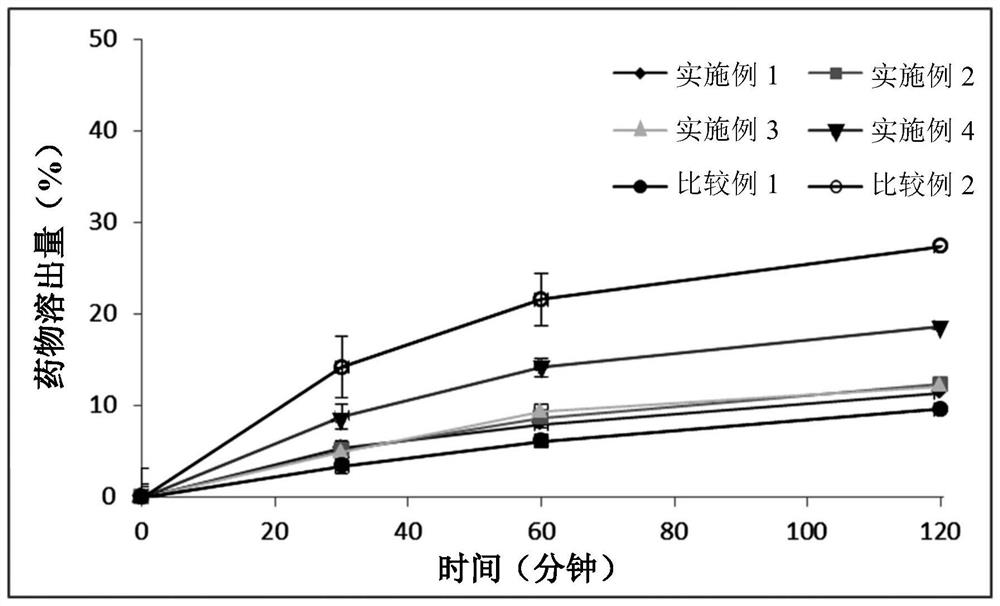
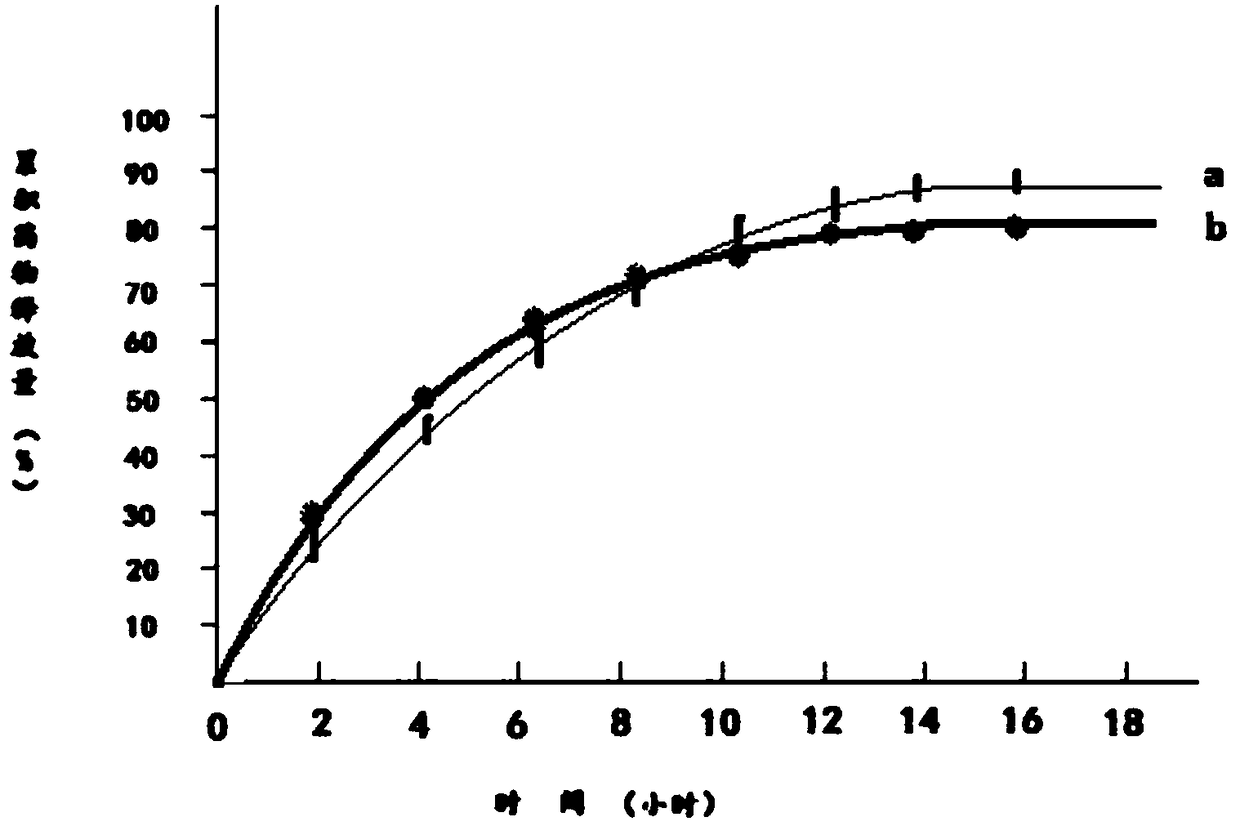
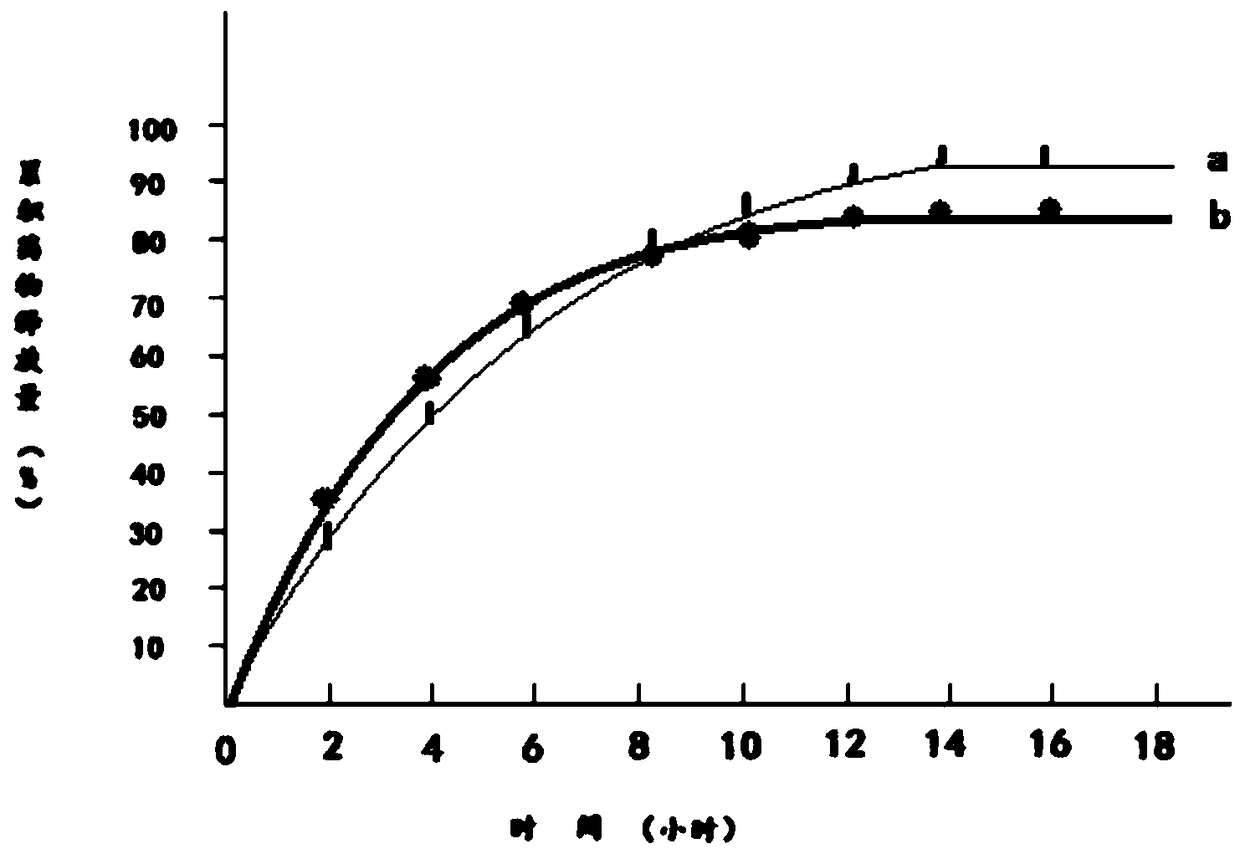
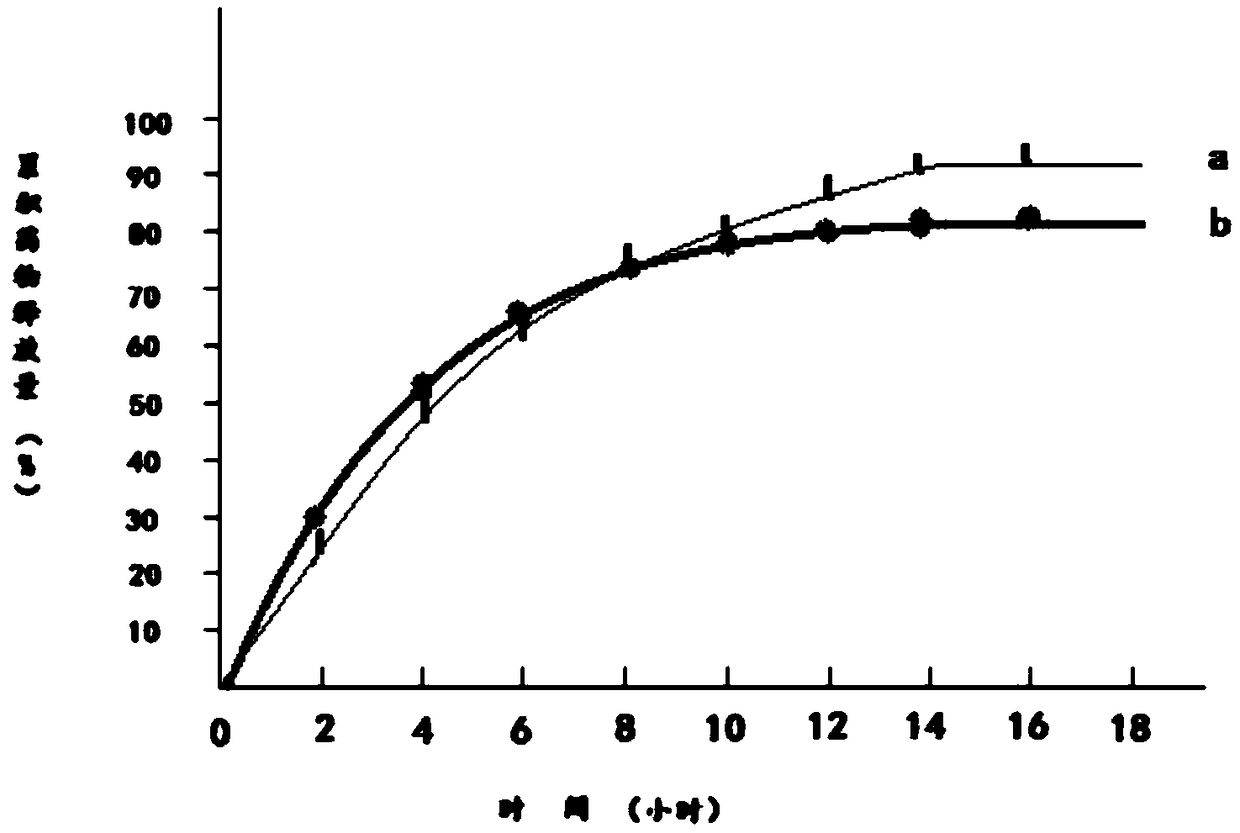
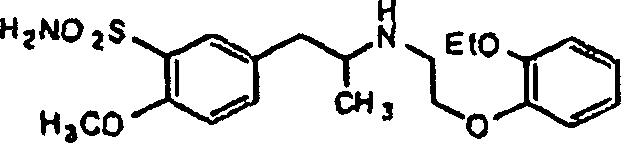
Patents
Literature
33 results about "Tamsulosine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Formulations of a nanoparticulate finasteride, dutasteride or tamsulosin hydrochloride, and mixtures thereof
Described are nanoparticulate compositions of finasteride, dutasteride, tamsulosin hydrochloride, or a combination thereof. The formulations exhibit unexpectedly prolonged release and can be maintained in a depot for release to a patient for a period of up to six months.
Owner:ELAN PHRMA INT LTD
Injectable depot compositions and its process of preparation
Novel injectable compositions are provided comprising an active agent which is tamsulosin or letrozole or its pharmaceutically acceptable salts, derivatives, isomers, polymorphs, solvates, hydrates, analogues, enantiomers, tautomeric forms or mixtures thereof and one or more pharmaceutically acceptable excipient(s) wherein the compositions are preferably formulated as biodegradable microparticles or nanoparticles which can optionally be reconstituted with an aqueous, hydro-alcoholic or oily liquid vehicle prior to administration. The novel injectable compositions of the present invention preferably form a depot upon administration in vivo and are in the form of an in situ gelling composition or an implant composition which provides a prolonged release of tamsulosin or letrozole for extended periods of time. Also described are process for preparation of such novel compositions and method of using them.
