Tamsulosin hydrochloride sustained release microgranules and preparation method thereof
A technology of tamsulosin hydrochloride and slow-release microparticles, which can be used in pharmaceutical formulations, microcapsules, medical preparations of non-active ingredients, etc., and can solve problems such as unfavorable taking by patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
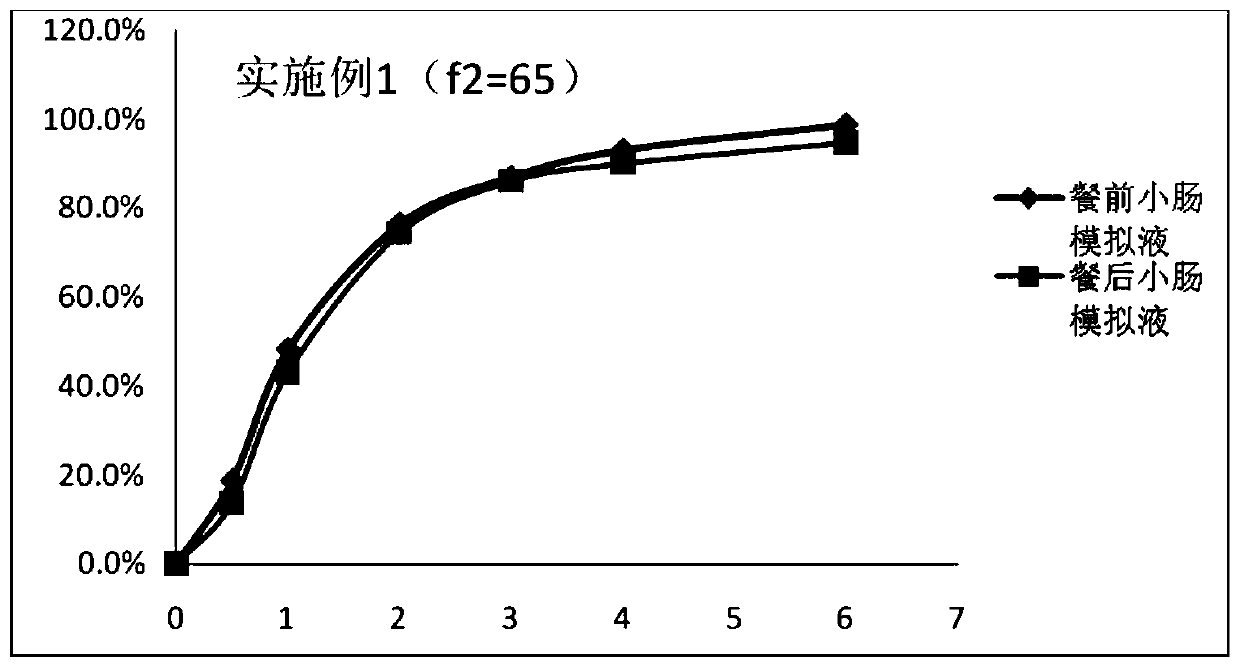
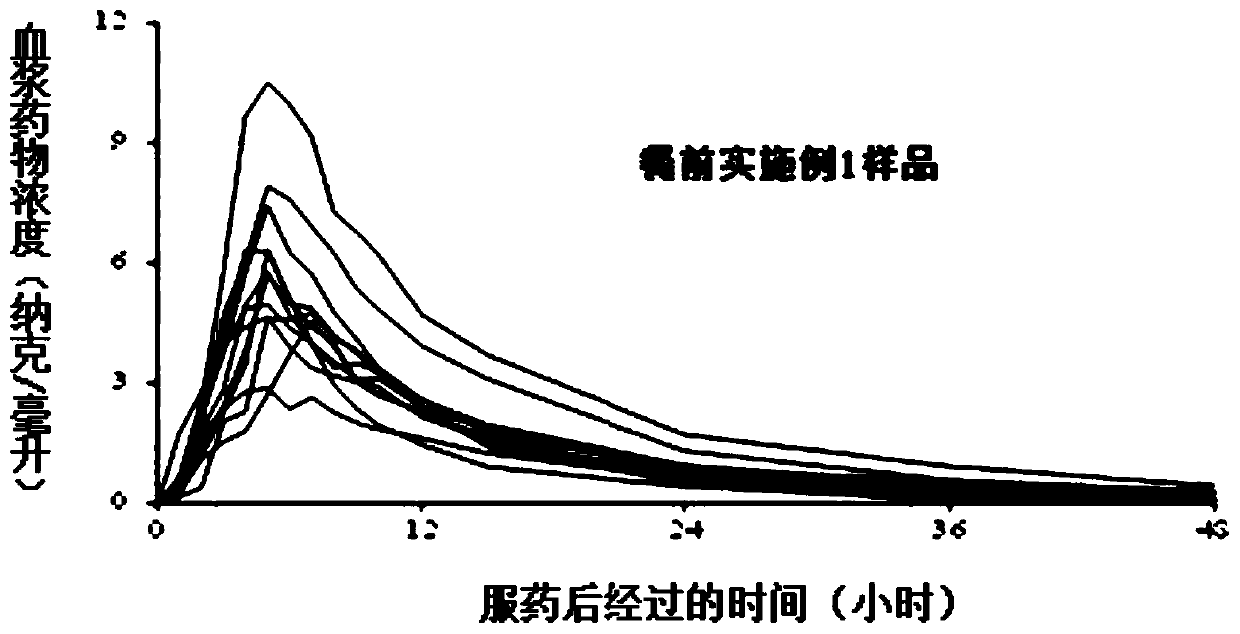
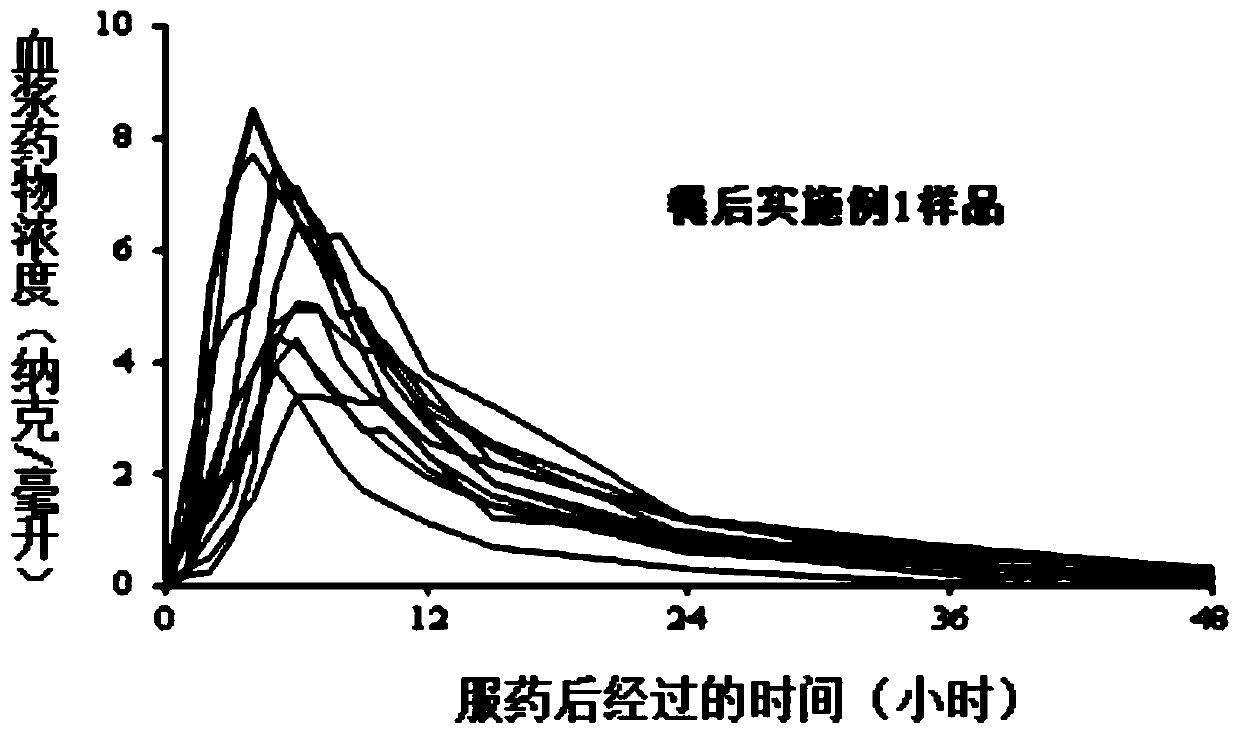
Embodiment 1
[0047] Prescription of pill core containing drug coating layer:
[0048]
[0049] Enteric coating layer coating solution prescription (weight gain 2.5%):
[0050]
[0051] Sustained-release coating layer coating solution prescription (weight gain 6%):
[0052]
[0053] The preparation method of tamsulosin hydrochloride slow-release microparticles, the steps are as follows:
[0054] Step (1), take the prescribed amount of tamsulosin hydrochloride and hypromellose, add purified water, stir until dissolved, and obtain a drug-containing solution;
[0055] Step (2), taking a blank microcrystalline cellulose pellet core and placing it in a fluidized bed, setting the coating parameters of the fluidized bed, and using the drug-containing solution prepared in step (1) for coating to obtain a drug-containing pellet core;
