A kind of medicine for treating cerebral ischemic dementia and preparation method thereof
A technology for cerebral ischemia and ischemic brain, applied in drug combinations, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve the lack of objective laboratory evidence for diagnosis and efficacy judgment, and the lack of large-scale placebo control Clinical research, the selection of positive control drugs is not representative, etc., to achieve the effect of reducing the rate of brain lipid peroxidation, improving bioavailability, and repairing dynamic balance disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
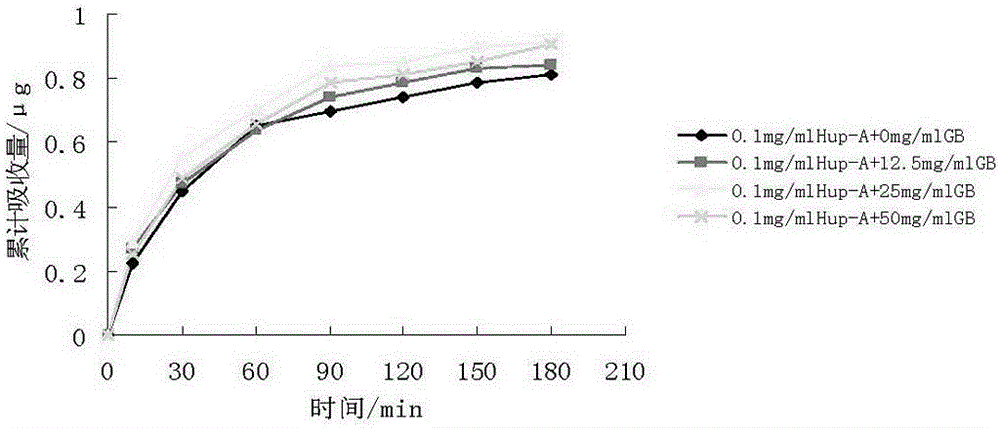
[0057] Embodiment 1: Preparation of Huperzine A and Ginkgolide B Solid Lipid Nanoparticles
[0058] The ingredients are effective doses of huperzine A 0.1 mg, ginkgolide B 10 mg, glyceryl monostearate 2.0 g, sodium taurocholate 0.2 g, poloxamer 1880.6 g, and the rest is liquid phase water, 100 ml in total.
[0059] Preparation:
[0060] a. In the preparation tank, stir the prescribed amount of emulsifier at 75°C for 30 minutes, and disperse evenly to form a water phase;
[0061] b. In the preparation tank, melt the prescribed amount of solid lipid phase at 65°C, then add the prescribed amount of huperzine A and ginkgolide B, and stir evenly to form the lipid phase;
[0062] c. Quickly add the water phase to the lipid phase at one time, stir at high speed (6000rpm) for 10 minutes, and make colostrum;
[0063] d. Pass through a homogenizer, adjust the homogenization pressure to 100MPa, repeat the homogenization of the solution 5 times to obtain a uniform emulsion, pass through...
Embodiment 2
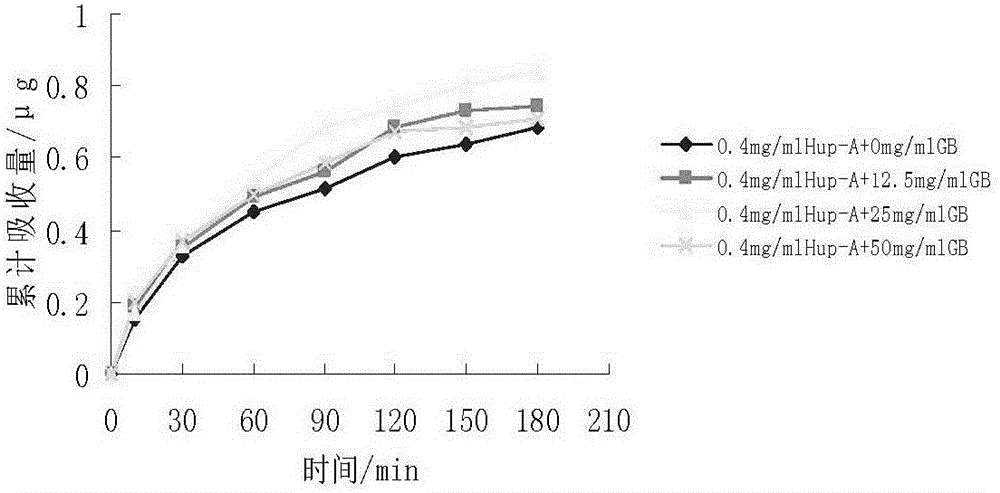
[0064] Embodiment 2: Preparation of Huperzine A and Ginkgolide B Solid Lipid Nanoparticles
[0065] The ingredients are effective doses of huperzine A 0.15 mg, ginkgolide B 5 mg, glycerol monostearate 1.0 g, lecithin 0.5 g, poloxamer F68 1.6 g, and the rest is liquid phase water, 100 ml in total.