Owner:PANACEA BIOTEC
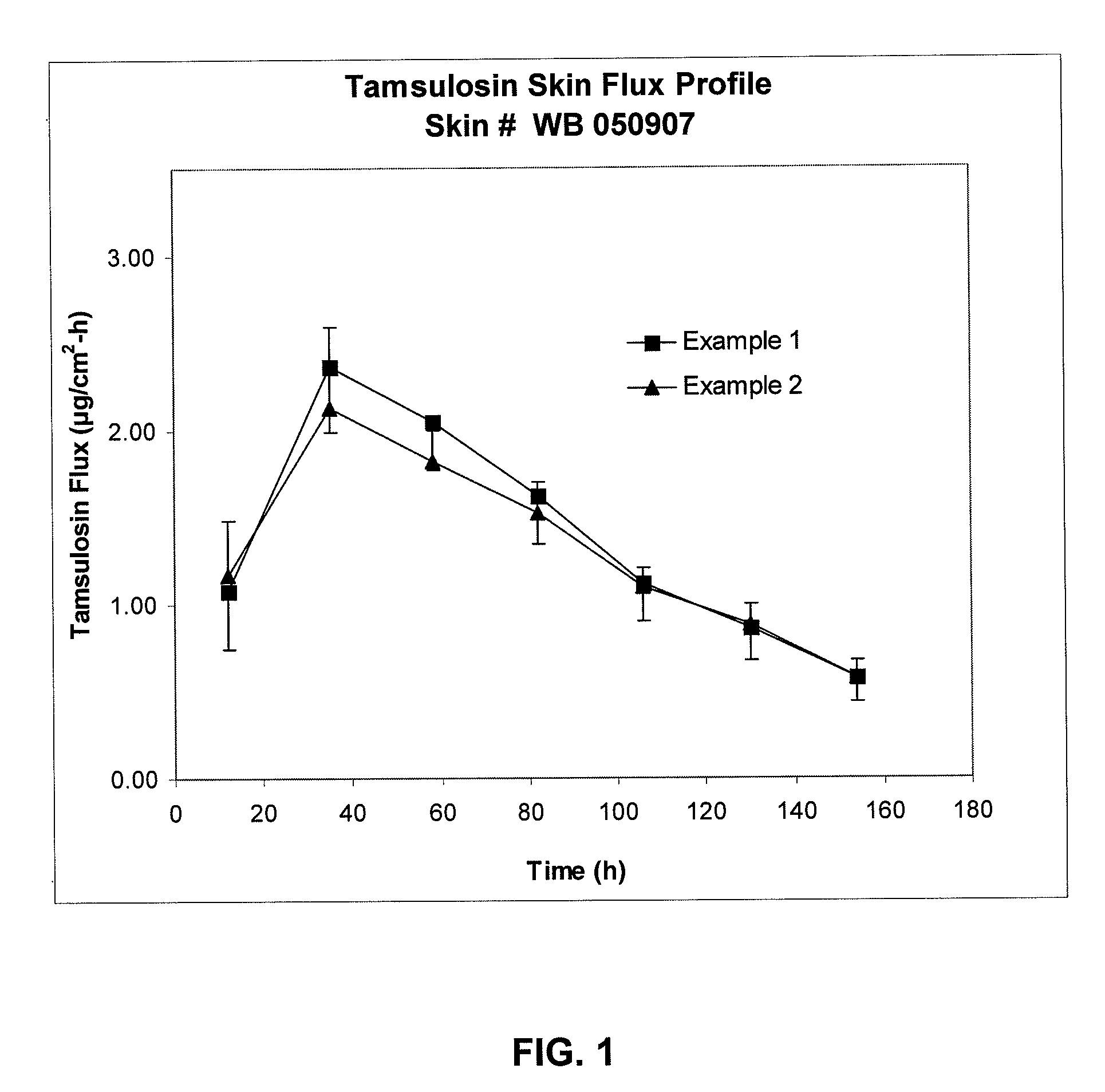
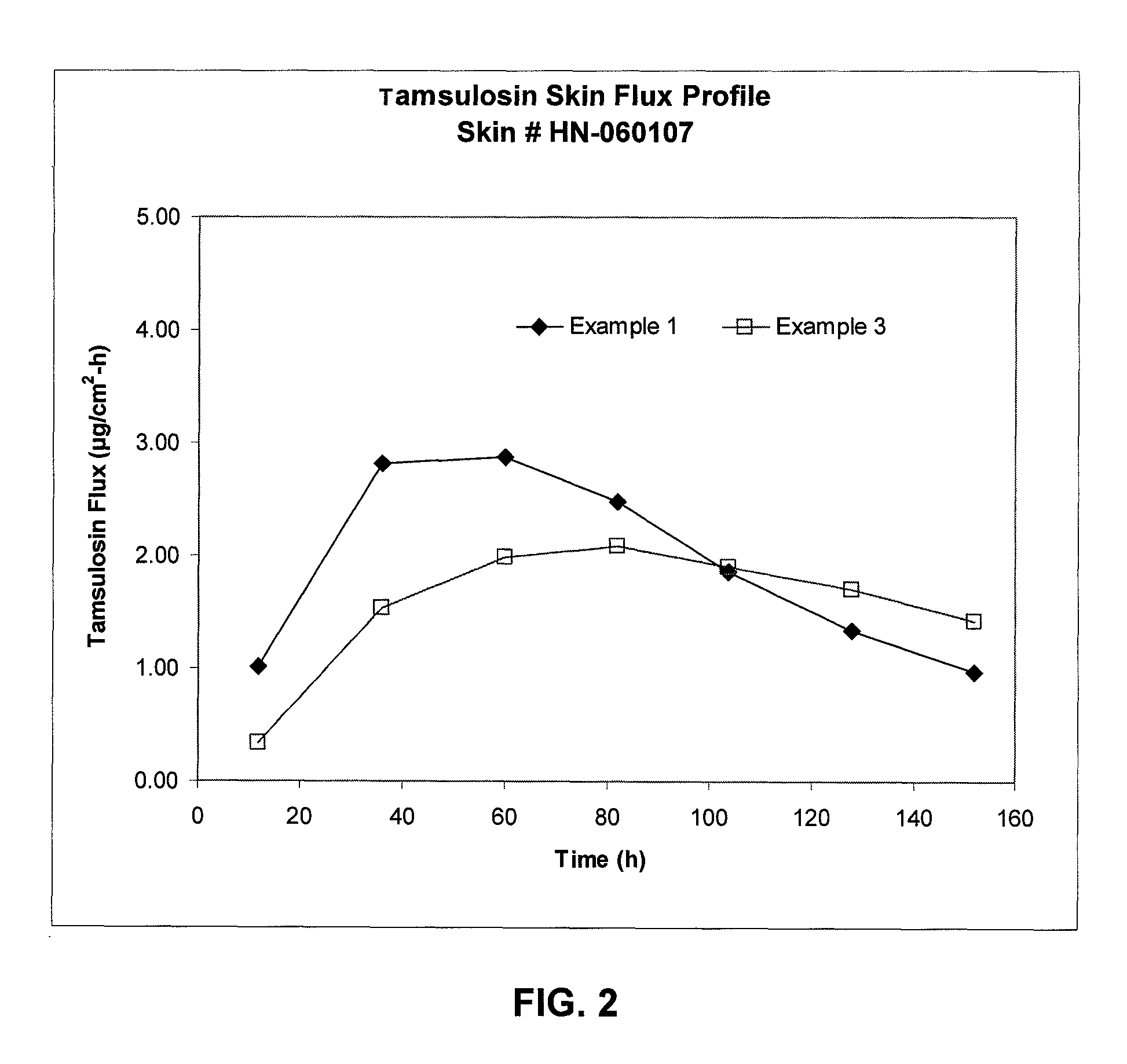
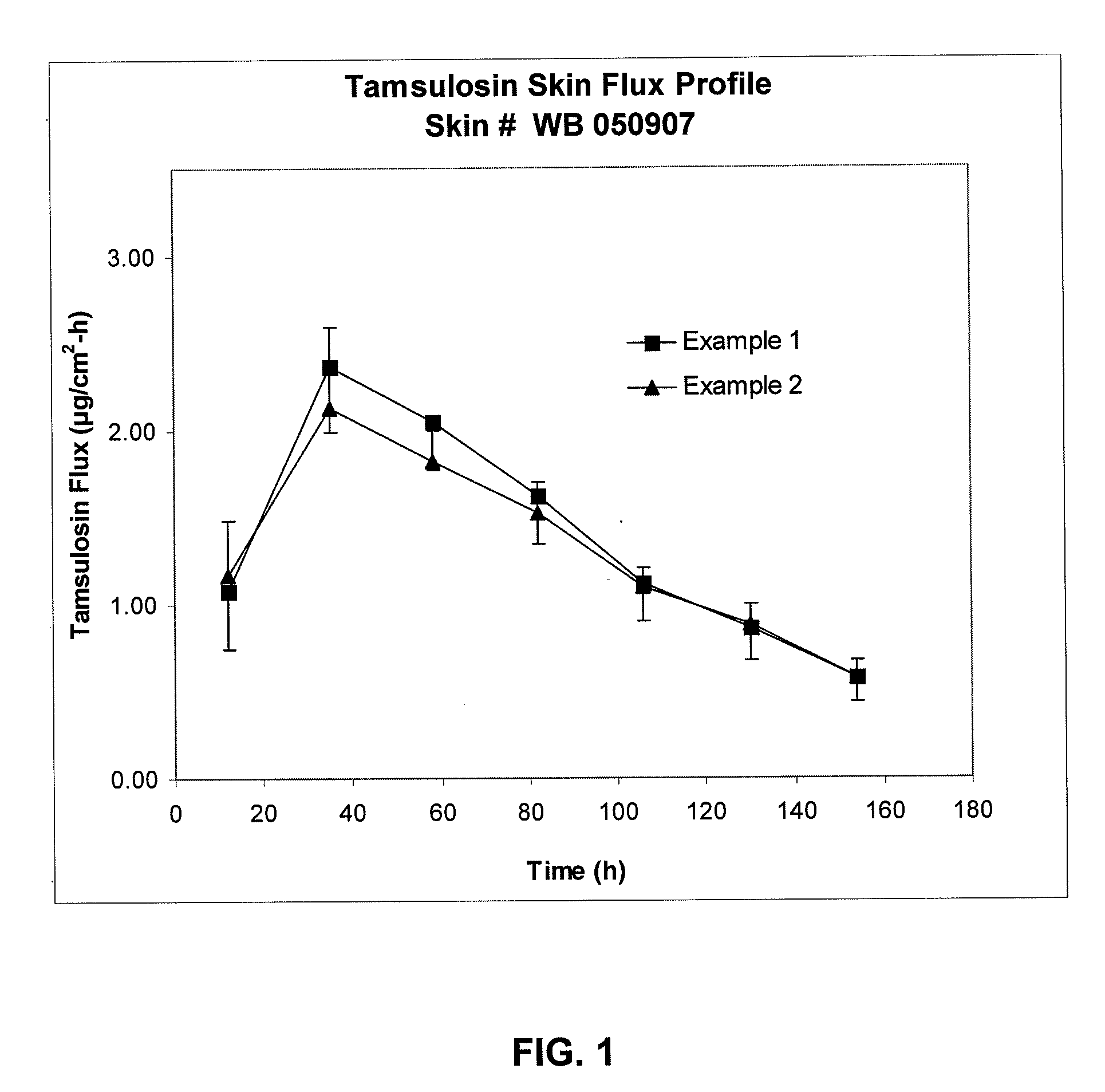
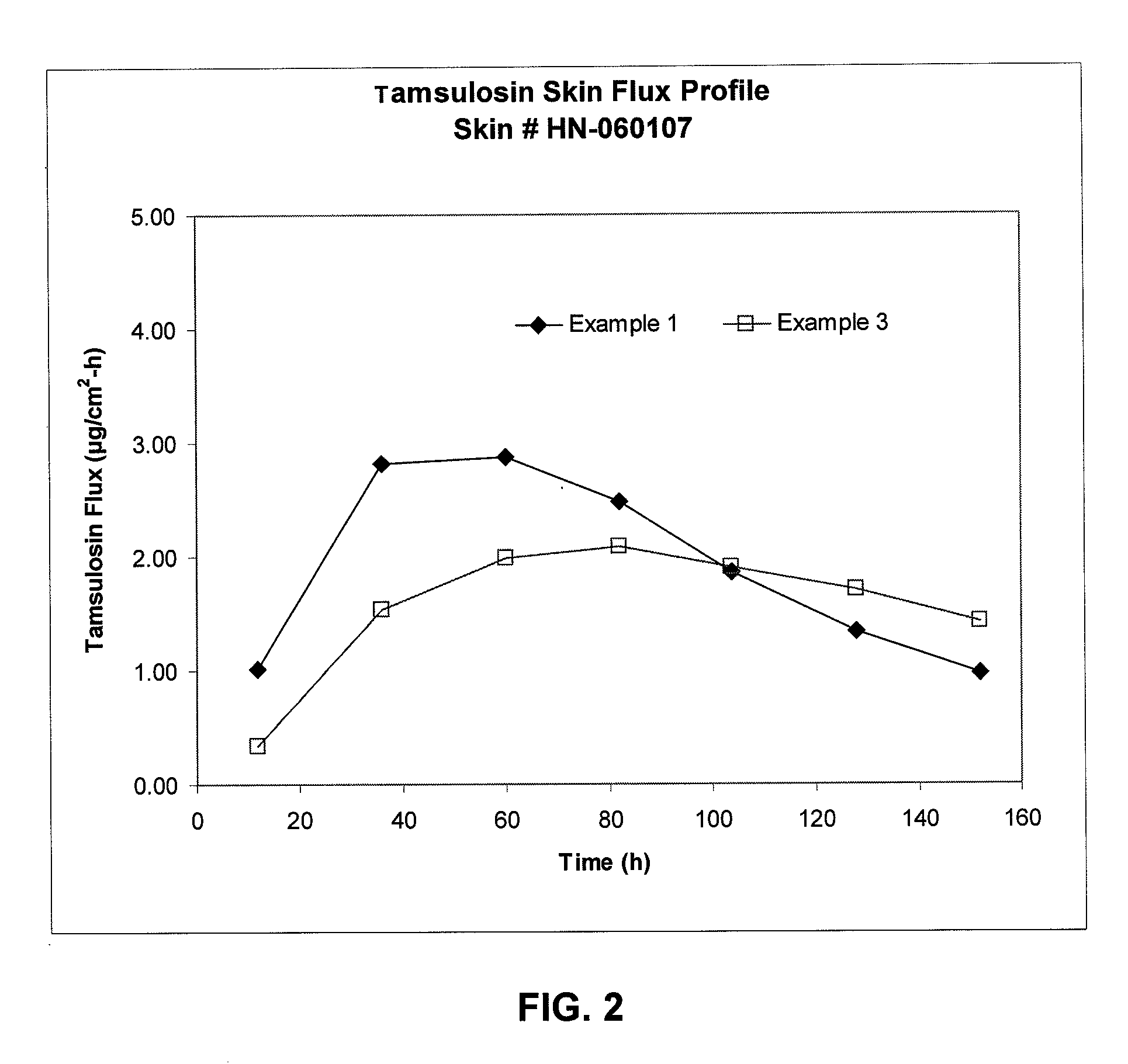
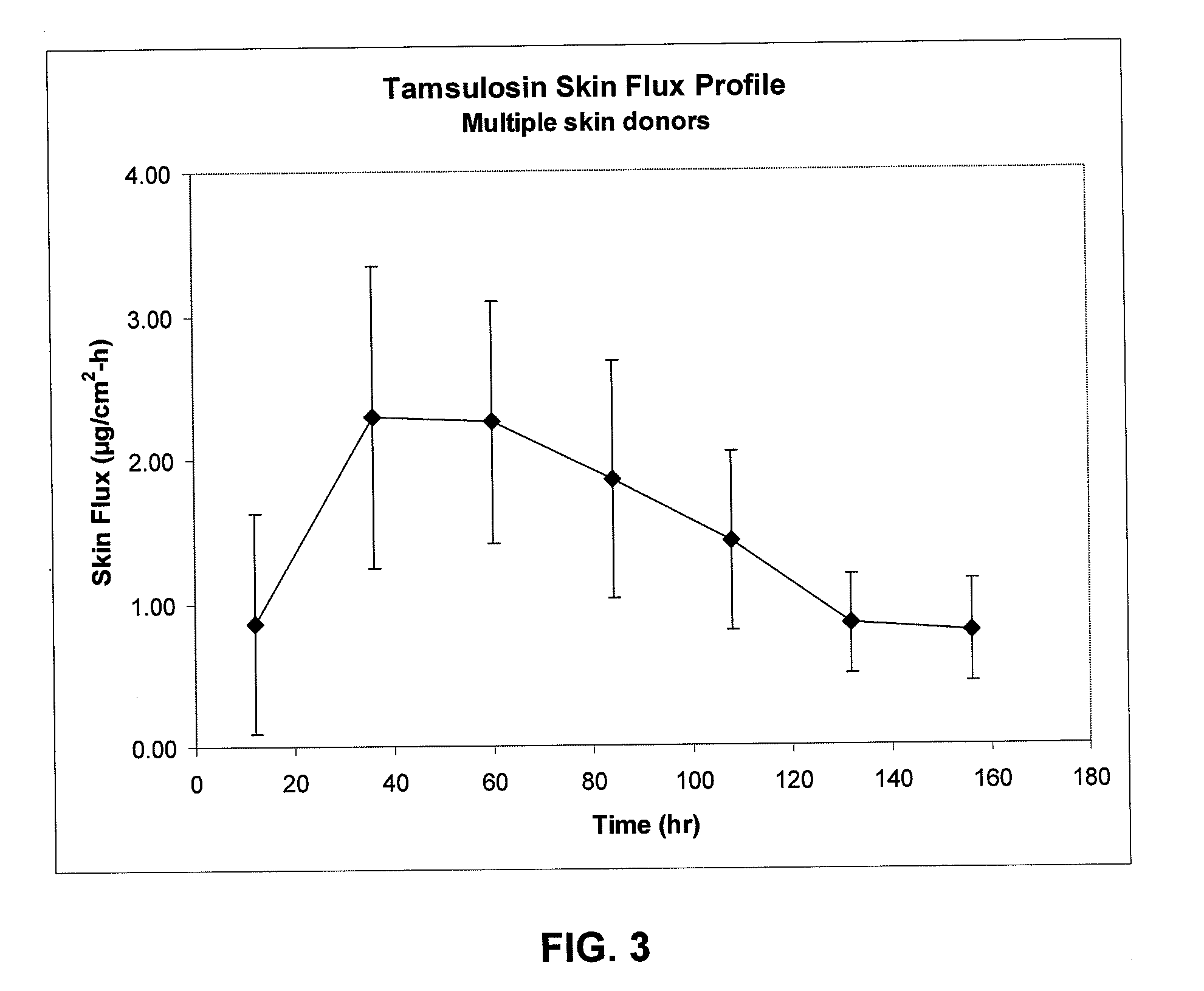
Transdermal administration of tamsulosin
In an aspect of the invention, a composition for making a patch for the transdermal delivery of tamsulosin is provided. The composition comprises (a) at least about 1 wt % tamsulosin or a pharmaceutically acceptable salt of tamsulosin, (b) at least about 40 wt % polyisobutylene adhesive or hydrophobic synthetic rubber adhesive, (c) about 1-20 wt % of an aprotic solvent in which tamsulosin dissolves readily, (d) about 1-20 wt % of an unsaturated fatty acid or an alpha-hydroxy acid or a mixture of unsaturated fatty acids or alpha-hydroxy acids or of both unsaturated fatty acids and alpha-hydroxy acids, (e) a lipophilic permeation enhancer, and (f) a matrix modifier.
Owner:CORIUM PHARMA SOLUTIONS INC
Transdermal Administration of Tamsulosin
In an aspect of the invention, a composition for making a patch for the transdermal delivery of tamsulosin is provided. The composition comprises (a) at least about 1 wt % tamsulosin or a pharmaceutically acceptable salt of tamsulosin, (b) at least about 40 wt % polyisobutylene adhesive or hydrophobic synthetic rubber adhesive, (c) about 1-20 wt % of an aprotic solvent in which tamsulosin dissolves readily, (d) about 1-20 wt % of an unsaturated fatty acid or an alpha-hydroxy acid or a mixture of unsaturated fatty acids or alpha-hydroxy acids or of both unsaturated fatty acids and alpha-hydroxy acids, (e) a lipophilic permeation enhancer, and (f) a matrix modifier.
