Preparation method of pH-responsive adriamycin nano-drug capsule with surface positive charge
A nano-drug, doxorubicin technology, applied in the direction of drug combination, microcapsule, capsule delivery, etc., can solve the problems of degradation cycle and degradation efficiency, low drug loading, tissue toxicity and side damage, etc., and achieves a small average particle size. , The effect of fast dissolution rate and large dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Add 0.1 mol of OP-10, 0.12 mol of triethylamine and 80 mL of tetrahydrofuran into the three-necked flask, place the three-necked flask in ice water, and stir with an electromagnetic stirrer. Dissolve 0.12 mol of AC in tetrahydrofuran, and then add it dropwise into a three-neck flask. After the addition is complete, react for 5 hours, and keep the temperature at 0-5°C. The tetrahydrofuran was removed from the reaction product by a rotary evaporator, and the temperature was set at 50 °C. Then add 200 mL of acetone, separate to obtain the supernatant containing OP-AC, then use a rotary evaporator (set temperature at 40° C.) to remove the acetone, and centrifuge to obtain OP-AC. Dry in a vacuum oven to obtain OP-AC.
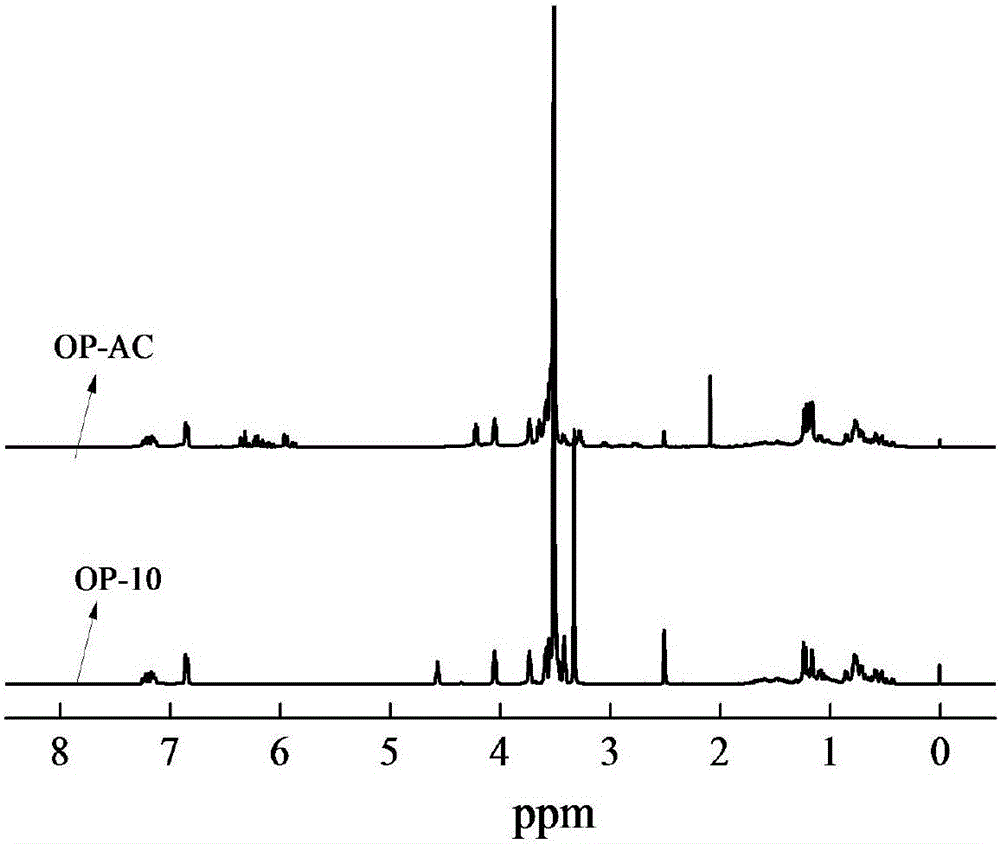
[0046] From figure 1 It can be seen from the NMR diagram shown that there is an obvious characteristic peak around 3.5 ppm, indicating that there is a double bond in the experimentally synthesized OP-AC.