[0056] Step (3), take the prescribed amount of triethyl citrate and talcum powder and disperse them in an appropriate amount of purified water, place them ...
Embodiment 2
[0081] Prescription of pill core containing drug coating layer:
[0082]
[0083] Enteric coating layer coating solution prescription (weight gain 2%):
[0084]
[0085] Sustained-release coating layer coating solution prescription (weight gain 6%):
[0086]
[0087] Preparation method: with embodiment 1.
[0088] Determination of release rate: according to the second method of appendix XD of the 2015 edition of Chinese Pharmacopoeia, the release test method under the WS1-(X-333)-2003Z item is measured (n=12), and the average release rate is 23% (2h), respectively. 54% (3h) and 89% (5h), the release rate meets the regulations.
Embodiment 3
[0090] Prescription of pill core containing drug coating layer:
[0091]
[0092] Enteric coating layer coating solution prescription (weight gain 3%):
[0093]
[0094] Sustained-release coating layer coating solution prescription (weight gain 6%):
[0095]
[0096] Preparation method: with embodiment 1
[0097] Determination of release rate: According to the second method of appendix XD of the 2015 edition of the Chinese Pharmacopoeia, the release test method under the item WS1-(X-333)-2003Z is measured (n=12), and the average release rate is 21% (2h), respectively. 57% (3h) and 83% (5h), comply with the regulations.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com