Owner:CORIUM PHARMA SOLUTIONS INC
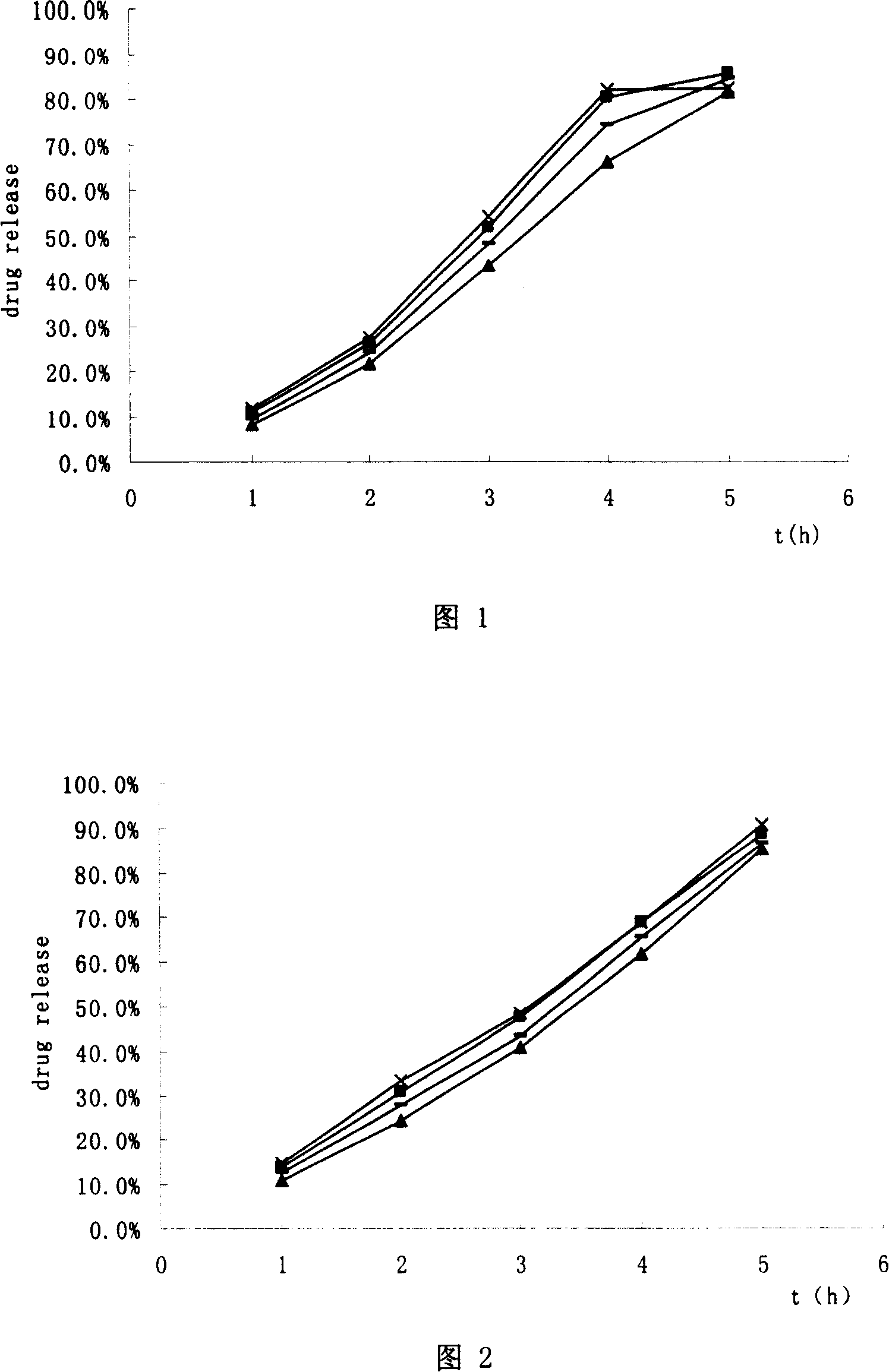
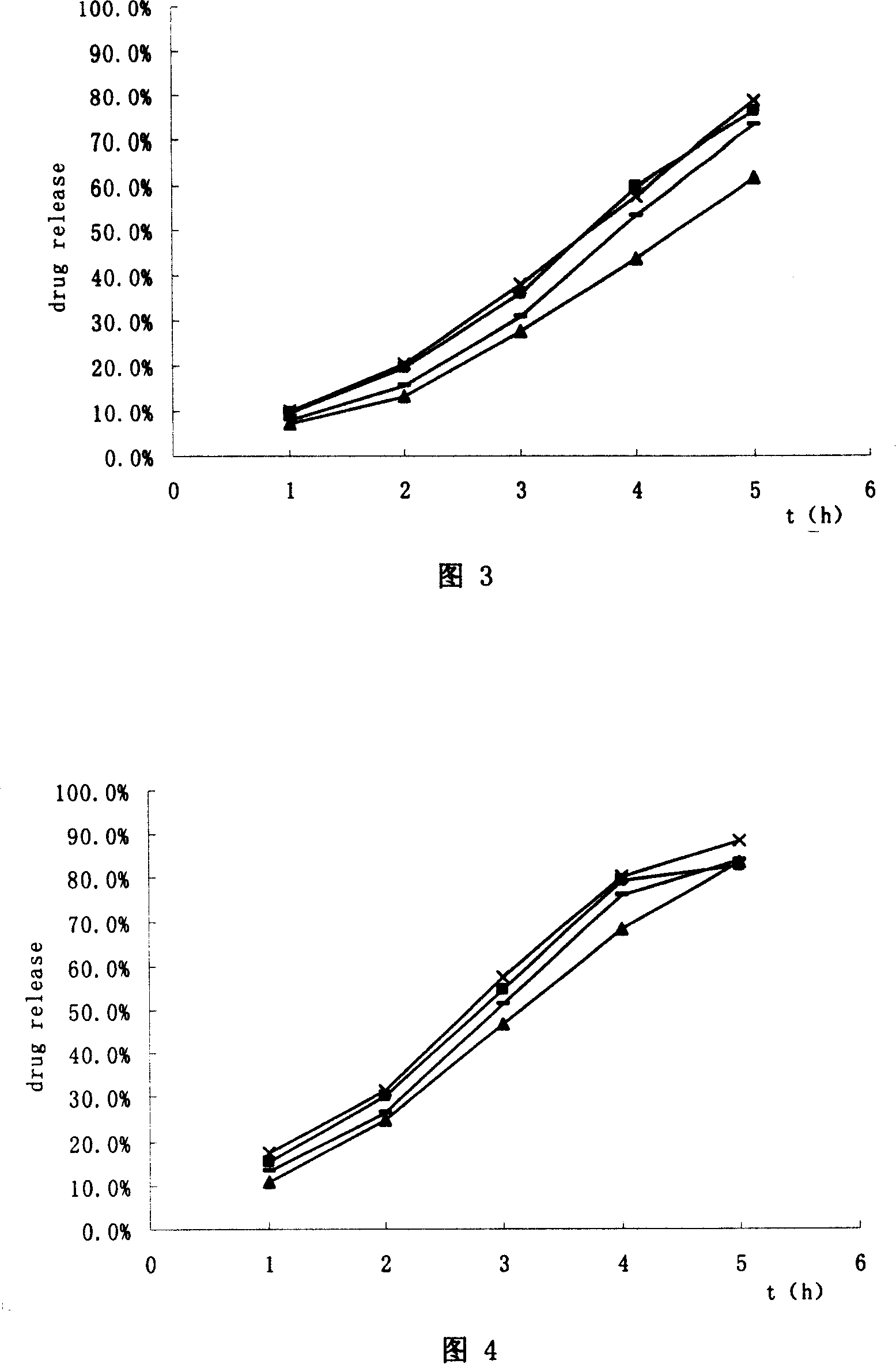
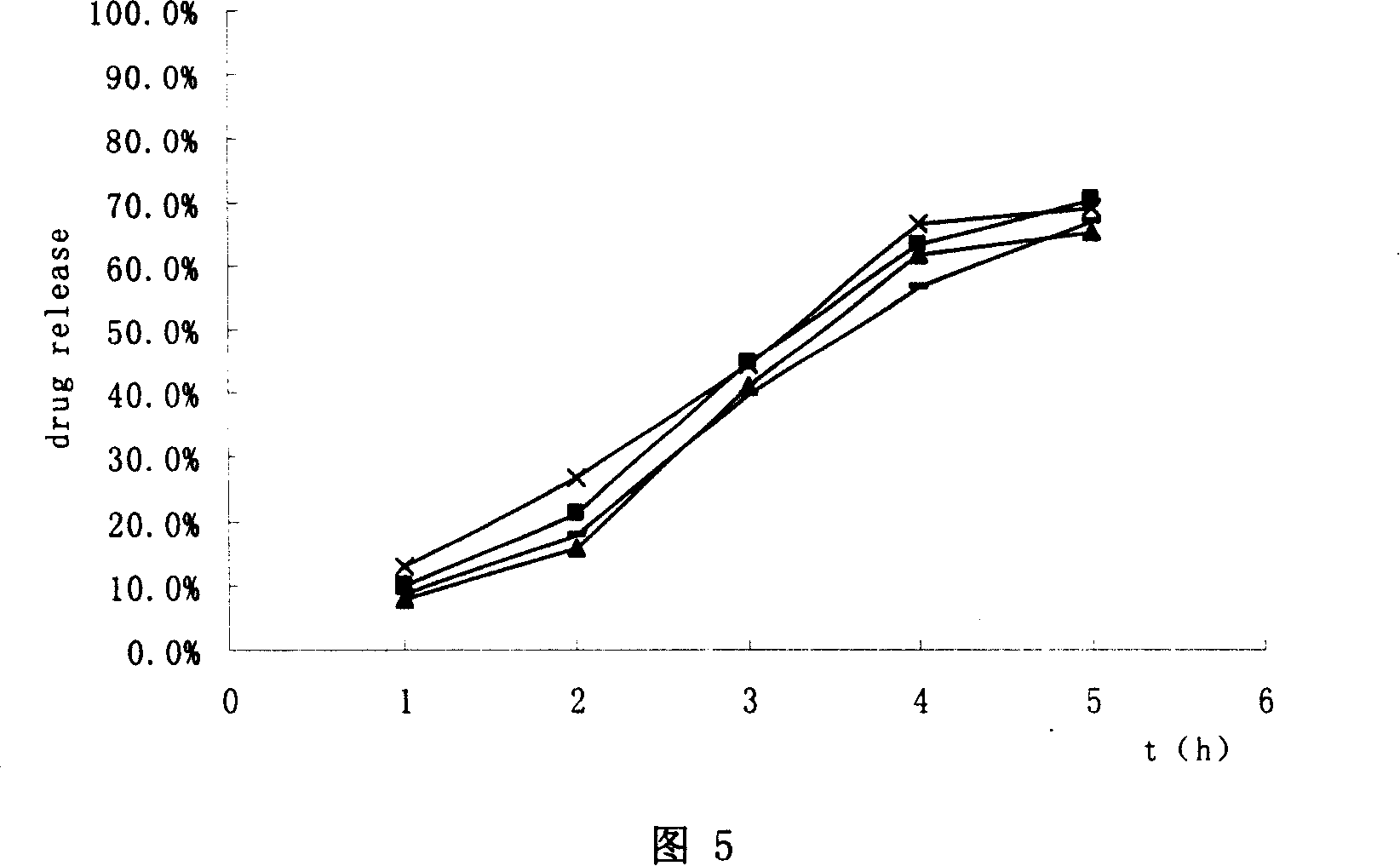
Hydrochloric tamsulosin sustained-release capsule and its preparation method
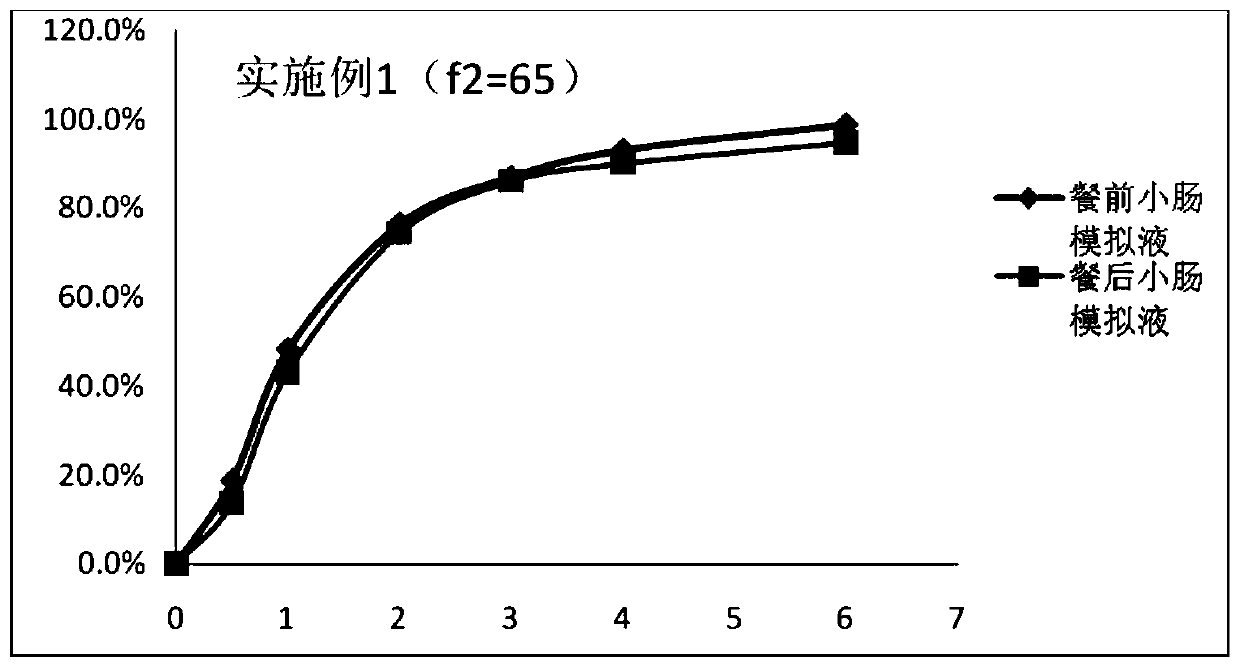
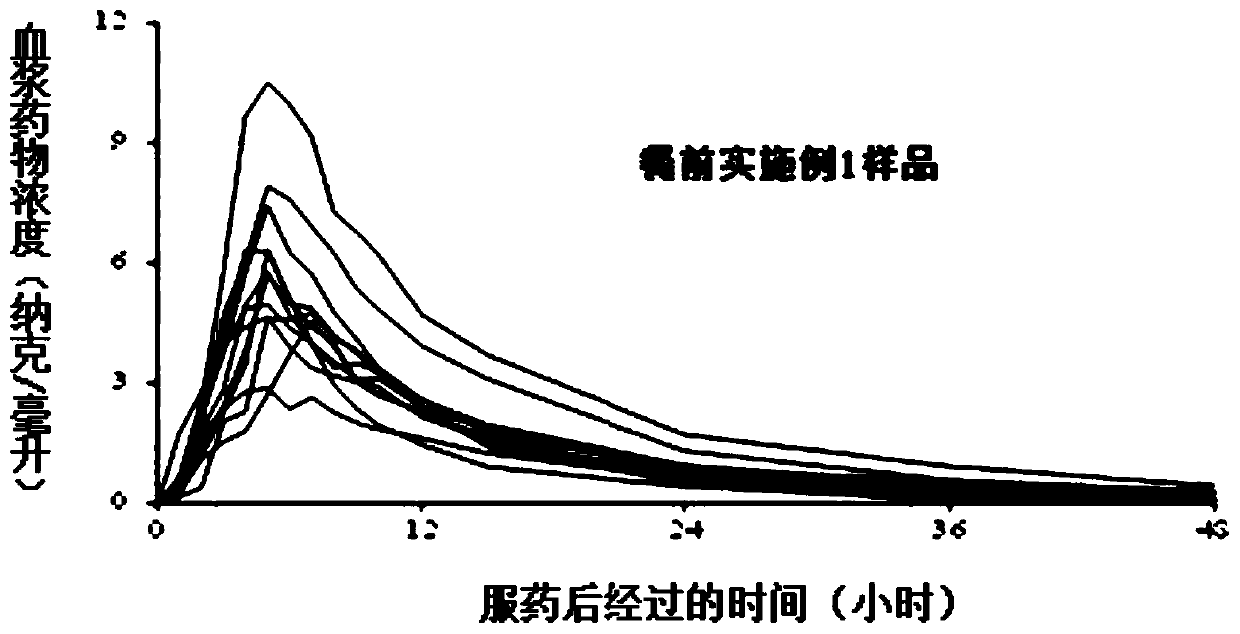
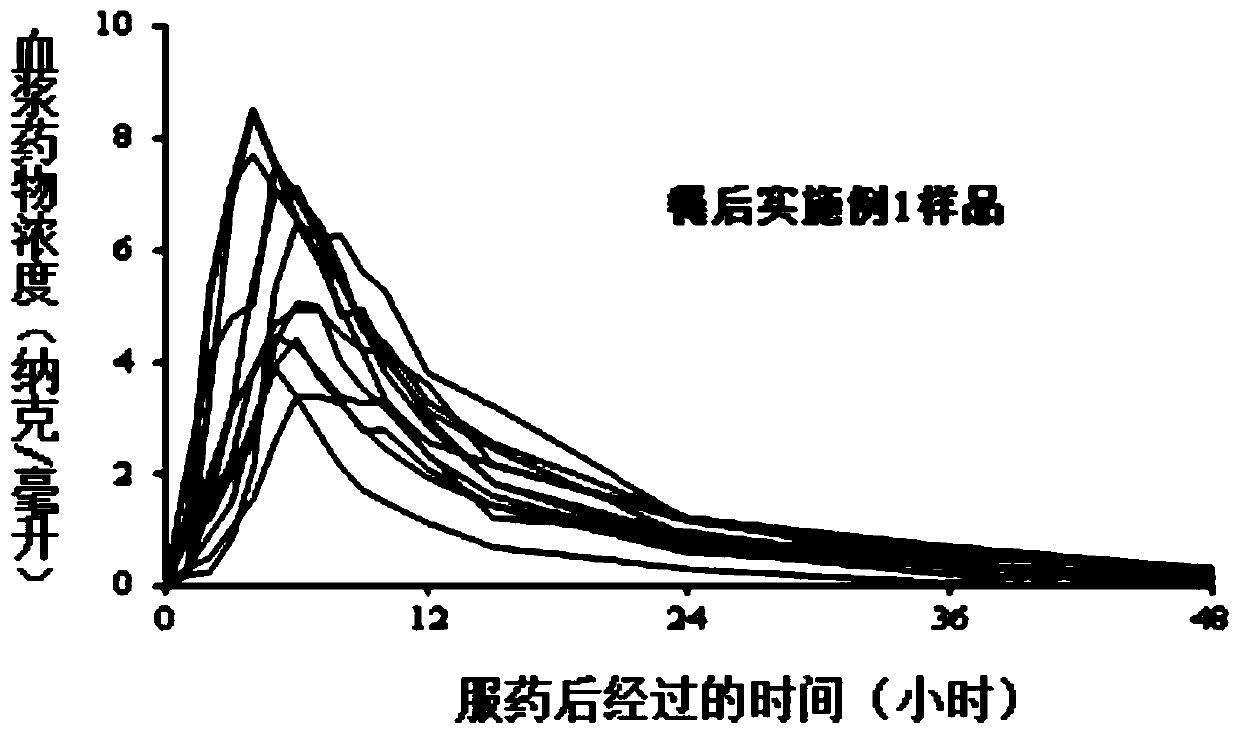
ActiveCN101125134APrecise Controlled ReleaseControl releaseOrganic active ingredientsPharmaceutical delivery mechanismSide effectOral medication
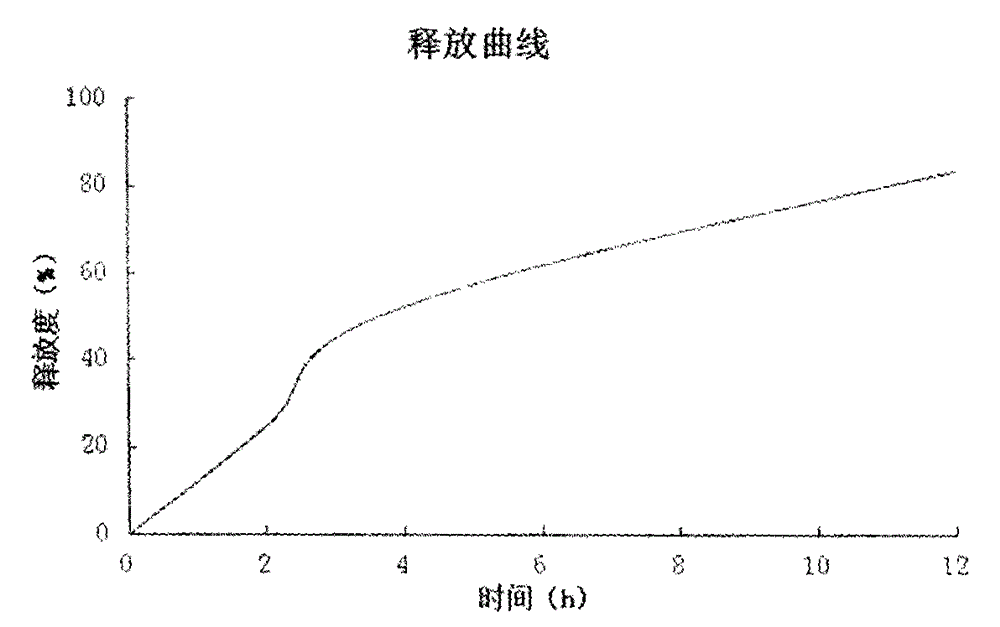
The present invention provides a tamsulosin hydrochloride sustained-release capsule. The tamsulosin hydrochloride sustained-release capsule of the present invention can avoid the sudden release of the drug tablets and the performance differences generated from the gastric emptying differences, display minor food effect or do not display food effect, and obtain the stable curve of the plasma drug concentration and longer action time simultaneously, so as to reduce the occurrence of cardiovascular side effects, greatly improve the safety, effectiveness and compliance of the medication for the patients. The tamsulosin hydrochloride sustained-release capsule of the present invention can ensure the sustained and regular release of the main ingredient tamsulosin hydrochloride after the oral administration, and the present invention is characterized by convenient administration, durable function, stable efficacy, fewer side effects and so on.
Owner:CHANGZHOU NO 4 PHARMA FACTORY
Controlled release formulation of tamsulosin hydrochloride and preparation process thereof
InactiveUS20050100606A1Maintain validityLong effectivenessGranular deliveryTamsulosin hclEthyl ester
Provided is a controlled-release formulation of tamsulosin hydrochloride, which includes a granular core, and a drug-coating layer coated on the granular core, including the tamsulosin hydrochloride and a release-controlling agent selected from the group consisting of (a) a first copolymer of 1 part by weight of ethylacrylate, 2 parts by weight of methylmethacrylate, and 0.1 parts by weight of trimethylammonioethyl methacrylate chloride, (b) a mixture of the