[0066] Preparation:
[0067] a. In the preparation tank, stir and disperse the prescribed amount of emulsifier at 80°C to make a water phase;
[0068] b. In the preparation tank, melt the prescribed amount of solid lipid phase at 70°C, then add the prescribed amount of huperzine A and ginkgolide B, and stir evenly to form a lipid phase;
[0069] c. Quickly add the water phase to the lipid phase at one time, stir at high speed (6000rpm) for 10 minutes, and make colostrum;
[0070] d. Pass through a homogenizer (or microfluidizer), adjust the homogenization pressure to 100MPa, repeat the homogenization of the solution 4 times to obtain a uniform emulsion, pass through a microporous ...
Embodiment 3
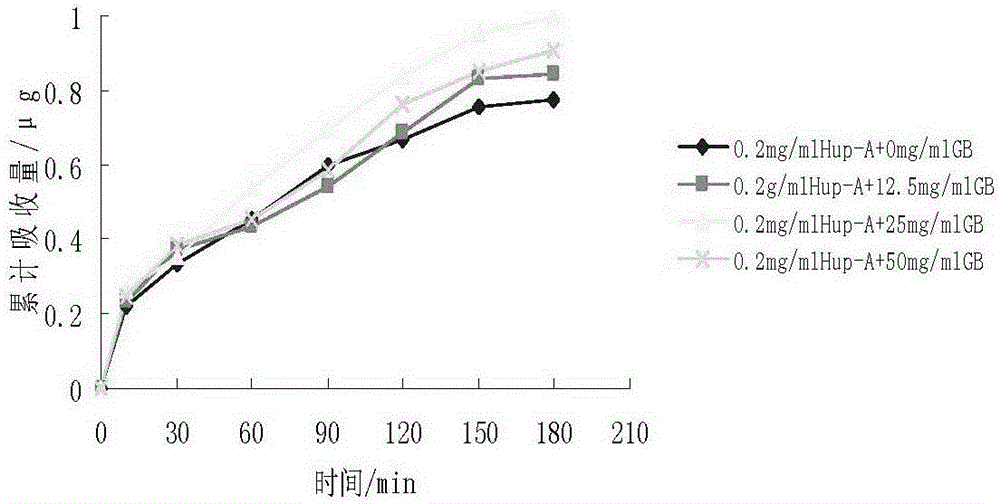
[0071] Embodiment 3: Preparation of Huperzine A and Ginkgolide B Solid Lipid Nanoparticles
[0072] The ingredients are effective doses of huperzine A 0.2mg and ginkgolide B 15mg, glyceryl monostearate 1.0g, sodium taurocholate 0.5g, poloxamer F68 1.6g, and the rest is liquid phase water, 100ml in total .
[0073] Preparation:
[0074] a. In the preparation tank, stir and disperse the prescribed amount of emulsifier at 90°C to make a water phase;
[0075] b. In the preparation tank, melt the prescribed amount of solid lipid phase at 80°C, then add the prescribed amount of huperzine A and ginkgolide B, and stir evenly to form the lipid phase;
[0076] c. Quickly add the water phase to the lipid phase at one time, stir at high speed (6000rpm) for 10 minutes, and make colostrum;
[0077] d. Ultrasound the colostrum for 5 minutes with a probe at a power of 400W, keep at 70°C to obtain a uniform emulsion, pass through a microporous membrane, and cool down to room temperature to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com