A kind of sustained and controlled release preparation of tamsulosin hydrochloride and preparation method thereof
A technology for tamsulosin hydrochloride and controlled-release preparations, which is applied in the directions of inactive medical preparations, pharmaceutical formulations, inorganic inactive ingredients, etc. The effect of short cycle, convenient use and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
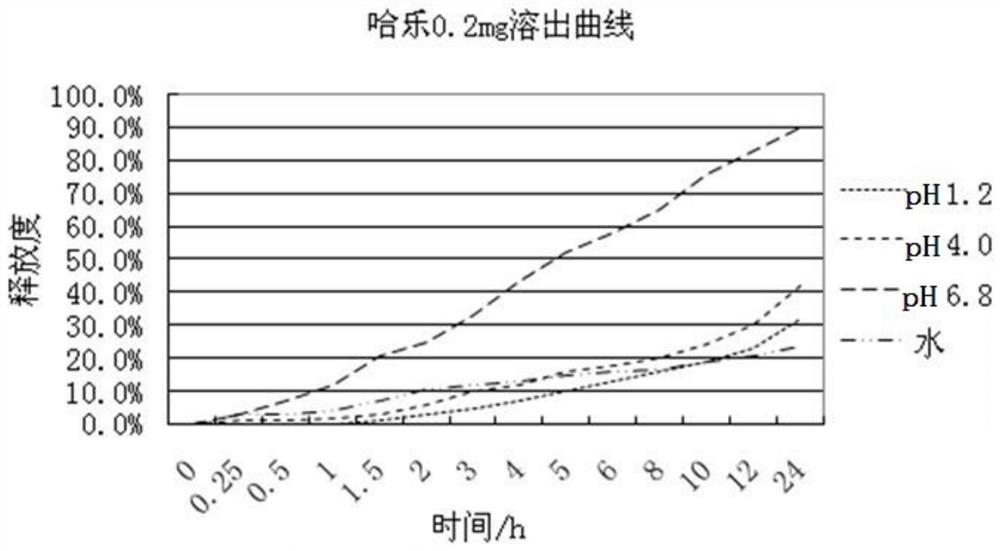
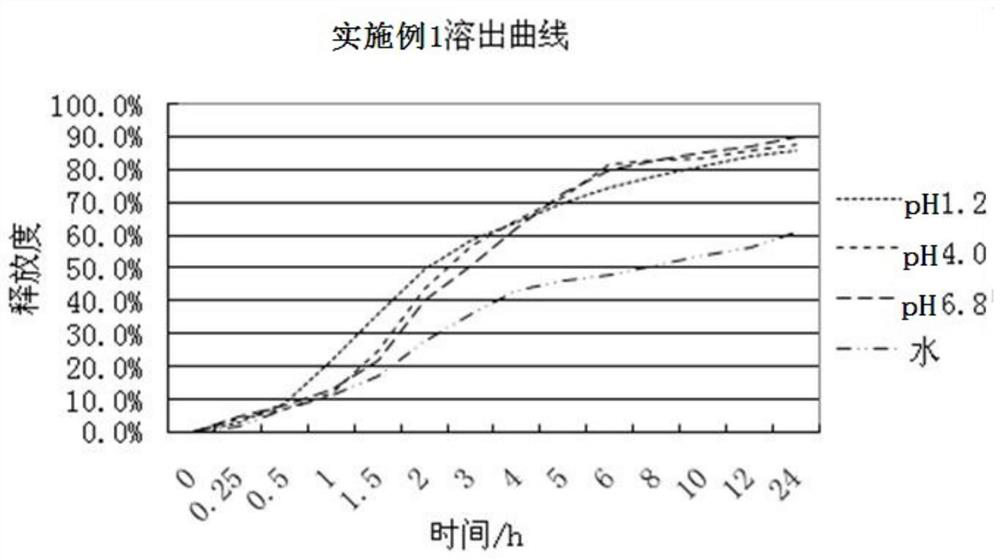
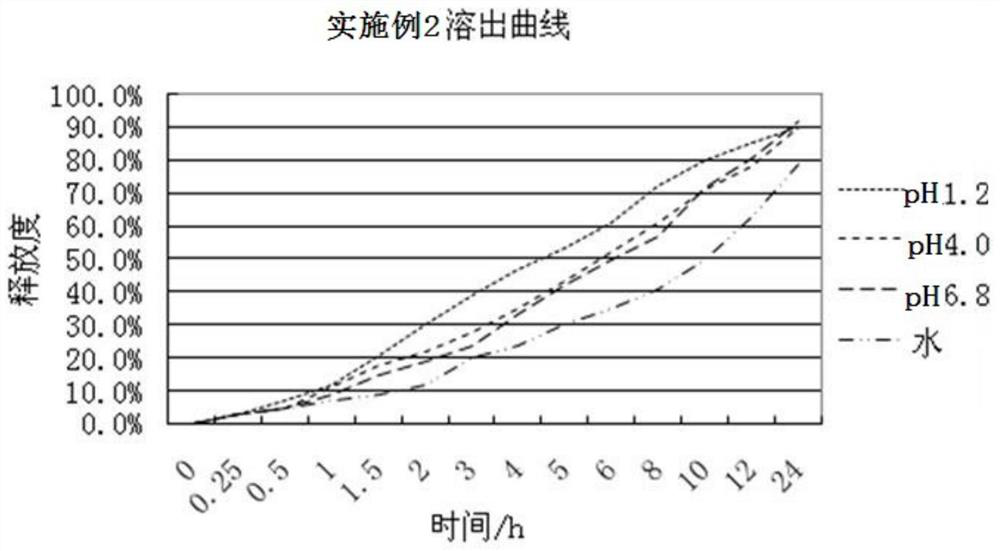
Image
Examples
Embodiment 2000
[0058] The prescription of the contents of 2000 tamsulosin hydrochloride sustained-release capsules of the present embodiment is as follows:
[0059]
[0060]
[0061] The preparation method of the capsule content of the present embodiment is as follows:
[0062] (1) Dissolve 0.4g tamsulosin hydrochloride in 120g water, stir until completely dissolved;
[0063] (2) Take the solution prepared in step (1), add 10 g of hypromellose under stirring, and stir for 30 minutes;
[0064] (3) Add 150g of Surelease aqueous dispersion to the solution prepared in step (2), and stir for 30min;
[0065] (4) cross 80 mesh sieves to obtain coating solution;
[0066] (5) Use the fluidized bed bottom spray coating technology to spray liquid on the blank microcrystalline cellulose pellet core. After the coating is finished, the pellets are dried to obtain sustained and controlled release pellets.
Embodiment 2
[0068] The prescription of the contents of 2000 tamsulosin hydrochloride sustained-release capsules of the present embodiment is as follows:
[0069]
[0070] The preparation method of the capsule content of the present embodiment is as follows:
