Berberine hydrochloride caffeic acid eutectic compound, preparation method thereof, composition of berberine hydrochloride caffeic acid eutectic compound, and applications
A technology of berberine hydrochloride and caffeic acid, which is applied in the directions of drug combination, separation/purification of carboxylic acid compounds, organic chemical methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Preparation method 1 of berberine hydrochloride and caffeic acid cocrystal:
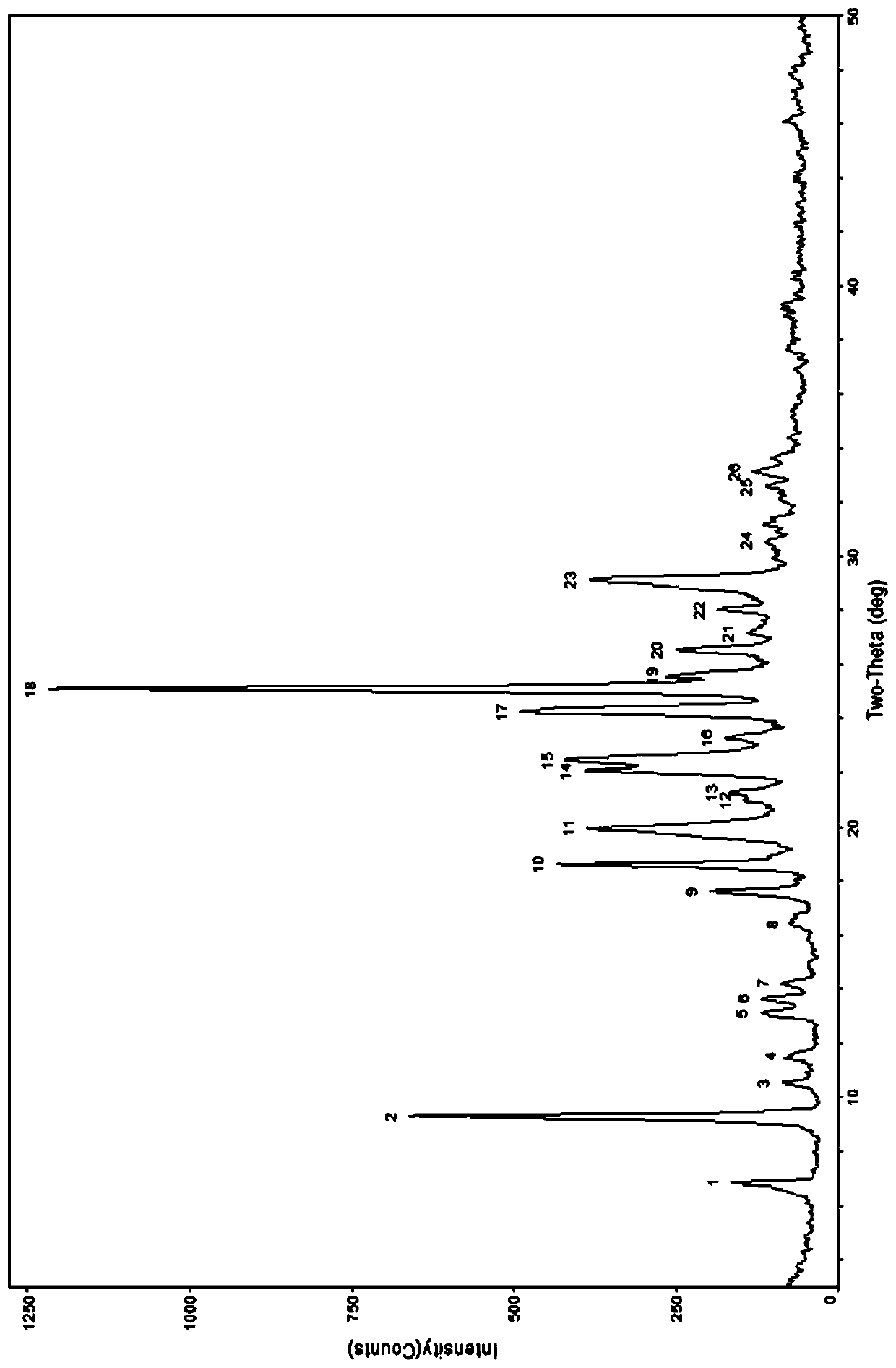
[0056] According to the table below, take an appropriate amount of berberine hydrochloride and caffeic acid and put them into a mortar with a molar ratio of 2:1, add an appropriate amount of organic solvent, and manually grind for an appropriate time. Carry out powder X-ray diffraction analysis to it, its diffraction pattern and figure 1 Consistent, indicating that the obtained sample is a co-crystal of berberine hydrochloride and caffeic acid.