first copolymer and a second copolymer of 1 part by weight of ethylacrylate, 2 parts by weight of methylmethacrylate, and 0.2 parts by weight of trimethylammonioethyl methacrylate chloride, the weight ratio between the first copolymer and the second copolymer being 1: 0.05 to 0.2, and (c) polyvinylacetate. A process for preparing the controlled-release formulation of the tamsulosin hydrochloride is also provided.
Owner:CTC BIO INC +1
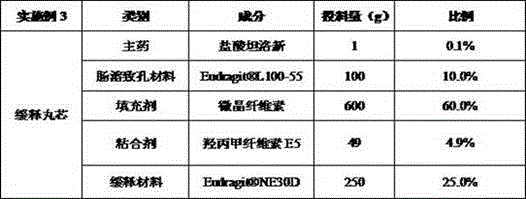
Tamsulosin Hydrochloride sustained-release preparation, preparation method and applications thereof
ActiveCN104814923AReduce adverse reactions and side effectsOvercoming large fluctuations in releasePharmaceutical delivery mechanismUrinary disorderDrugLong lasting
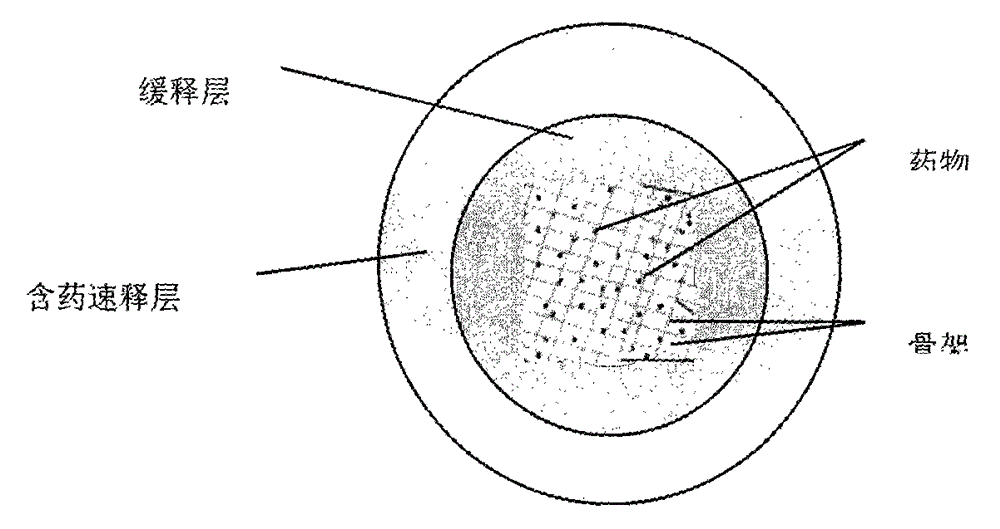
The present invention relates to a tamsulosin hydrochloride sustained-release preparation, a preparation method and applications thereof. The preparation comprises, by weight, 5-95% of framework type medicine-containing sustained-release micro-pills, 1-20% of a film control type sustained-release coating film, and 1-10% of a medicine-containing rapid-release layer, wherein the framework type medicine-containing sustained-release micro-pills comprise 0.01-1% of tamsulosin hydrochloride, 5-50% of a sustained-release material, 1-10% of an adhesive, and 5-50% of a pore forming agent, the film control type sustained-release coating film comprises 1-40% of a sustained-release material, 1-5% of a plasticizer, 10-60% of a pore forming agent, and 1-5% of an anti-adhesion agent, and the medicine-containing rapid-release layer comprises 0.01-1% of tamsulosin hydrochloride, 1-95% of a rapid-release material, and 1-10% of an adhesive. The tamsulosin hydrochloride sustained-release preparation has advantages of fast, stable, sustained, and long-lasting effect, low side effect, controllable quality, suitability for mass production and the like.
Owner:ZHEJIANG HAILISHENG PHARM CO LTD
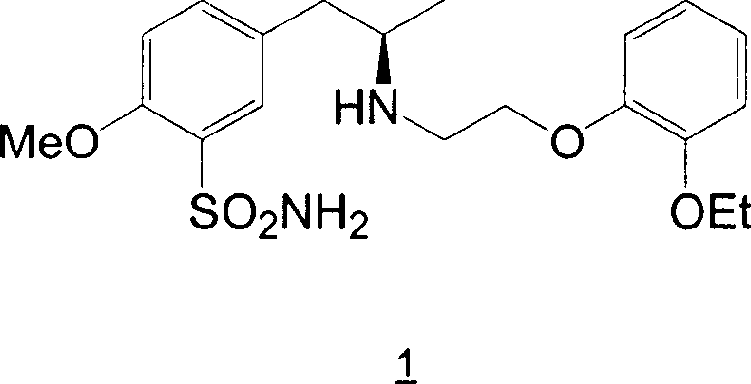
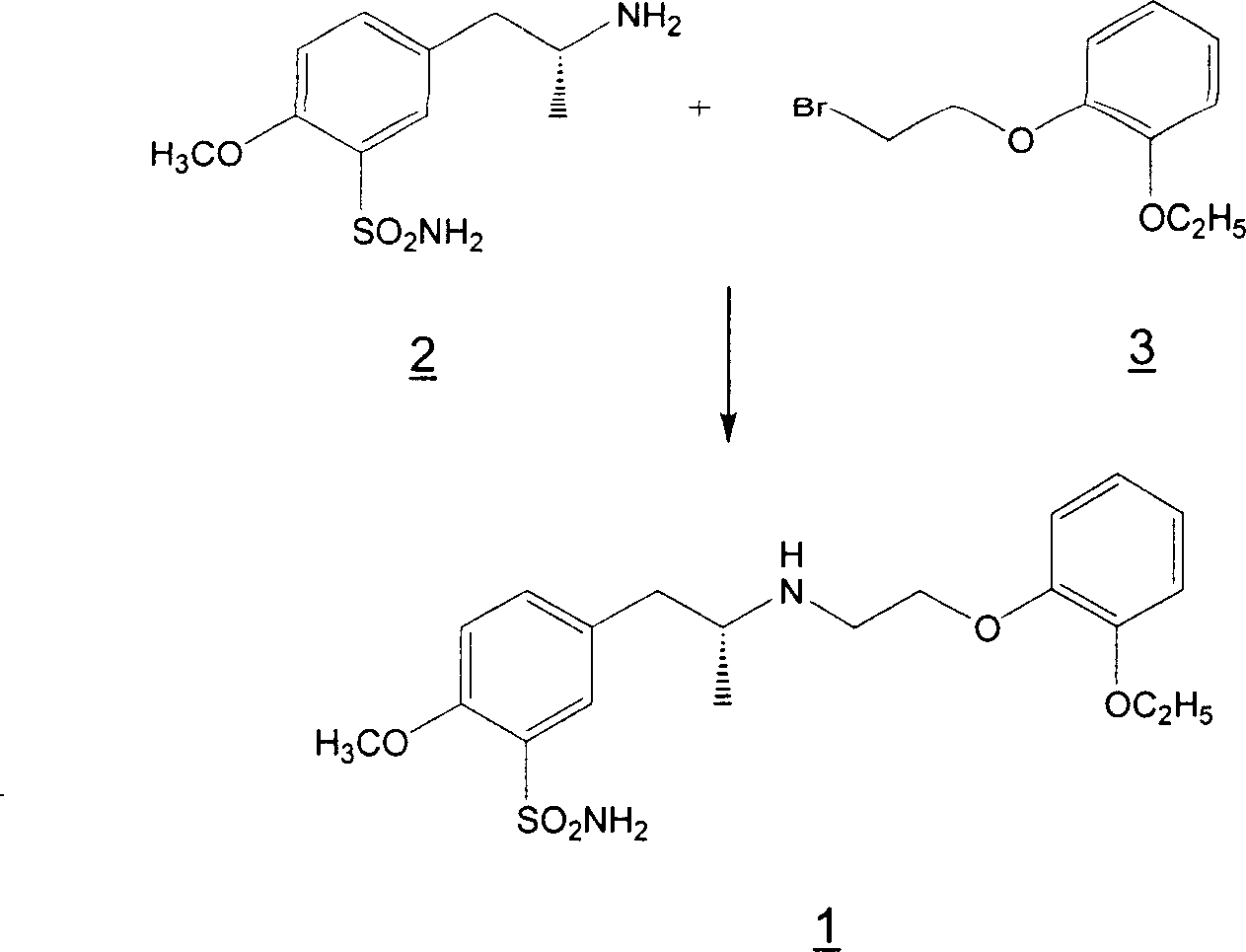
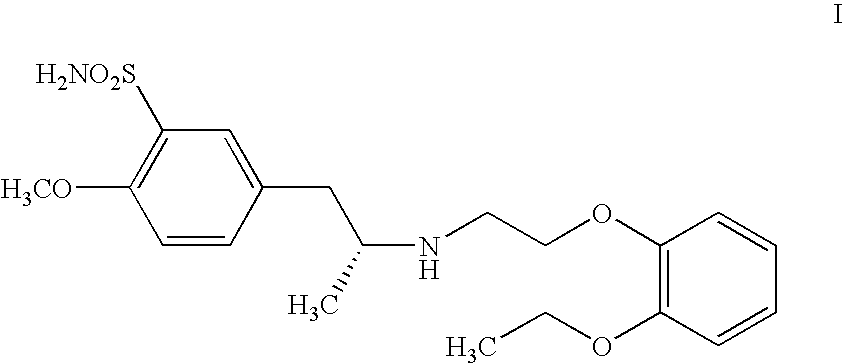
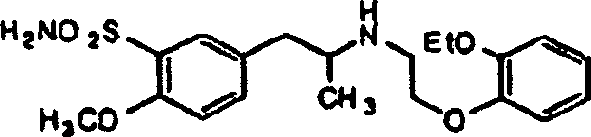