[0071] (1) Dissolve 0.3g tamsulosin hydrochloride in 60g water and stir until completely dissolved;
[0072] (2) Take the solution prepared in step (1), add 5 g of hypromellose under stirring, and stir for 30 minutes;
[0073] (3) Add 75g of Surelease aqueous dispersion to the solution prepared in step (2), and stir for 30min;
[0074] (4) cross 80 mesh sieves to obtain coating liquid A;
[0075] (5) The preparation method of coating liquid B is the same as that of coating liquid A;
[0076] (6) Use the spray coating technology at the bottom of the fluidized bed to spray liquid on the blank microcrystalline cellulose pellet core, first spray liquid with coating liquid A, then spray liquid continuously with coating liquid B, and...
Embodiment 3
[0079] The prescription of the contents of 2000 tamsulosin hydrochloride sustained-release capsules of the present embodiment is as follows:
[0080]
[0081] The preparation method of the capsule content of the present embodiment is as follows:
[0082] (1) Dissolve 0.3g tamsulosin hydrochloride in 90g water, stir until completely dissolved;
[0083] (2) Take the solution prepared in step (1), add 2.5 g of triethyl citrate under stirring, and stir for 30 min;
[0084] (3) Add 95g of Eudragit NE30D into the solution prepared in step (2), and stir for 30min;
[0085] (4) 28g of talcum powder is added to the dispersion liquid prepared in step (3), and stirred until completely dispersed;
[0086] (5) cross 80 mesh sieves to obtain coating solution A;
[0087] (6) The preparation method of coating liquid B is the same as that of coating liquid A;
[0088] (7) Use fluidized bed bottom spray coating technology to spray liquid on the blank microcrystalline cellulose pellet cor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com