Medicine composition for Jardiance solid dispersion and preparation method thereof
A technology of solid dispersion and composition, applied in the field of empagliflozin oral solid preparation, empagliflozin pharmaceutical composition and its preparation, can solve the problems of cost increase, low yield, large loss, etc., and achieve low production Cost, simple and easy preparation process, simple and easy to implement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Enpagliflozin solid dispersion preparation:
[0044] Weigh 10g of Enpagliflozin and 70g of polyethylene glycol respectively, add them to the solid dispersion machine, set the temperature to 160℃, after melting, take it out, quickly put it in the refrigerator to solidify, vacuum dry, and smash through 80 mesh sieve. .
Embodiment 2
[0046] Weigh 10g of Enpagliflozin and 80g of polyethylene glycol respectively, add them to the solid dispersion machine, set the temperature to 170℃, after melting, take it out, quickly put it in the refrigerator to solidify, vacuum dry, and smash through 80 mesh sieve. .
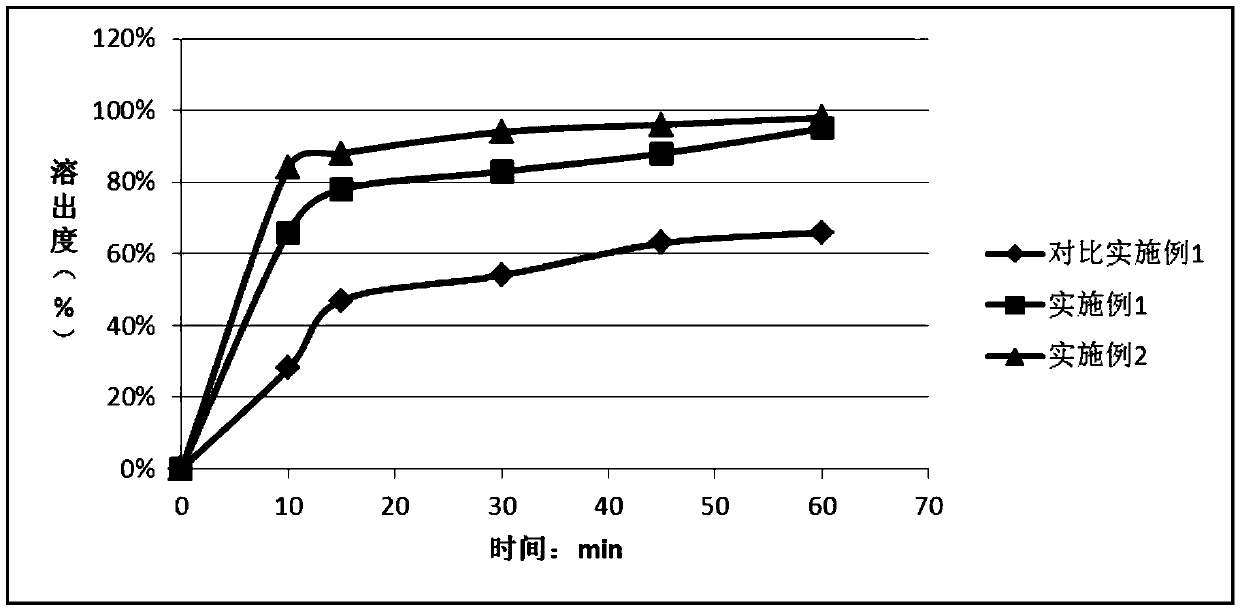
[0047] Determination of dissolution: Weigh 25mg of the pharmaceutical composition prepared in Examples 1 to 2 and 25mg of the raw material of Enpagliflozin in Comparative Example 1, respectively, and put them in 900ml pH1.0 medium by paddle method , 50rpm, samples were taken at 10, 15, 30, 45, and 60 minutes to detect dissolution.
[0048] The dissolution results are as follows:
[0049]
[0050] The results showed that the dissolution rate of Examples 1 to 2 made into solid dispersions was significantly faster than that of the bulk drug group of Comparative Example 1, and Example 2 with a large proportion of polyethylene glycol had a higher dissolution rate than Example 1.
Embodiment 3
[0052] For the preparation of Enpagliflozin tablets, the prescription used the composition prepared in Example 1, the prescription 2 used the composition prepared in Example 2, and the prescription 3 was a bulk drug that was not made into a solid dispersion.
[0053] See the following table for prescriptions:
[0054] Component
[0055] Determination of dissolution: Take prescription 1 to 3 samples, respectively, in 900ml pH1.0 medium, paddle method, 50rpm, and take samples at 10, 15, 30, 45, 60 minutes to detect dissolution.
[0056] The results are as follows:
[0057]
[0058] The results showed that the dissolution rate of the formulations 1 to 2 made into solid dispersions was significantly better than that of the formulation 3 made without solid dispersions.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com