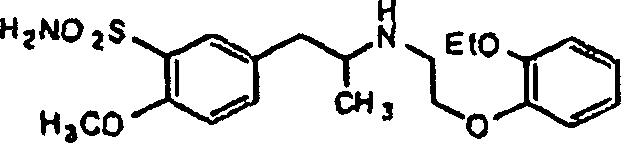
Tamsulosin tablets
A technology of tamsulosin and tablet, applied in unit dosage form for treating or improving benign prostatic hyperplasia, the field of preparing tamsulosin tablet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Three batches of monolithic tablets were prepared by step-blending and direct compression methods:
[0064] (%)
0.4mg
0.5
26.4mg
33.0
26.4mg
33.0
Hydroxypropyl Methyl Cellulose (HPMC)
26.4mg
33.0
0.4mg
0.5
total
80mg
100
[0065] The difference from batch to batch is only the viscosity value of the selected hydroxypropyl methylcellulose:
[0066] Batch A contains METHOCEL K4M CR PREMIUM
[0067] Batch B contains METHOCEL K15M CR PREMIUM
[0068] Batch C contains METHOCEL K100M CR PREMIUM
[0069] b) Operation method
[0070] Tamsulosin hydrochloride was blended (15 minutes) with anhydrous lactose in a 1:9 ratio (10% active substance), ground (15 seconds) and then blended again (5 minutes). This pre-blend was then mixed with the remainder of the lactose,...
Embodiment 3
[0078] Tamsulosin Hydrochloride
Embodiment 4
[0080] Monolithic tablets prepared by dry compaction
[0081] Two batches of tablets were produced by a process involving compression, milling, blending and tabletting.
[0082] Batch 4a.: Compressed monolithic tablets, 6 mm diameter. Contains 33.0% METHOCEL K15M P.
[0083] Batch 4b. Compressed monolithic erodible sheet, 9mm diameter. Contains 13.2% METHOCEL K15MP.
[0084] Batch 4a
Batch 4b
0.4mg
0.4mg
lactose
65.6mg
215.6 mg
Hydroxypropyl Methyl Cellulose (HPMC)
33.0mg
33.0mg
1.0mg
1.0mg
total
100mg
250mg
[0085] b) Operation method
[0086] the batch
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com