Core-coated tablet for treating hypercholesteremia
A chip-packed technology for hypercholesterolemia, which is applied in the field of medicine and can solve problems such as the instability of atorvastatin calcium raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Preparation of Atorvastatin Calcium Granules
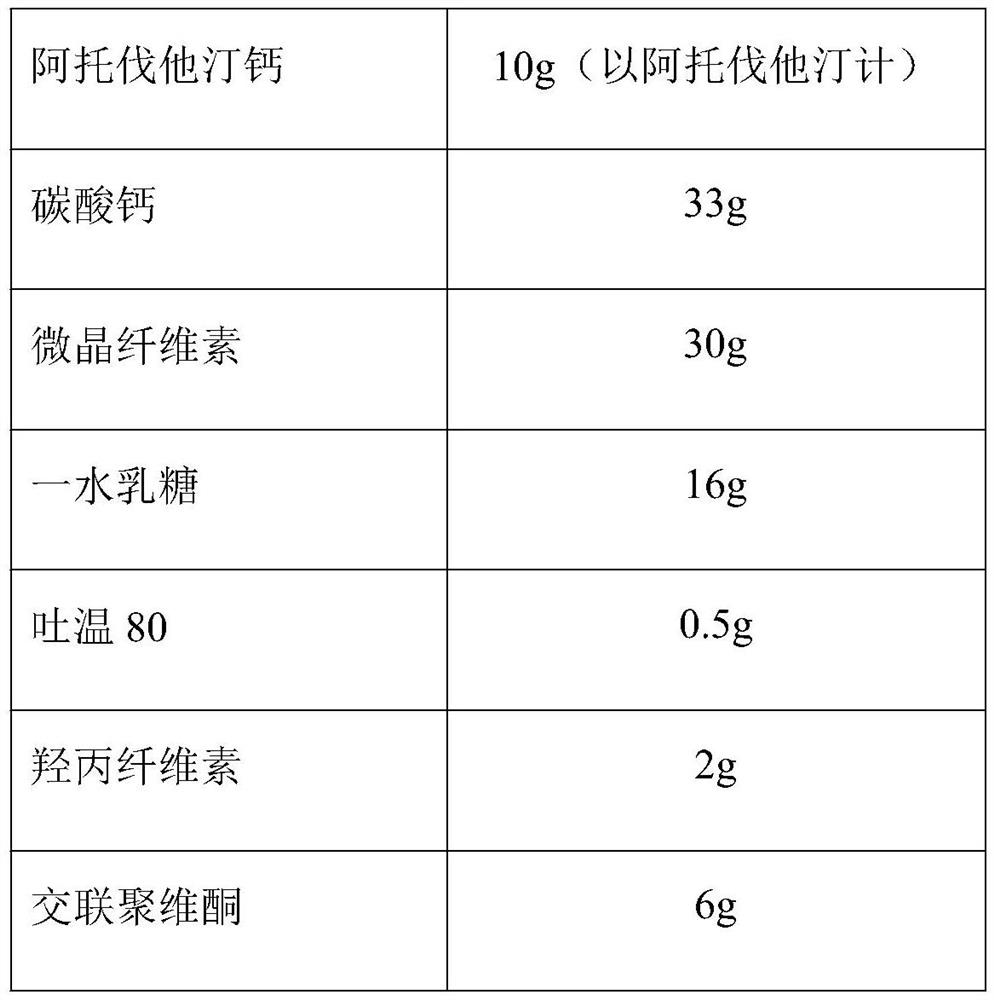
[0023] The prescription consists of:
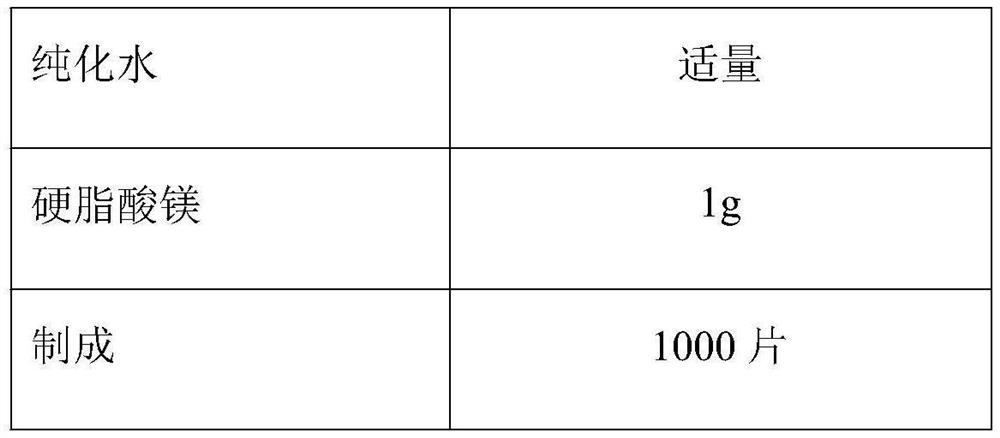
[0024] Atorvastatin Calcium 10g (calculated as atorvastatin) calcium carbonate 33g microcrystalline cellulose 30g lactose monohydrate 16g Tween 80 0.5g Hypromellose 2g Crospovidone 6g purified water Appropriate amount Magnesium stearate 1g Opadry 03k28487 3g production 1000 pieces
[0025] Preparation:
[0026] (1) Weigh Tween 80, prepare a 1% (W / W) solution with purified water, and stir until completely dissolved; (2) Granulation: add microcrystalline cellulose, Lactose, croscarmellose sodium, hydroxypropyl cellulose, atorvastatin calcium and calcium carbonate were mixed for 10 minutes; (3) adding the Tween 80 aqueous solution prepared in step (1), stirring and chopping (4) put the wet granules into the fluidized dryer and dry until the granule moisture is 1.0%-3.0%; Sieve for sizing, and collect the g...