Preparation of r-5-(2-(2-ethoxyphenoxyethylamino)propyl)-2-methoxybenzenesulphonamide hydrochloride of high chemical purity
Owner:LEK PHARMA D D
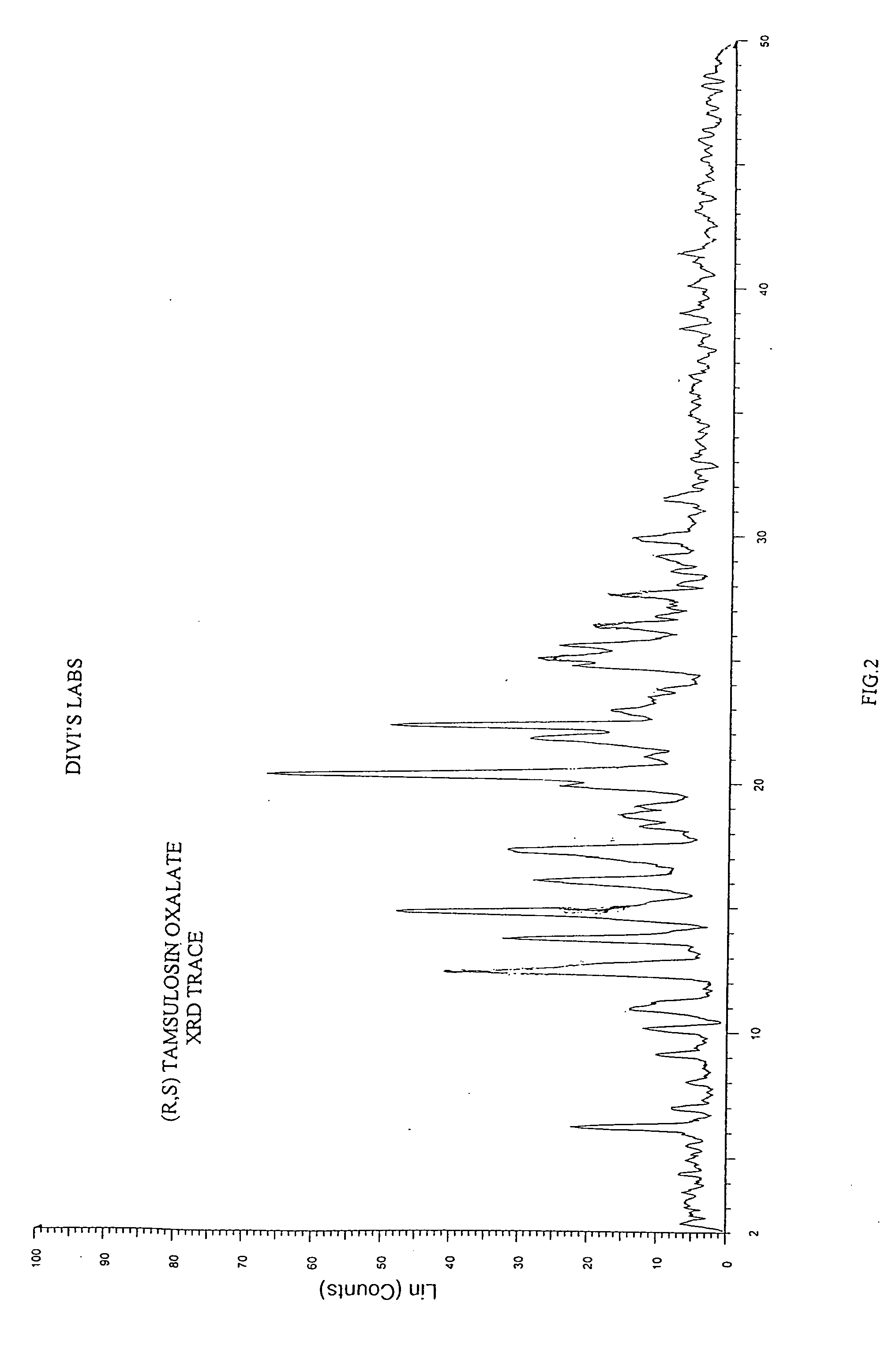
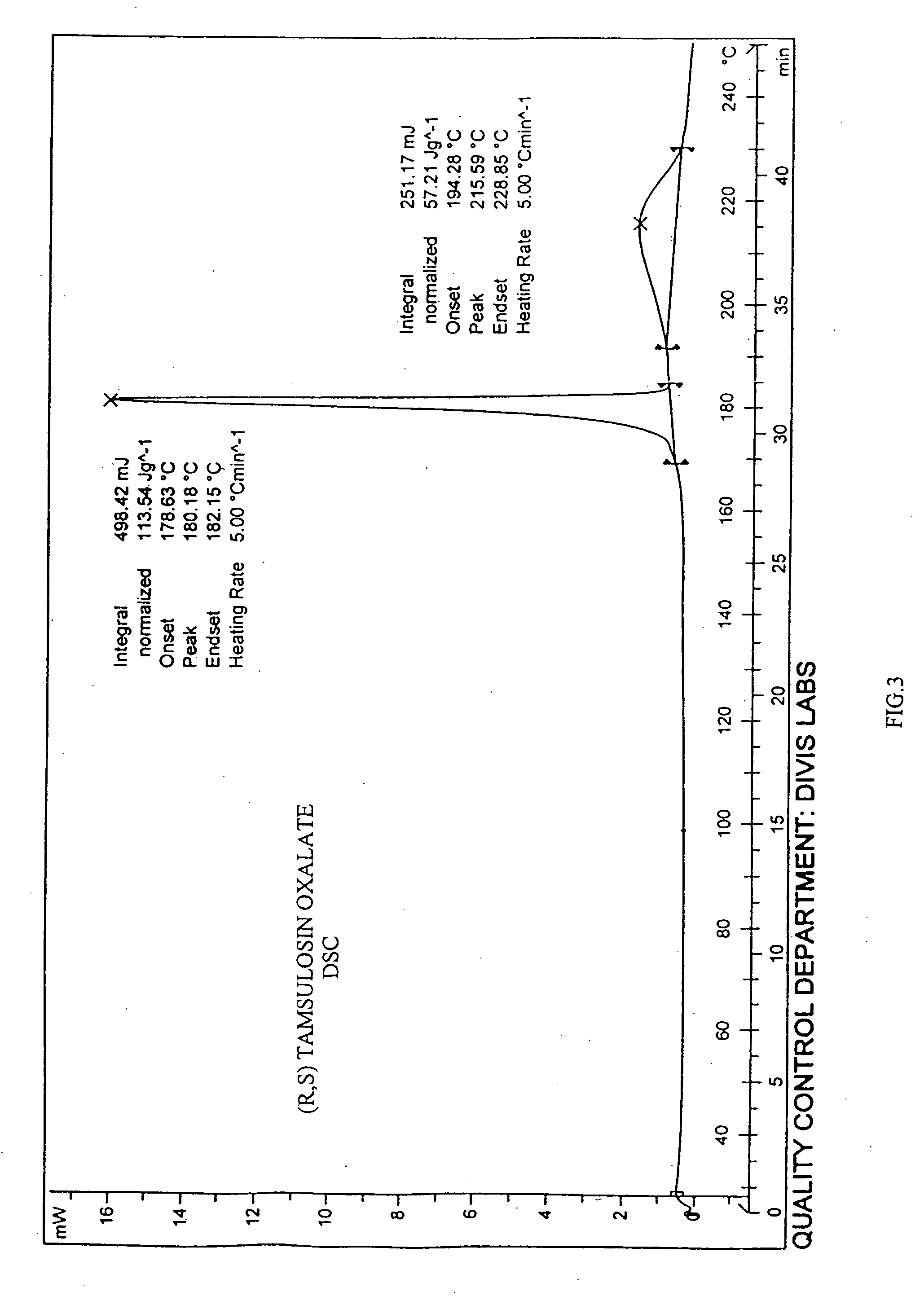
Process for the resolution of racemic (R,S) -5-(2-(2-(2- ethoxyphenoxy) ethylamino)propyl)-2-methoxybenzene sulfonamide (tamsulosin), its novel R and S isomers and their salts and processes for their preparation
ActiveUS20060079714A1Speed up the processOrganic compound preparationOrganic chemistry methodsBenzeneMedicinal chemistry
An improved process is described to resolve a racemic mixture in any proportion of 5-(2-(2-(2-ethoxyphenoxy)ethylamino)propyl)-2-methoxy benzene sulfonamide as a free base or some of its salts, with BPA either S or R form to obtain enantiomerically highly pure R and S-isomer as a well characterized free base or as a salt of the title compound. Also described are novel R and S-isomers of 5-(2-(2-(2-ethoxyphenoxy) ethylamino)propyl)-2-methoxy benzene sulfonamide and their salts and the processes for their preparation.
Owner:DIVI S LAB LTD
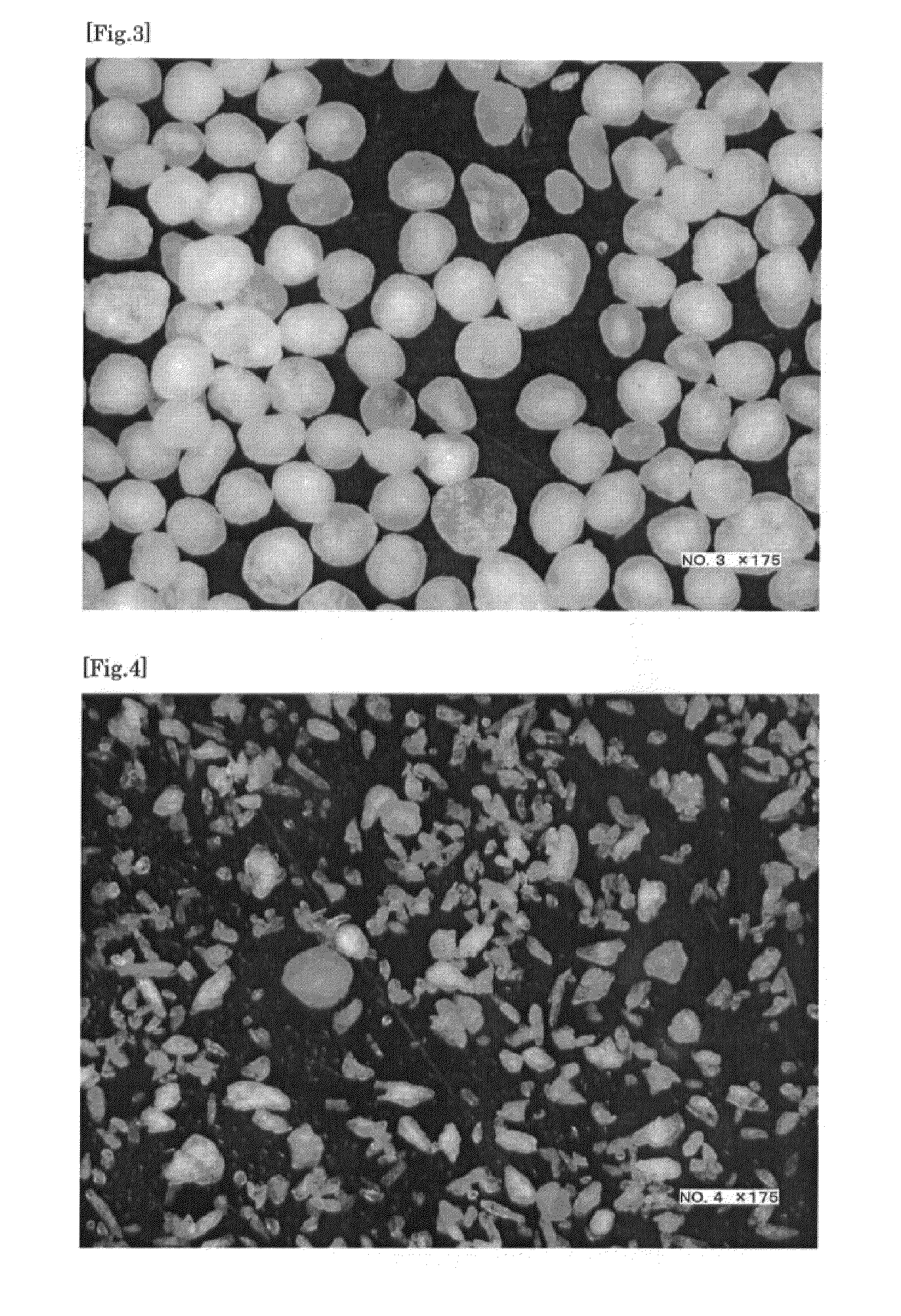
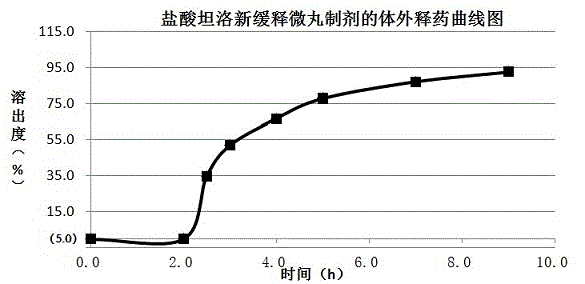
Process for production of spherical microparticles comprising tamsulosin hydrochloride
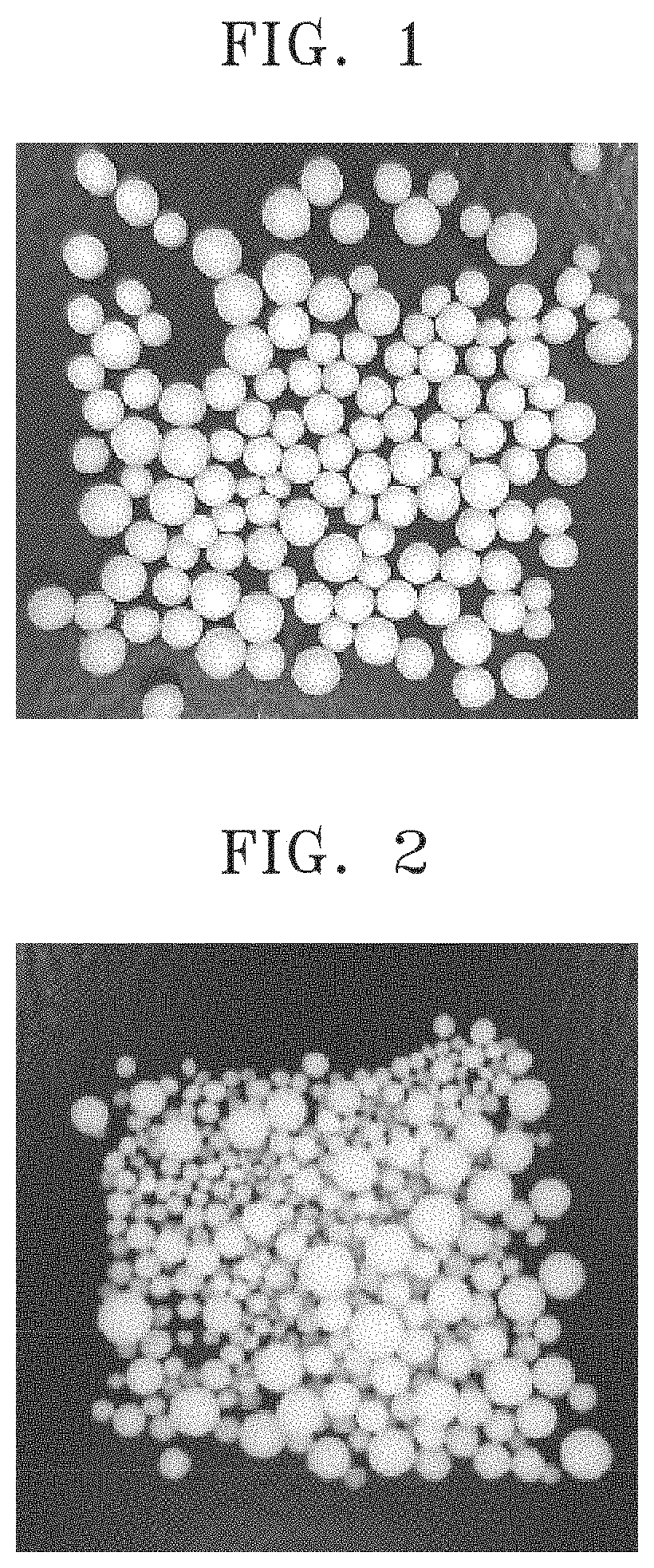
InactiveUS20110104270A1Narrow particle size distributionRough textureBiocidePowder deliveryTamsulosin hclOrally disintegrating tablet
The present invention provides a method for producing spherical fine particles containing tamsulosin hydrochloride, the method includes the steps of: (1) mixing and stirring tamsulosin hydrochloride (a), microcrystalline cellulose (b), and water until a mixture of the component (a) and the component (b) is uniformly impregnated with the water; (2) granulating the mixture obtained in step (1) using an stirring granulator whose peripheral speed is set to be 5.5 to 9.0 m / s; and (3) drying the granules obtained in step (2). The present invention also provides spherical fine particles obtained according to the method, coated fine particles obtained by applying a coating to the spherical fine particles, and an orally disintegrating tablet containing the coated fine particles.