[0057]
[0058]
[0059] Preparation method 2 of berberine hydrochloride and caffeic acid cocrystal:
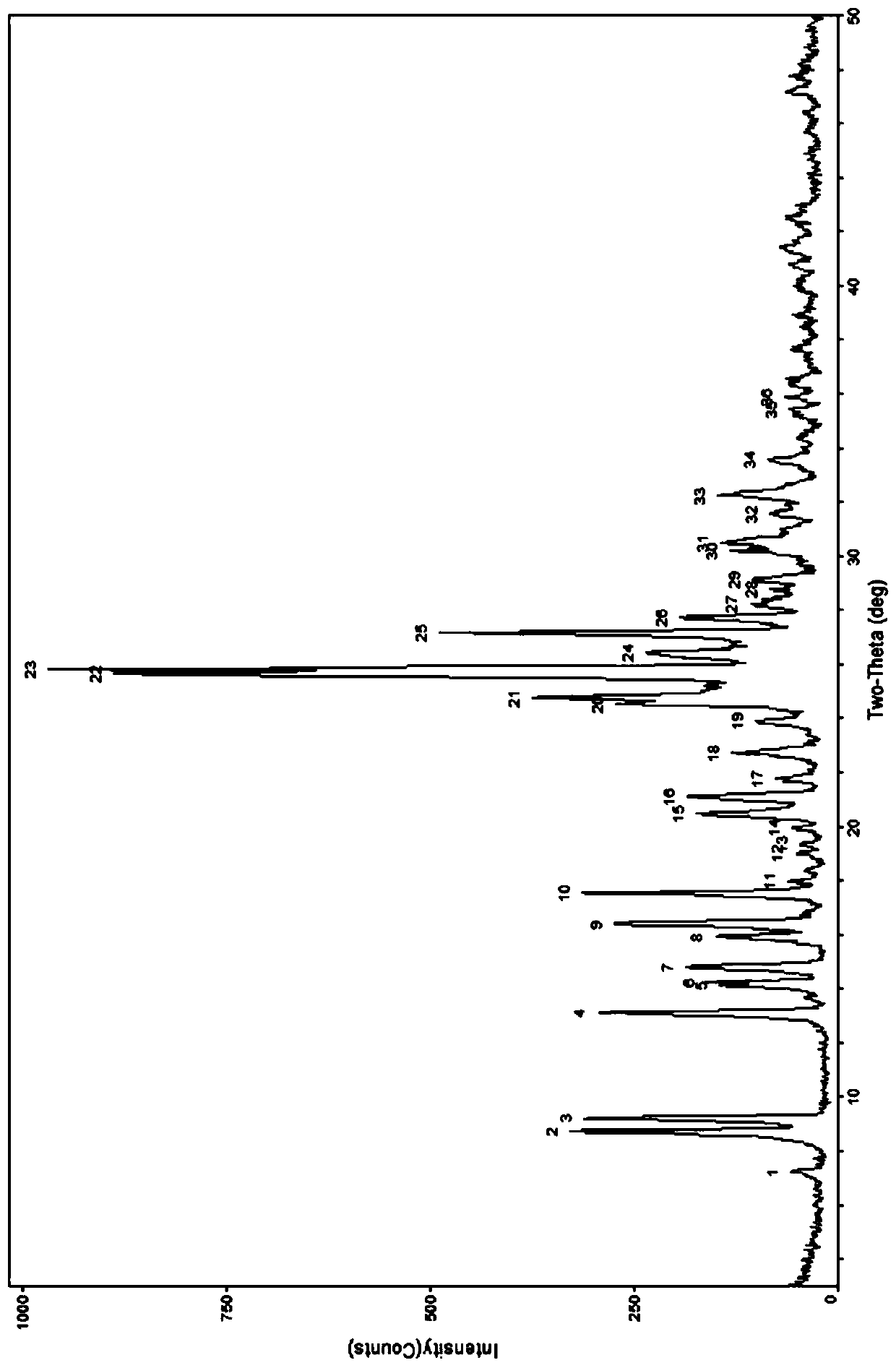
[0060] As shown in the table below, take appropriate amount of berberine hydrochloride and caffeic acid and put them into a ball mill tank with a molar ratio of 2:1, add an appropriate amount of organic solvent, select an appropriate ball-to-material ratio, set an appropriate speed, and grind for an appropriate time. Carry out powder X-ray di...