Embodiment 3
[0035] 1. Preparation of atorvastatin calcium inner chip
[0036] The prescription consists of:
[0037]
[0038]
[0039] Preparation:
[0040] (1) Weigh Tween 80, prepare a 1% (W / W) solution with purified water, and stir until completely dissolved; (2) Granulation: add microcrystalline cellulose, Lactose, croscarmellose sodium, hydroxypropyl cellulose, atorvastatin calcium and calcium carbonate were mixed for 10 minutes; (3) adding the Tween 80 aqueous solution prepared in step (1), stirring and chopping (4) put the wet granules into the fluidized dryer and dry until the granule moisture is 1.0%-3.0%; Sieve for sizing, and collect the granules after sizing; (6) Add the granules and magnesium stearate in step (2), mix them with a three-dimensional mixer, discharge, and collect the mixed granules. (7) Tablet pressing: use a rotary tablet press machine, Round dimpled die-pressed tablet.
[0041] 2. Preparation of outer layer granules containing ezetimibe
[0042] Th...
Embodiment 4
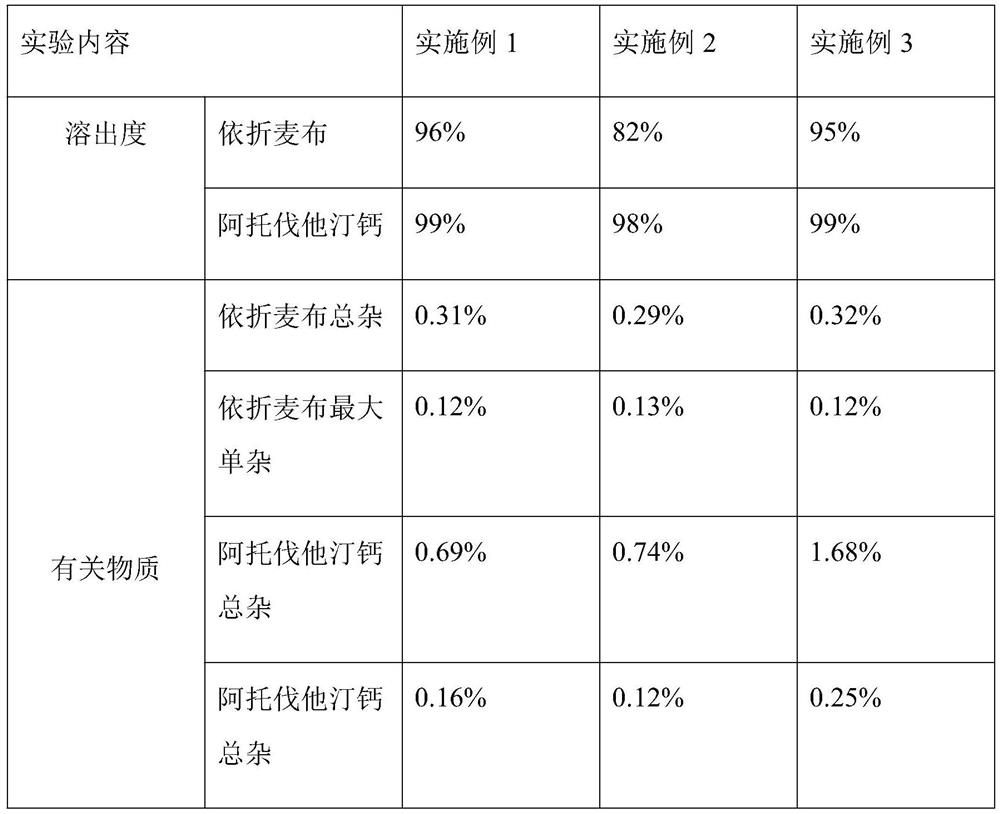
[0049] After removing the packages of the packaged chips prepared in the above examples, they were placed at 60° C. for 15 days for investigation to measure the impurities. In addition, each embodiment was taken to make a coated chip, and the dissolution rate was measured respectively (0.3% sodium lauryl sulfate, pH4.5 acetate buffer, paddle method, 50 rpm, volume 900ml, sampling in 30 minutes). The results are shown in the table below.
[0050]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com