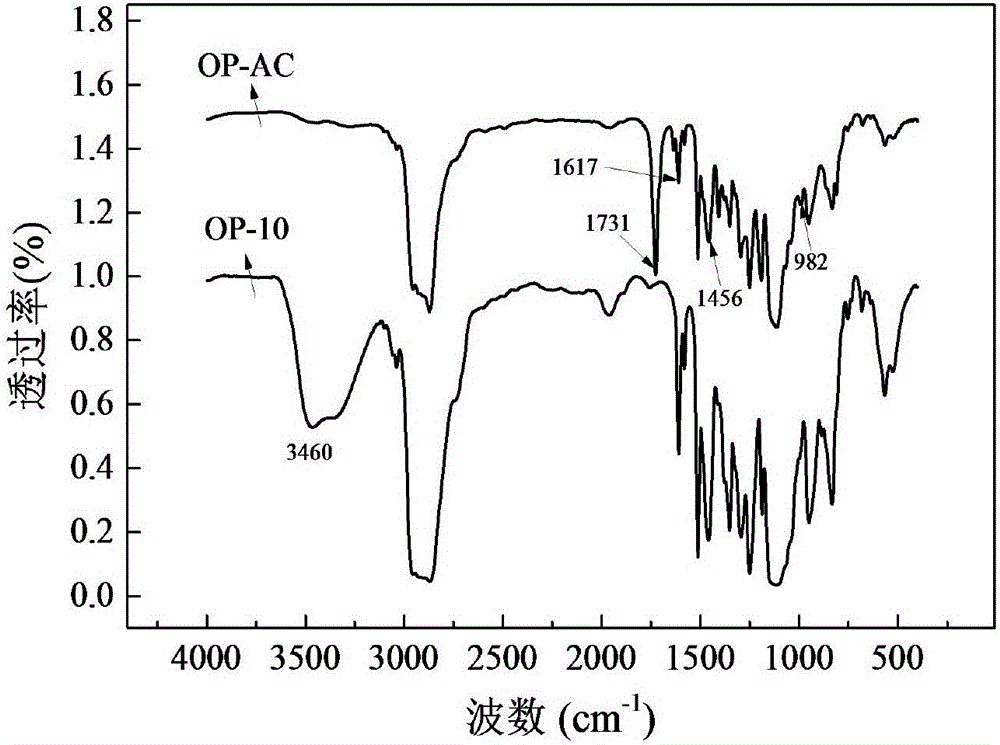
[0047] From figure 2 It can be seen from the infr...
Embodiment 2
[0049]Weigh 5 mg of doxorubicin hydrochloride, dissolve it in 1 mL of dimethyl sulfoxide at room temperature, add 4 μL of triethylamine to remove hydrochloric acid according to the molar ratio of triethylamine: doxorubicin hydrochloride is 3:1, react for 10 min, record For the mixture ①. Dissolve 1 mg of OP-AC in 25 mL of deionized water, stir for 10 min, and record it as the mixture ②. Add the mixed solution ① to the mixed solution ②, react for 20 minutes, and record it as the mixed solution ③. According to the ratio of doxorubicin / (AM+GDM) as 1 / 0.03 (mass ratio), and the mass ratio of AM and GDM as 1 / 0.4, add AM to the mixture ③, add GDM after 10 minutes, and then add 0.2 mg ammonium persulfate, add TEMED according to the mass ratio of monomer and TEMED as 1 / 5, react for 1.5h, and record it as the mixed solution ④. Put the mixed solution ④ into a dialysis bag (MW=3500), dialyze with deionized water, store in the dark at 4°C, change the deionized water every 4 hours, and re...
Embodiment 3
[0052] Weigh 5 mg of doxorubicin hydrochloride, dissolve it in 1 mL of dimethyl sulfoxide at room temperature, add 4 μL of triethylamine to remove hydrochloric acid according to the molar ratio of triethylamine: doxorubicin hydrochloride is 3:1, react for 10 min, record For the mixture ①. Dissolve 1 mg of OP-AC in 25 mL of deionized water, stir for 10 min, and record it as the mixture ②. Add the mixed solution ① to the mixed solution ②, react for 20 minutes, and record it as the mixed solution ③. According to the ratio of doxorubicin / (AM+GDM) as 1 / 0.05 (mass ratio), and the mass ratio of AM and GDM as 1 / 0.4, add AM to the mixture ③, add GDM after 10 minutes, and then add 0.2 mg ammonium persulfate, add TEMED according to the mass ratio of monomer and TEMED as 1 / 5, react for 1.5h, and record it as the mixed solution ④. Put the mixed solution ④ into a dialysis bag (MW=3500), dialyze with deionized water, store in the dark at 4°C, change the deionized water every 4 hours, and r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com