Owner:SAWAI PHARMA
Tamsulosin hydrochloride sustained-release pellet preparation
ActiveCN104586771AControl releaseEfficient releaseUrinary disorderAmide active ingredientsTamsulosin hclControlled release
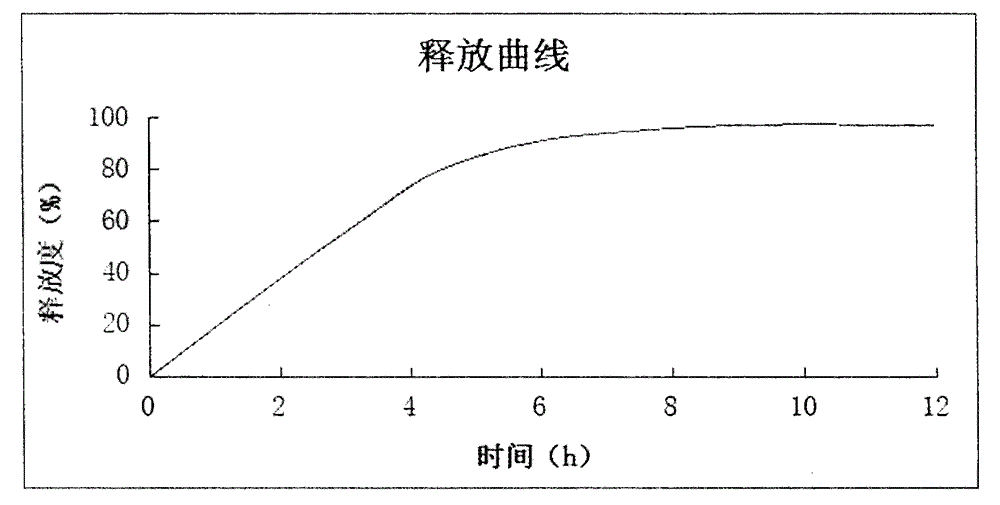
The invention discloses a tamsulosin hydrochloride sustained-release pellet preparation. The tamsulosin hydrochloride sustained-release pellet preparation comprises a tamsulosin hydrochloride sustained-release pellet core, a sustained-release coating layer, and an enteric soluble coating layer; and is a capsule preparation. Three slow release methods including skeleton slow-release, membrane control slow-release, and enteric coating are adopted for preparation of the tamsulosin hydrochloride sustained-release pellet preparation; the obtained tamsulosin hydrochloride sustained-release pellet capsule preparation is capable of controlling releasing from the stomach to the intestine, so that adverse reaction caused by large amount absorption of tamsulosin hydrochloride in a short time is prevented. A preparation method of the tamsulosin hydrochloride sustained-release pellet preparation is simple; and in vivo release of tamsulosin hydrochloride can be controlled stably and effectively.
Owner:REGENEX PHARMA LTD
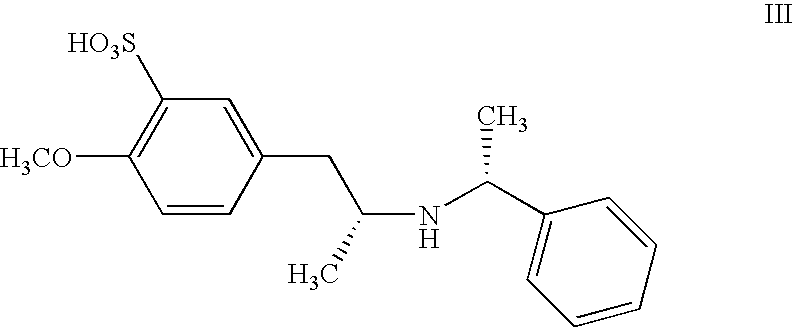
Process for the preparation of tamsulosin and intermediates thereof
InactiveUS20090234154A1Organic compound preparationOrganic chemistry methodsSulfonyl chlorideChlorosulfuric acid
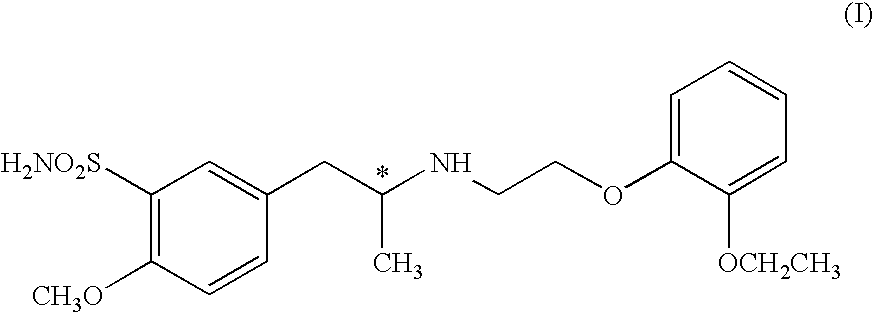
A process for producing tamsulosin of formula Iand pharmaceutically acceptable addition salts, thereof comprises the steps of:a) Reacting compound R,R-[2-(4-methoxy-phenyl)-1-methyl-ethyl]-(1-phenyl-ethyl)-amine of formula II or a salt thereof with chlorosulfonic acid with or without an organic solvent, to obtain compound R,R-2-methoxy-5-[2-(1-phenyl-ethylamino)-propyl]-benzenesulfonic acid of formula IIIb) Hydrogenolysis of compound R,R-2-methoxy-5-[2-(1-phenyl-ethylamino)-propyl]-benzenesulfonic acid of formula III or a salt thereof carried out in an alcohol in the presence of a palladium catalyst using hydrogen or a source of hydrogen, to obtain compound R-(−)-5-(2-amino-propyl)-2-methoxy-benzenesulfonic acid of formula IVc) Reacting primary amine R-(−)-5-(2-amino-propyl)-2-methoxy-benzenesulfonic acid of formula IV, or a salt thereof, with a compound of formula V wherein X represents an halogen atom selected from the group consisting of Cl; Br and I, to obtain 5-{(2R)-2-[2-(2-ethoxy-phenoxy)-ethylamino]-propyl}-2-methoxy-benzenesulfonic acid compound of formula VId) Reacting compound of formula VI with an halogenating agent, to obtain the corresponding sulfonylchloride of formula VII.e) Reacting compound VII with ammonia to obtain compound I.
Owner:HOVIONE INTER
Controlled-release pharmaceutical composition including tamsulosin or pharmaceutically acceptable salts thereof, and oral formulation including the same
InactiveUS20150086623A1Shield bitter tasteMinimize side-effectsBiocidePill deliverySide effectRelease pattern
A controlled-release pharmaceutical composition including first and second groups of microparticles, each of the microparticles including a core including tamsulosin or pharmaceutically acceptable salts thereof, a controlled-release polymer coating layer formed on the core, and an enteric polymer outer layer formed on the controlled-release polymer coating layer, wherein the average thickness of the controlled-release polymer coating layer is different in each of the first and second groups of microparticles, and an oral formulation including the same, are provided. This pharmaceutical composition can easily control the extent of release of an active ingredient depending on changes in pH in the intestinal tract and the release pattern of the active ingredient in the small intestine, thus preventing the active ingredient from being rapidly transferred into the blood to thereby minimize side-effects, and maintaining the effective blood concentration of the active ingredient for a predetermined period of time. Furthermore, this composition can shield the bitter taste of the active ingredient even when exposed to the inside of the mouth, thus increasing the therapeutic effects for patients upon oral administration.
Owner:SAMYANG BIOPHARMLS CORP
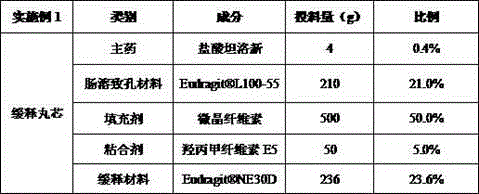
Slow/controlled-release preparation of tamsulosin hydrochloride and preparation method thereof
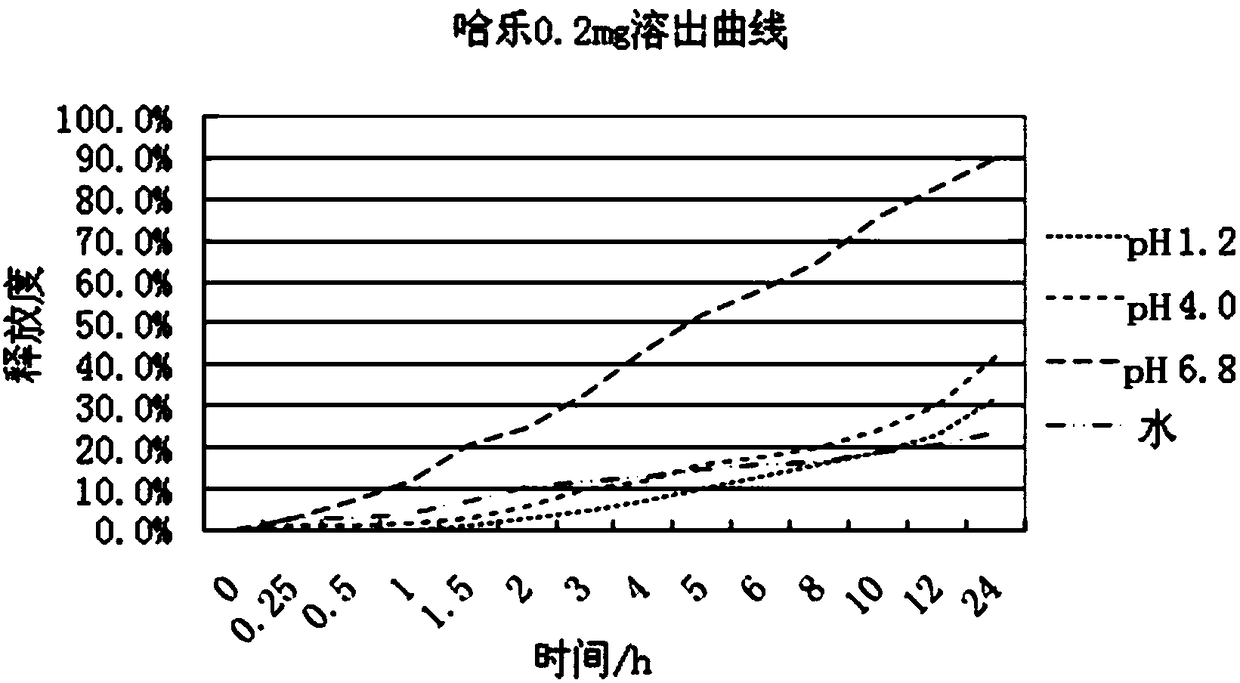
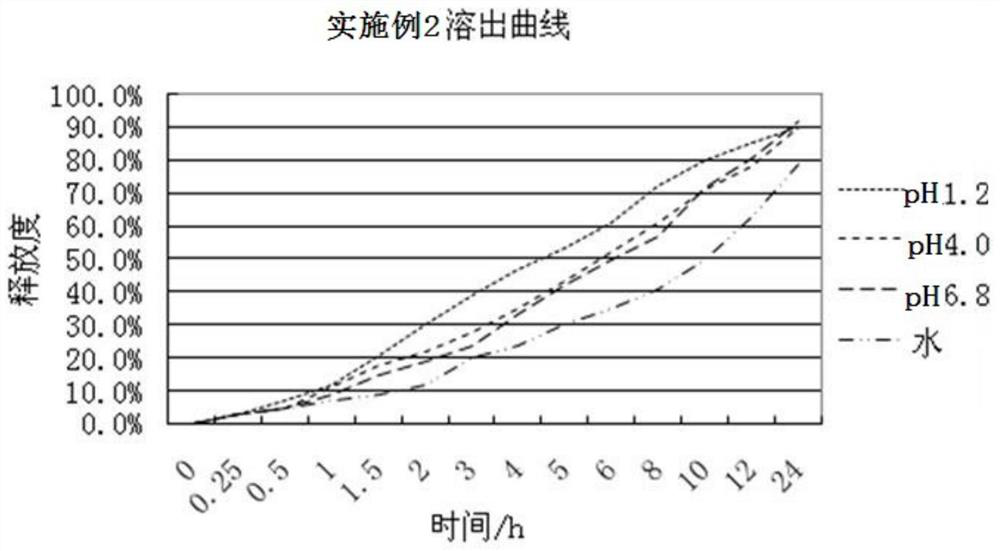
ActiveCN108096220AAffect absorptionProblems Affecting AbsorptionInorganic non-active ingredientsUrinary disorderCelluloseDissolution
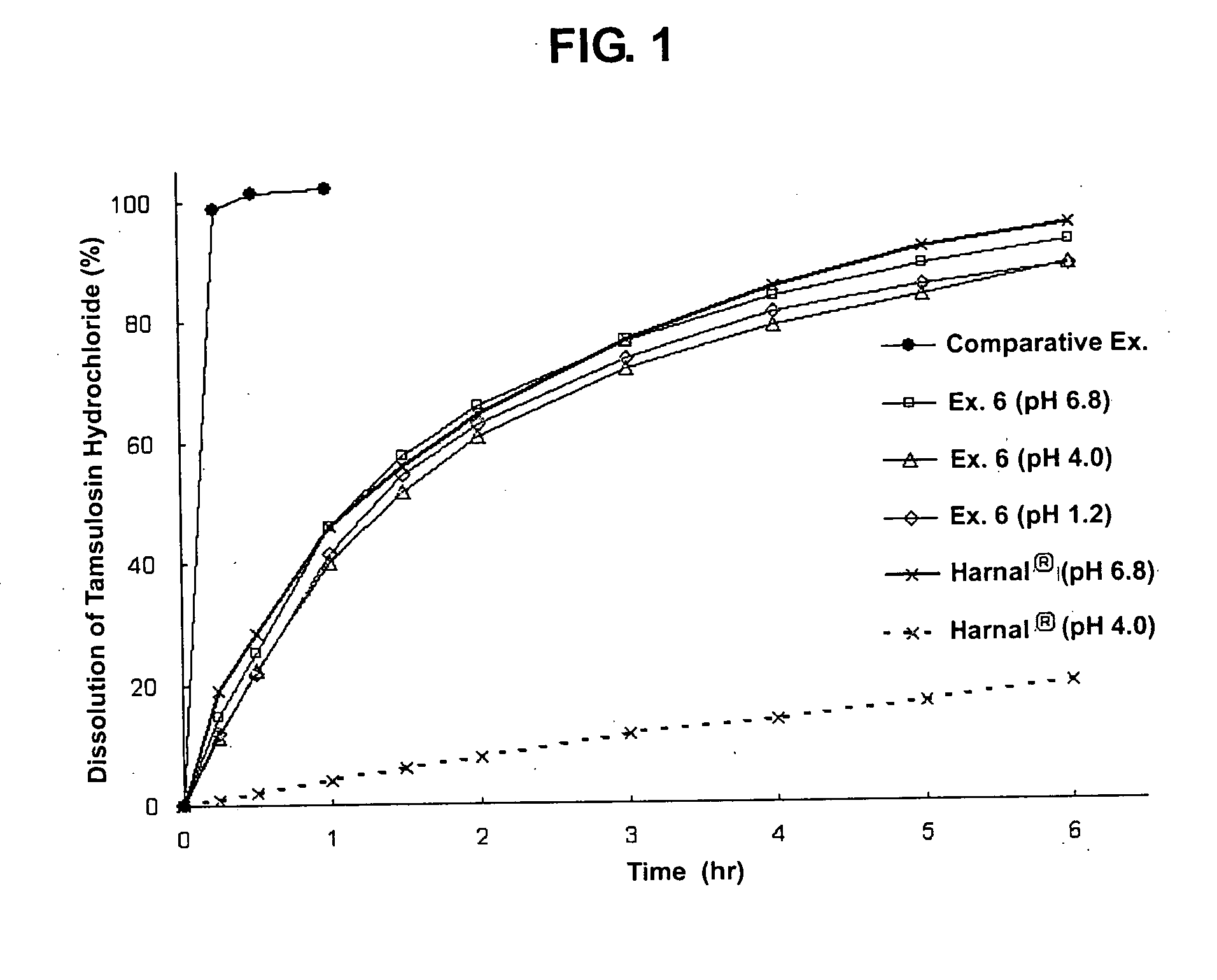
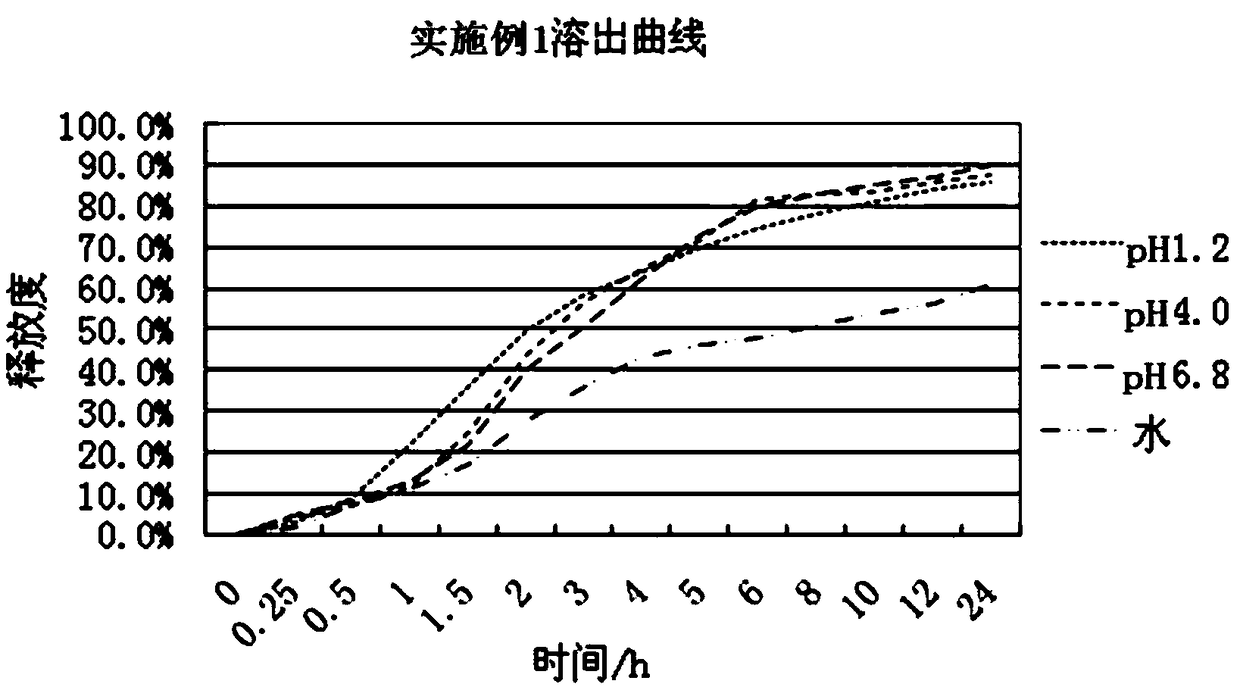
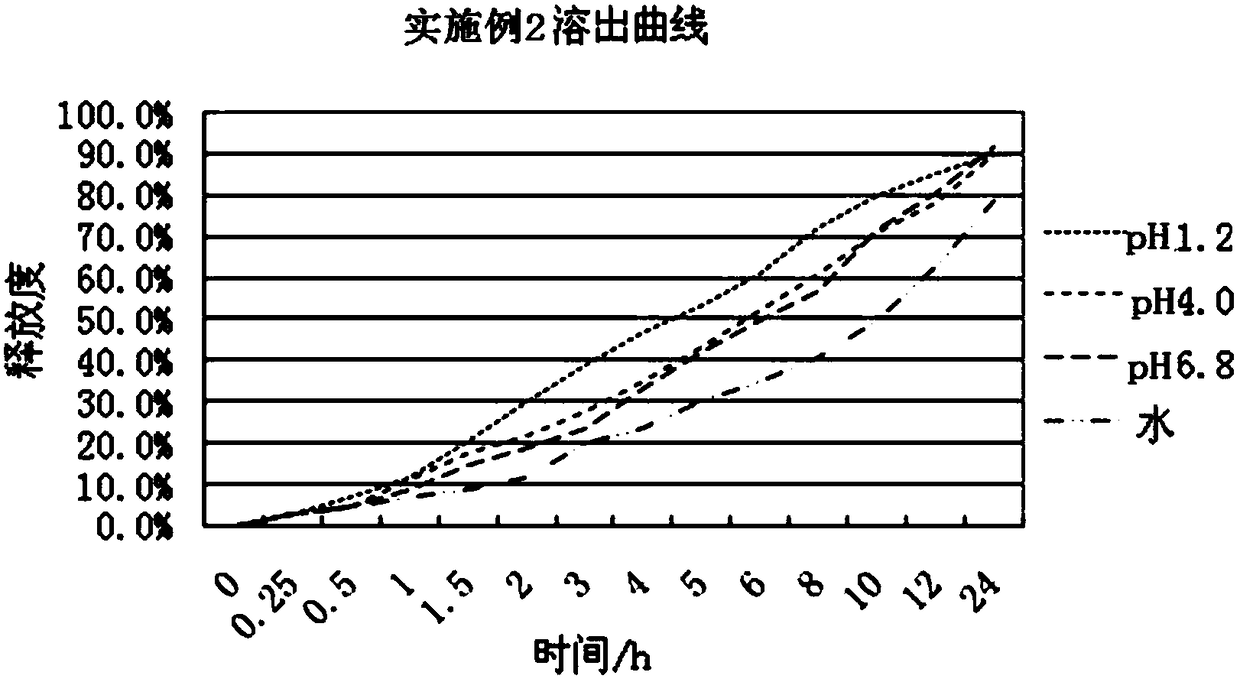
The invention relates to a slow / controlled-release preparation of tamsulosin hydrochloride and a preparation method thereof. The slow / controlled-release preparation comprises (i) a pill core which ismade of the following raw material in parts by weight: 135-165 parts of a microcrystal cellulose micro-pill cores; (ii) a slow-release layer which is made of the following raw materials in parts by weight: 0.36-0.44 parts of tamsulosin hydrochloride, 145-200 parts of a slow / controlled release material and 3.8-58 parts of a pore forming agent. Dissolution degree tests show that the slow / controlled-release tamsulosin hydrochloride preparation consisting of a blank pill core and a medicine-carrying slow / controlled release coating layer has a slow-release effect within a relatively pH value range(the pH value is 1.2, 4 or 6.8), has a pH value independent slow-release property, and is capable of solving the problem that absorption of tamsulosin hydrochloride is affected as the pH value of a gastrointestinal tract is changed if a patient takes foods, therefore, the slow / controlled-release preparation of the tamsulosin hydrochloride, which is provided by the invention, can be taken both before or after a meal, and is relatively convenient to take, and absorption of the tamsulosin hydrochloride is not affected.