Embodiment 2
[0066] Stability characteristics of berberine hydrochloride and caffeic acid co-crystal:
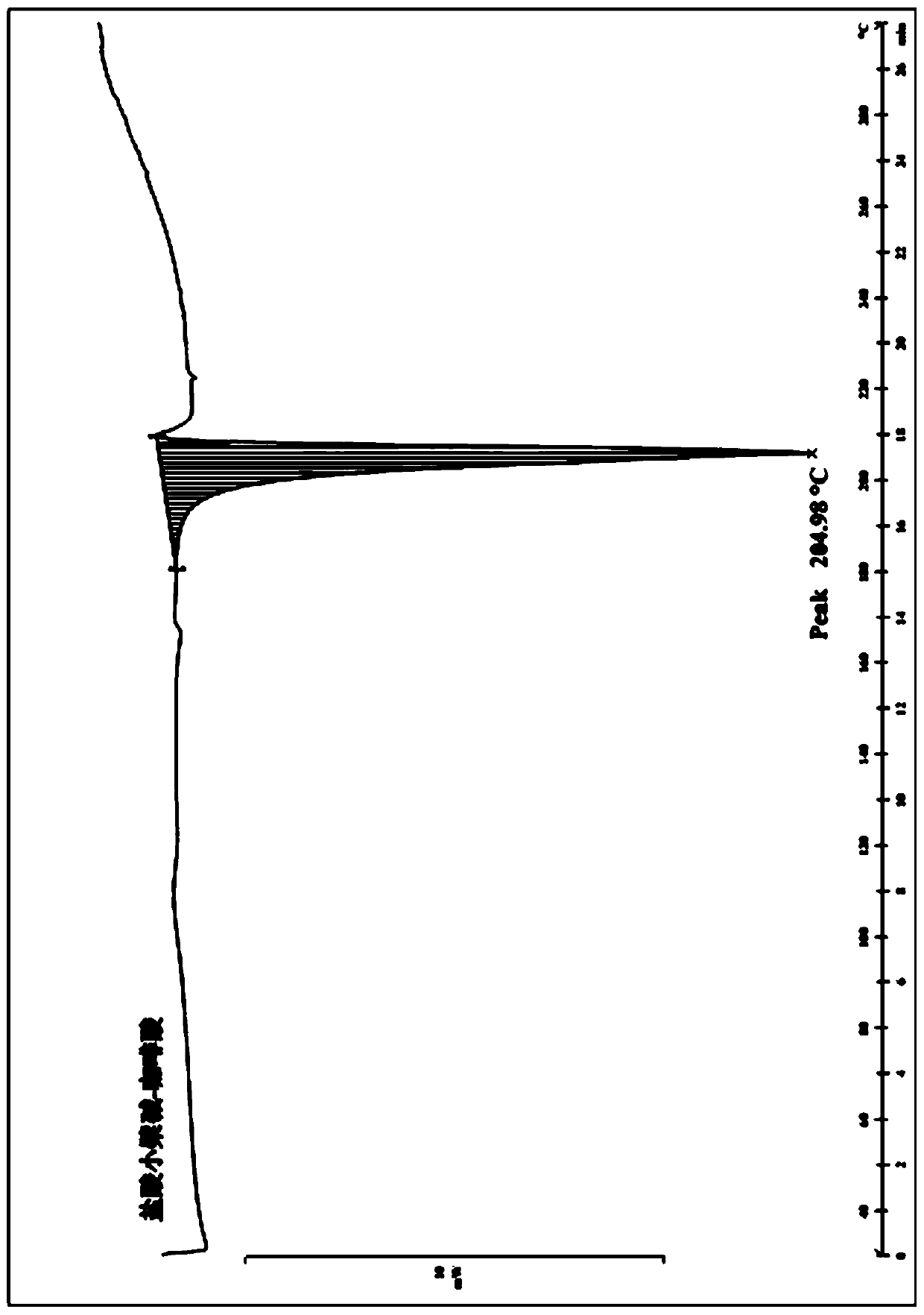
[0067] High temperature test: Put the samples of berberine hydrochloride and berberine hydrochloride and caffeic acid eutectic in an open clean watch glass, place them at 60°C for 10 days, and take samples on the 0th day, the 5th day and the 10th day . Powder X-ray diffraction analysis was performed on the samples obtained at the above sampling points, and the results showed that berberine hydrochloride and the eutectic of berberine hydrochloride and caffeic acid were stable under the high temperature influence factor test.
[0068] High-humidity test: put samples of berberine hydrochloride and berberine hydrochloride and caffeic acid eutectic in an open clean surface dish, and place them at 25°C for 10 days at a relative humidity of 90%±5%, and on the 0th day , Sampling on the 5th and 10th day. Powder X-ray diffraction analysis was performed on the samples obtained at the above sampli...
Embodiment 3
[0072] Solubility characteristics of berberine hydrochloride and caffeic acid co-crystal and berberine hydrochloride API in four solvent systems: selection of solvent system: ① refer to the solvent system used in the dissolution method in the appendix of the Pharmacopoeia; ② refer to The pH value of the digestive juice of different organs in the organism; according to the above two references, four solvent systems were set up: 0.1N hydrochloric acid solution with a pH value of 1.0; acetate buffer solution with a pH value of 4.5; phosphoric acid with a pH value of 6.8 Saline buffer; aqueous solution. Refer to the "Technical Guidelines for Dissolution Test of Ordinary Oral Solid Preparations" for determination, the model-independent similarity factor (f2) method is used for the comparison of dissolution curves, and the comparison of berberine hydrochloride and berberine hydrochloride and caffeic acid eutectic is carried out through the calculation of f2 value The similarity of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com