Sirolimus preparation and preparation process thereof
A technology of sirolimus and preparations, which is applied in the field of medicine, can solve the problems of poor solubility of sirolimus, narrow therapeutic window, and large difference in dissolution of solid preparations, and achieve low oral bioavailability, simple prescription, and good operability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Prescription: Sirolimus 2g
[0058] Povidone K30 6g
[0059] Microcrystalline Cellulose 200g
[0060] 95% ethanol 170ml
[0061] Appropriate amount of water
[0062] Makes 1000 pieces
[0063] Preparation Process:
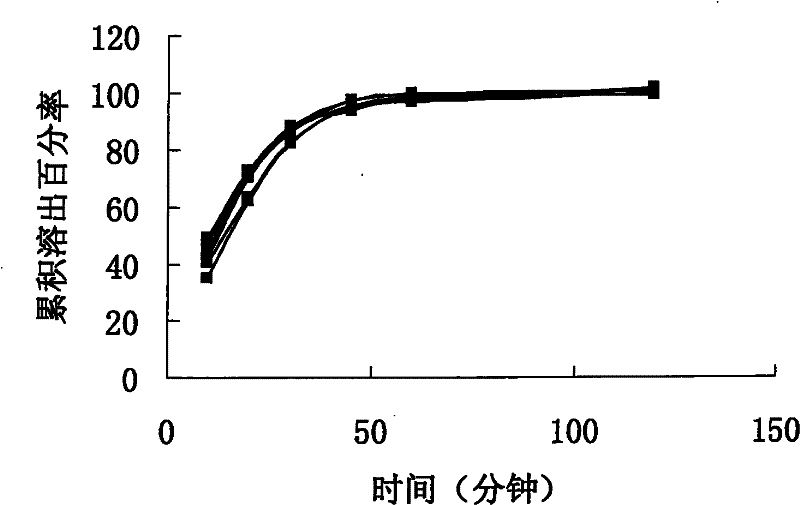
[0064] Take sirolimus and povidone K30, mix well, add 95% ethanol, stir in a water bath at 60°C to dissolve, add this solution into microcrystalline cellulose, stir and granulate, dry in a fluidized bed, measure the moisture 5% or less, then add a concentration of 3% povidone K30 aqueous solution to the dry granules by weight 0.8:1 to make a soft material, extrude with a pore size of 0.6 mm, spheroidize for 1 minute, and dry in a fluidized bed at a speed of 600 rpm. Measure moisture below 3%, get the granule that is thicker than 80 meshes, add 0.5% magnesium stearate. Fill to capsule. That is, sample 1 was obtained.
[0065] The dissolution profile of sample 1 (6 grains) is as follows figure 1 shown. Depend on figure 1 It can be seen that the unif...
Embodiment 2
[0067] Prescription: Sirolimus 2g
[0068] Povidone K30 6g
[0069] Microcrystalline Cellulose 200g
[0070] Cross-linked PVP 10g
[0071] 95% ethanol 170ml
[0072] Appropriate amount of water
[0073] Makes 1000 pieces
[0074] Preparation Process:
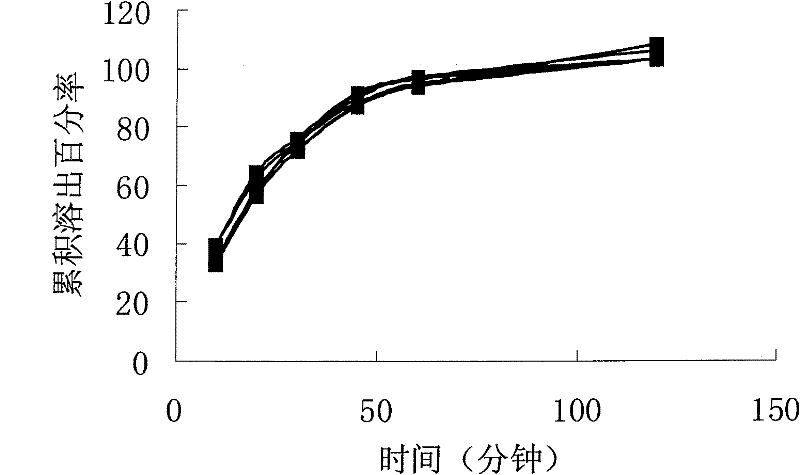
[0075] Take sirolimus and povidone K30, mix well, add 95% ethanol, stir in a water bath at 60°C to dissolve, add this solution to microcrystalline cellulose and cross-linked PVP, stir to granulate, and use a fluidized bed Dry, measure the water content below 5%, then add water-based soft material to the dry granules according to the weight of 1:1, extrude with 1mm aperture, spheroidize for 3 minutes, rotate at 800rpm, dry in a fluidized bed, measure the water content below 3%, take For particles coarser than 80 meshes, 0.5% magnesium stearate is added. Fill to capsule. Sample 2 is obtained.
[0076] The dissolution of sample 2 (6 capsules) is as follows figure 2 shown. Depend on figure 2 It can be seen that the uni...
Embodiment 3
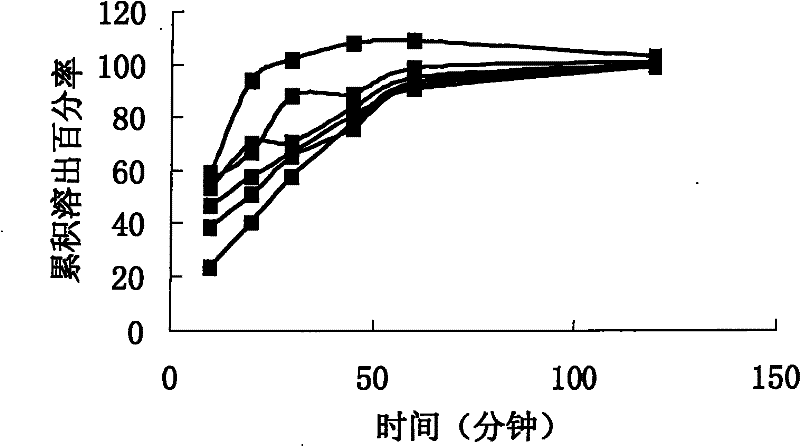
[0077] Embodiment 3: in vitro cumulative dissolution comparative test:
[0078] According to CN200810071842.0 embodiment 2, the capsule is sample 3.
[0079] Commercially available product (produced by U.S. Wyeth, batch number: c70282) was purchased as sample 4
[0080] Solid dispersion without sustained release, that is, the preparation intermediate of Example 2 without the extrusion and spheronization process is directly encapsulated, which is sample 5.
[0081] The test method is: according to the dissolution method (Chinese Pharmacopoeia version two appendix XC first method in 2005), with 0.4% sodium lauryl sulfate solution 500ml as the dissolution medium, the rotating speed is 120 revolutions per minute, operated according to the law, after 10 , 20, 30, 45, 60, and 120 minutes of sampling, take 10ml of the solution and filter it, and take the subsequent filtrate as the test solution; take an appropriate amount of sirolimus reference substance, weigh it accurately, dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com