Owner:SUZHOU CHUNGHWA CHEM & PHARMA IND
Injectable depot compositions and process of preparation of such compositions
InactiveCN101541316APromote ease of injectabilitySulfur/selenium/tellurium active ingredientsOintment deliveryActive agentProlonged release
Novel injectable compositions are provided comprising an active agent which is tarnsulosin or letrozole or its pharmaceutically acceptable salts, derivatives, isomers, polymorphs, solvates, hydrates, analogues, enantiomers, tautomeric forms or mixtures thereof and one or more pharmaceutically acceptable excipient(s) wherein the compositions are preferably formulated as biodegradable microparticles or nanoparticles which can optionally be reconstituted with an aqueous, hydro-alcoholic or oily liquid vehicle prior to administration. The novel injectable compositions of the present invention preferably form a depot upon administration in vivo and are in the form of an in situ gelling composition or an implant composition which provides a prolonged release of tamsulosin or letrozole for extended periods of time.; Also described are process for preparation of such novel compositions and method of using them.
Owner:PANACEA BIOTEC
Process for the Preparation of Tamsulosin
InactiveUS20080262089A1Reduce amountEfficient separationBiocideOrganic compound preparationTriethylphosphiteTrimethyl phosphite
The invention includes an improved process for producing tamsulosin comprising reacting 5-(2-aminopropyl)-2-methoxybenzenesulfonamide with 2-(o-ethoxyphenoxy)ethyl bromide in an organic phosphite solvent to obtain tamsulosin. Optically pure (R)-5-(2-aminopropyl)-2-methoxybenzenesulfonamide can be employed to produce optically pure (R)-tamsulosin product. The organic phosphite solvent utilized in the reaction can include tri-alkyl phosphites such as triethyl phosphite, trimethyl phosphite, and tributyl phosphite. Additionally, processes for producing tamsulosin having a low concentration of by-product contaminants, such as 5-((R)-2-{Bis-[2-(2-ethoxyphenoxy)ethyl]amino}-propyl)-2-methoxybenzenesulfonamide, and the use of such by-products to monitor the chemical purity of tamsulosin, are provided.
Owner:MEDICHEM
Tamsulosin tablets
Owner:SYNTHON BV
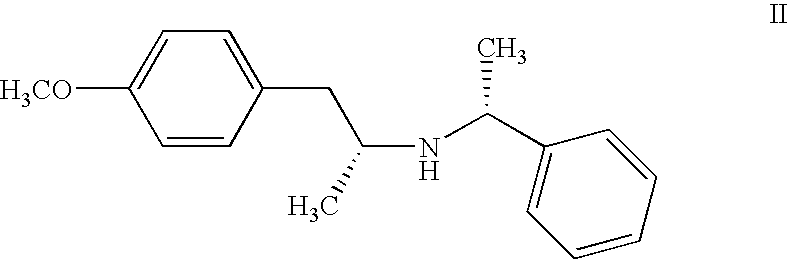
Process for preparing R- and S-isomers of (R)-5-(2-( (2-(2-ethoxyphenoxy)ethyl)amino)propyl)-2-methoxybenzenesulfonamide
A process for preparing optically pure enantiomers of R-(-)tamsulosin of formiula Ia and S-(+)tamsulosin of formula Ib by resolving racemic tamsulosin of formula I by means of (IR)-(-)-camphor-10-sulfonic acid and (1S)-(+)-camphor-10-sulfonic acid, resp., in an environment of organic solvents, water or mixtures thereof. ##STR00001##
Owner:FARMAK
A kind of sustained and controlled release preparation of tamsulosin hydrochloride and preparation method thereof
ActiveCN108096220BAffect absorptionProblems Affecting AbsorptionInorganic non-active ingredientsUrinary disorderTamsulosin hclTamsulosine
The invention relates to a slow / controlled-release preparation of tamsulosin hydrochloride and a preparation method thereof. The slow / controlled-release preparation comprises (i) a pill core which ismade of the following raw material in parts by weight: 135-165 parts of a microcrystal cellulose micro-pill cores; (ii) a slow-release layer which is made of the following raw materials in parts by weight: 0.36-0.44 parts of tamsulosin hydrochloride, 145-200 parts of a slow / controlled release material and 3.8-58 parts of a pore forming agent. Dissolution degree tests show that the slow / controlled-release tamsulosin hydrochloride preparation consisting of a blank pill core and a medicine-carrying slow / controlled release coating layer has a slow-release effect within a relatively pH value range(the pH value is 1.2, 4 or 6.8), has a pH value independent slow-release property, and is capable of solving the problem that absorption of tamsulosin hydrochloride is affected as the pH value of a gastrointestinal tract is changed if a patient takes foods, therefore, the slow / controlled-release preparation of the tamsulosin hydrochloride, which is provided by the invention, can be taken both before or after a meal, and is relatively convenient to take, and absorption of the tamsulosin hydrochloride is not affected.
Owner:SUZHOU CHUNGHWA CHEM & PHARMA IND
A kind of tamsulosin hydrochloride sustained-release microparticles and preparation method thereof
ActiveCN110711184BFast dissolution rateFast Diffusion ShrinkageUrinary disorderPharmaceutical non-active ingredientsTamsulosin hclAnti-Adhesion Agent
The invention discloses a tamsulosin hydrochloride slow-release granule. The slow-release granule uses a blank ball core as a ball core, and sprays a medicine coating layer, an enteric coating layer and a slow-release coating layer on the outside of the ball core in sequence; The layer is obtained by spraying the aqueous solution of tamsulosin hydrochloride and binder; the enteric coating layer is obtained by spraying the enteric coating solution containing enteric materials, plasticizers and anti-adhesive agents; It is obtained by spraying the sustained-release coating solution of insoluble sustained-release materials, plasticizers and anti-sticking agents. In the tamsulosin hydrochloride slow-release microparticles of the present invention, not only the absorption or bioavailability of tamsulosin is not affected by food effects and physiological changes of the gastrointestinal tract before and after meals, but also a stable blood drug concentration curve can be obtained And long-term action time, reduce the occurrence of cardiovascular side effects, greatly improve the safety, effectiveness and compliance of patients taking medicine.
Owner:CHINA PHARM UNIV +1
A pharmaceutical formulation for oral administration with improved content uniformity comprising sustained-release pellets containing tamsulosin hydrochloride
InactiveUS20210322319A1Good curative effectReduce biasUrinary disorderPharmaceutical non-active ingredientsTamsulosin hclSustained release pellets
Provided is an oral pharmaceutical formulation including sustained-release pellets containing tamsulosin hydrochloride and a pharmaceutically acceptable additive, wherein the sustained-release pellets includes about 50 wt % to about 100 wt % of particles having a particle size of about 0.50 mm to about 0.85 mm, and less than about 15 wt % of particles having a particle size less than about 0.50 mm. The oral pharmaceutical formulation may have a reduced deviation in dissolution rate of tamsulosin hydrochloride and improved content uniformity among unit dosage forms, and have ensured quality due to high reproducibility of unit dosage forms.
Owner:HANMI PHARMA
Method for detecting related substances of tamsulosin hydrochloride sustained-release capsule
PendingCN114689421AEasy to separateGood precisionComponent separationStrength propertiesTamsulosin hclGradient elution
The invention belongs to the field of pharmaceutical analysis, relates to a method for detecting related substances of tamsulosin hydrochloride sustained-release capsules, and particularly relates to a method for detecting related substances of tamsulosin hydrochloride sustained-release capsules by using a high performance liquid chromatography, which comprises the following steps: using a C18 column as a chromatographic column, using a buffer solution with the pH value of 1.5-3.0 as a mobile phase A, using acetonitrile as a mobile phase B, carrying out gradient elution, and controlling the detection wavelength to be 200-280nm. The detection method provided by the invention can effectively detect related substances in the tamsulosin hydrochloride sustained-release capsule, and has the advantages of high sensitivity, good separation degree, high precision and durability, simple operation and stable result, so that the method can be used for controlling impurities in the tamsulosin hydrochloride sustained-release capsule, and a powerful guarantee is provided for the product quality.
Owner:LUNAN PHARMA GROUP CORPORATION
Tamsulosin hydrochloride sustained release microgranules and preparation method thereof
ActiveCN110711184AFast dissolution rateFast Diffusion ShrinkageUrinary disorderPharmaceutical non-active ingredientsTamsulosin hclAnti-Adhesion Agent
The invention discloses tamsulosin hydrochloride sustained release microgranules. The tamsulosin hydrochloride sustained release microgranules take blank pill cores as pill cores, and the pill cores are successively coated with a medicine-containing coating layer, an enteric coating layer and a sustained release coating layer; the medicine-containing coating layer is obtained by being sprayed by an aqueous solution of tamsulosin hydrochloride and an adhesive; and the enteric coating layer is obtained by being sprayed by an enteric coating solution containing an enteric coating material, a plasticizer and an anti-viscosity agent. According to the tamsulosin hydrochloride sustained release microgranules, absorption or bioavailability of tamsulosin is not influenced by the food effect and physiological changes of gastrointestinal tract before and after meals, a stable blood concentration curve and long-term action time can be obtained, occurrence of cardiovascular side effects can be reduced, and the safety, effectiveness and compliance of patient medication can be greatly improved.
Owner:CHINA PHARM UNIV +1
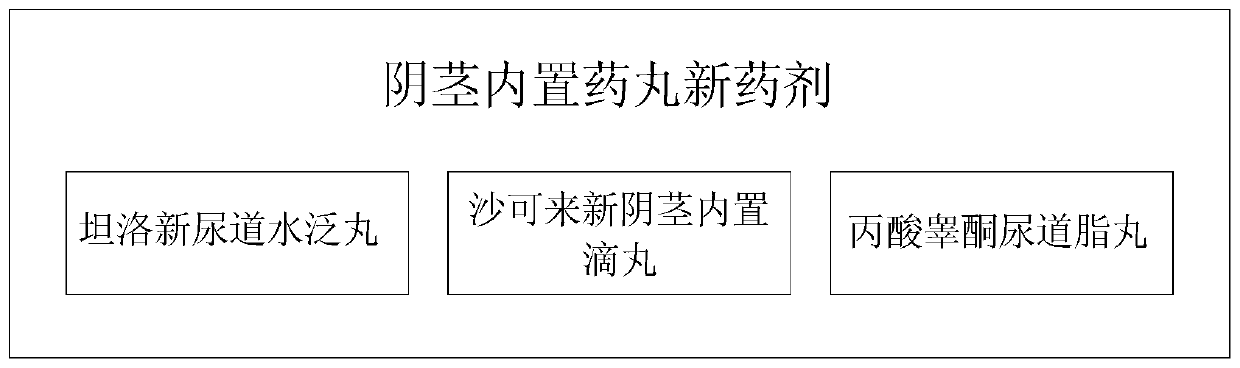
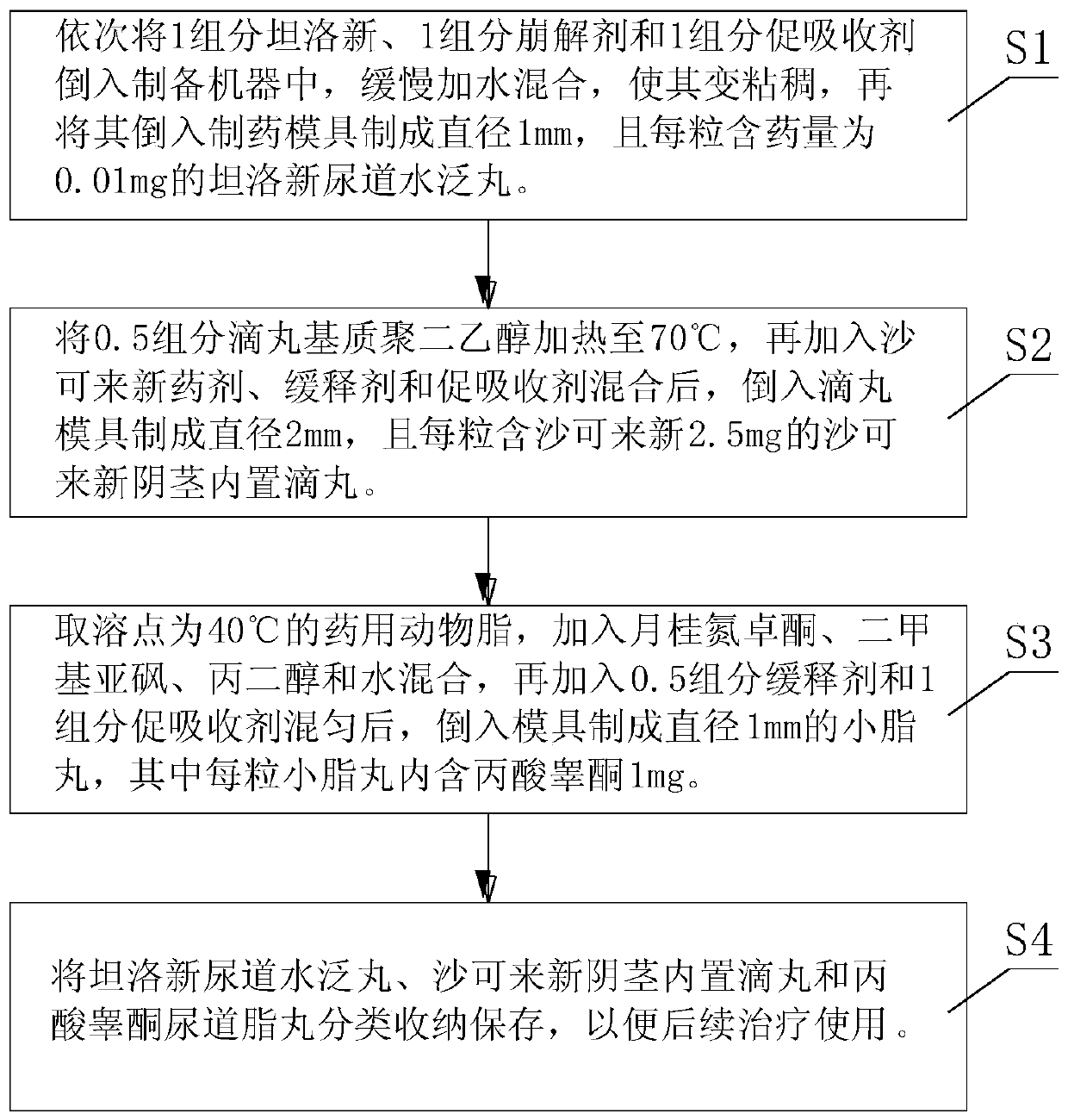
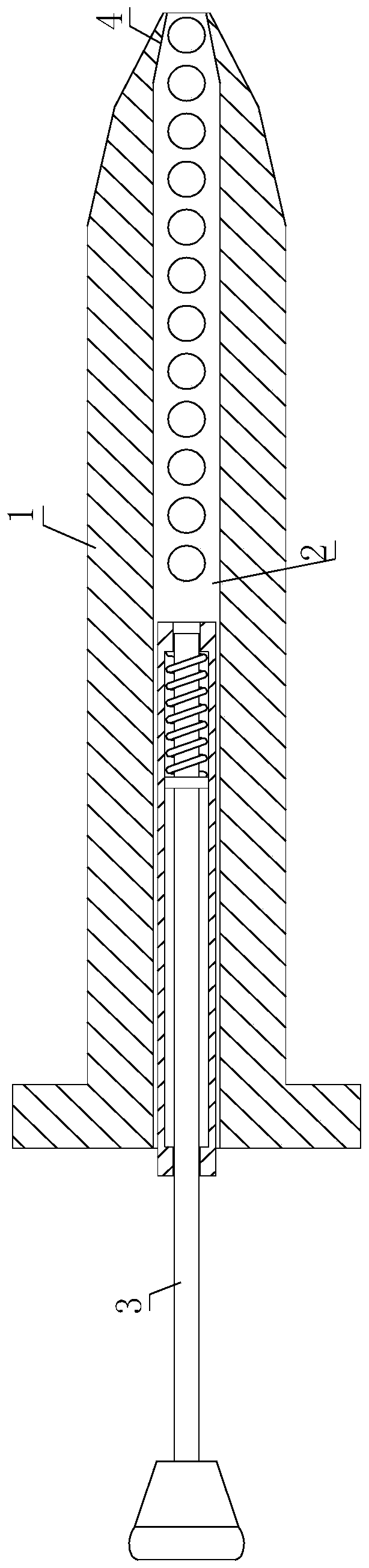
Novel medicament for penis built-in pills, preparation method and medicament placing device thereof
InactiveCN111544402AReduce degradationReduce or avoid side effectsPeptide/protein ingredientsMedical devicesPenisLiver and kidney
The invention discloses a novel medicament for penis built-in pills, a preparation method and a medicament placing device thereof. The novel medicament comprises a tamsulosin urethral water flooding pill, a sarcolysin penis built-in dropping pill and a testosterone propionate urethral lipid pill. When the medicament placing device is used, firstly, the penis is conventionally disinfected, the outer side of a medicament injection hose of the medicament placing device filled with pills is coated with glycerinum for lubrication, the medicament injection hose is inserted into the penis urethra, amedicament pushing rod is pushed forwards, and a corresponding number of pills are injected according to the required injection dosage. The administration mode and the administration path are changed,and rear-end medication of liver and kidney metabolism is adopted. In the treatment and health care process of men, a target organ can be used for direct administration, absorption and effect taking,the medicament composition is small, the medicament concentration in local blood is high, the medicament effect is strong, the medicament can take effect while being absorbed during urethra administration, the non-absorbed medicament is excreted in a harmless manner, and the medicament is a safe and efficient novel administration way, a novel medication mode and a novel medicament, and has a relatively high market prospect and a relatively strong medicament research and development prospect.
Owner:周宇
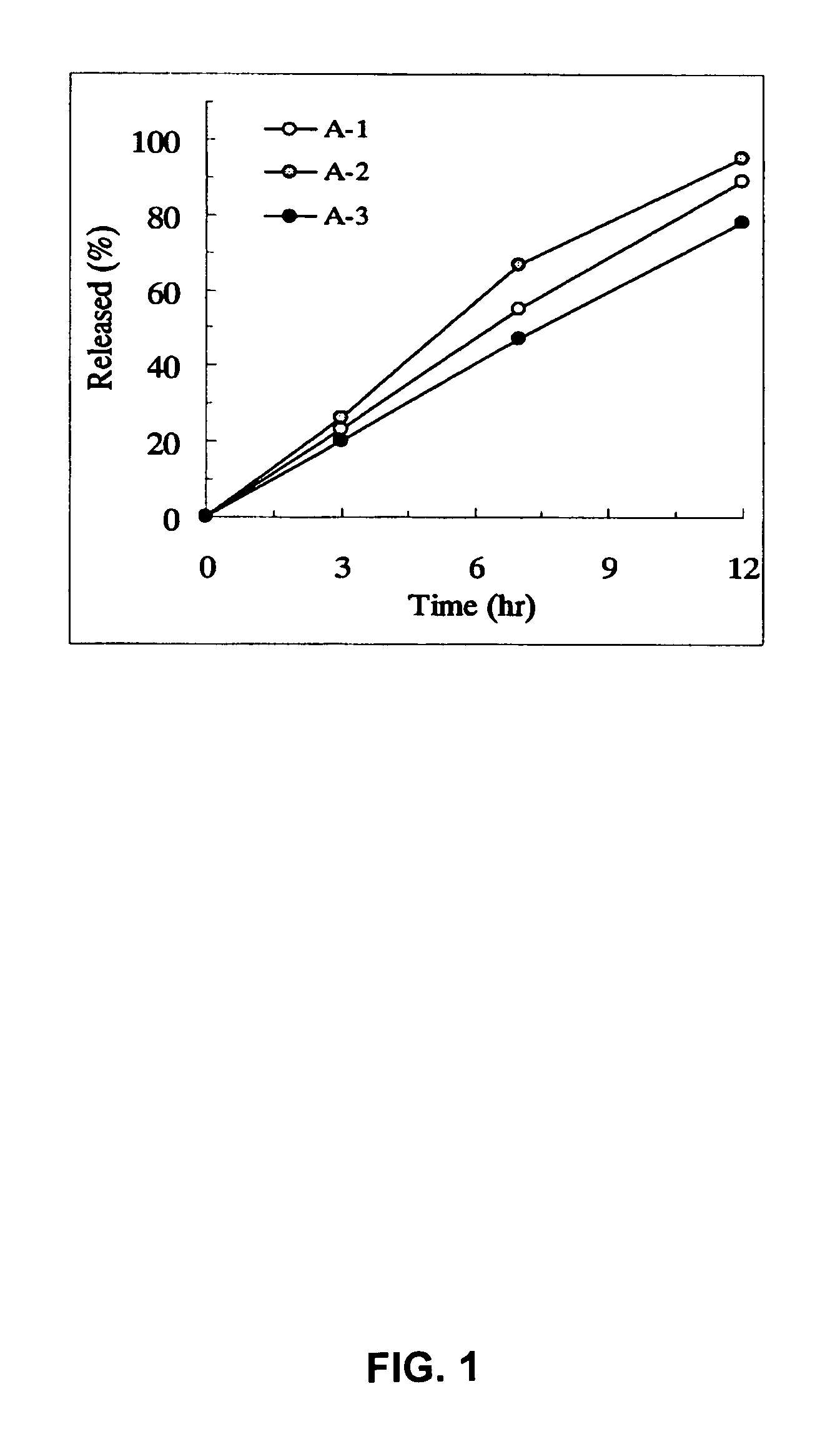
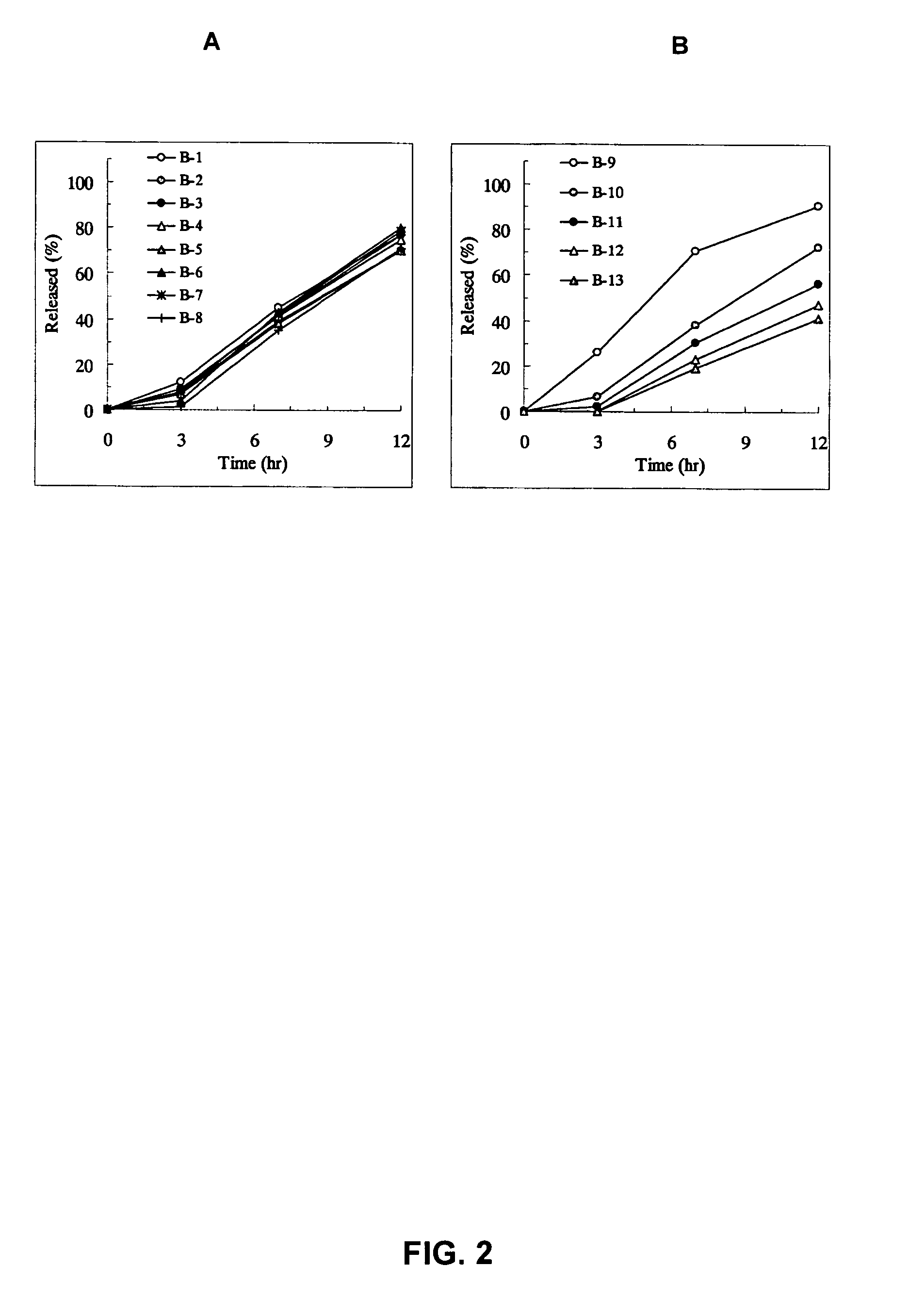
Sustained release pharmaceutical composition
InactiveUS8128958B2Equivalent and even more efficacyReduce the adverse eventsPowder deliveryBiocideTamsulosinDrug
The present invention provides a sustained-release pharmaceutical composition, characterized in that, there are contained tamsulosin or a pharmaceutically acceptable salt thereof and a carrier for a sustained-release pharmaceutical composition and the ratio (Cmin / Cmax ratio) of the plasma tamsulosin concentration at 24 hours after the administration of the preparation per os (Cmin) to the maximum plasma tamsulosin concentration after the administration (Cmax) is about 0.4 or more.
Owner:ASTELLAS PHARMA INC
Pharmaceutical composition containing tamsulosin hydrochloride with excellent acid resistance and preparation method therefor
PendingCN113347963AHigh dissolution rateDissolution, which contributes to better acid resistancePill deliveryAmide active ingredientsTamsulosin hclTamsulosine
The present invention relates to a pharmaceutical composition containing tamsulosin hydrochloride with excellent acid resistance and a preparation method therefor, and to a pharmaceutical composition in which, by coating prepared core beads, which comprise tamsulosin hydrochloride as an active ingredient, with an enteric coating liquid prepared using a certain amount of a plasticizer, adding a buffer base and the like to adjust density, and then mixing and tableting a mixed portion and a lubricant, even after the tableting, the enteric coating is not broken and acid resistance is maintained. Thus, the present invention has good tabletability, excellent content uniformity, excellent acid resistance due to a low dissolution rate of tamsulosin hydrochloride, and no significant decomposition of tamsulosin hydrochloride, and thus, stability can be ensured.
Owner:HANMI PHARMA
Pharmaceutical composition containing tamsulosin or hydrochloride thereof and preparation method therefor
PendingCN114051407AReduce contentImprove productivityInorganic non-active ingredientsUrinary disorderTamsulosin hclTamsulosine
The present invention provides a pharmaceutical composition containing tamsulosin or hydrochloride thereof, and a preparation method therefor. When an enteric-coated layer is formed, a fluid-bed system can be used to form the enteric-coated layer on core particles by adjusting a content of talc, with the resultant improvement of productivity. Even though the content of talc is reduced, aggregation of enteric-coated beads can be minimized; and after coating, the enteric-coated layer does not decrease in function and acid resistance. Therefore, the pharmaceutical composition can be applied to an oral preparation.
Owner:HANMI PHARMA
A kind of tamsulosin sustained-release pellets and preparation method thereof
ActiveCN102579359BUrinary disorderPharmaceutical non-active ingredientsSustained release pelletsTamsulosine
The invention provides a tamsulosin sustained-release pellet, comprising a pill core and a sustained-release coating layer, the sustained-release coating layer comprising a hydrophobic matrix and a pH-sensitive hydrophilic polymer, the polymer polymerized The pH value of the change in solubility of the substance is between 1 and 14, so that the drug can be released at different pH values, and it can also be used as a carrier for controlling the release of tamsulosin, which simplifies the prescription, reduces the difficulty of preparation, and is easier for industrial mass production. The inventors have surprisingly found that the formulation can be used to prepare various sustained-release dosage forms of tamsulosin, independent of the physicochemical properties of tamsulosin.
Owner:COSCI MED TECH CO LTD
Preparation method of tamsulosin hydrochloride sustained-release preparation
InactiveCN108992423AGood slow release functionRelease stabilityUrinary disorderAmide active ingredientsPolyethylene glycolDrug release
The invention provides a preparation method of a tamsulosin hydrochloride sustained-release preparation. The preparation method comprises the following steps: dissolving tamsulosin hydrochloride in 95% ethanol to obtain a solution A, and adding hydroxypropyl beta-cyclodextrin and mannitol into the solution A to obtain a solution B; dissolving polylactic acid and polyethylene glycol 200 in acetoneto obtain a solution C, mixing the solution B with the solution C to obtain a solution D, transferring the solution D to a magnetic stirrer, continuously stirring the solution D for 12 hours, loweringthe temperature of the solution D to 0-1 DEG C within 2 hours, allowing to stand still for 12 hours, and maintaining the temperature of the solution D at 0-1 DEG C during the standing period; heatingthe solution D after standing till for 12 hours, continuously stirring when the temperature of the solution D is raised to 15-18 DEG C, controlling the temperature of the solution D at 15-18 DEG C when stirring, and preparing the tamsulosin hydrochloride sustained-release preparation with a low-temperature spray drying method after continuously stirring for 12 hours. According to the invention, the dosage of a capsule wall material is moderate, the drying temperature of materials is low, and the prepared drug-loading preparation is uniform in size and stable in drug release, and has the characteristic of slow release.
Owner:刘丽
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com