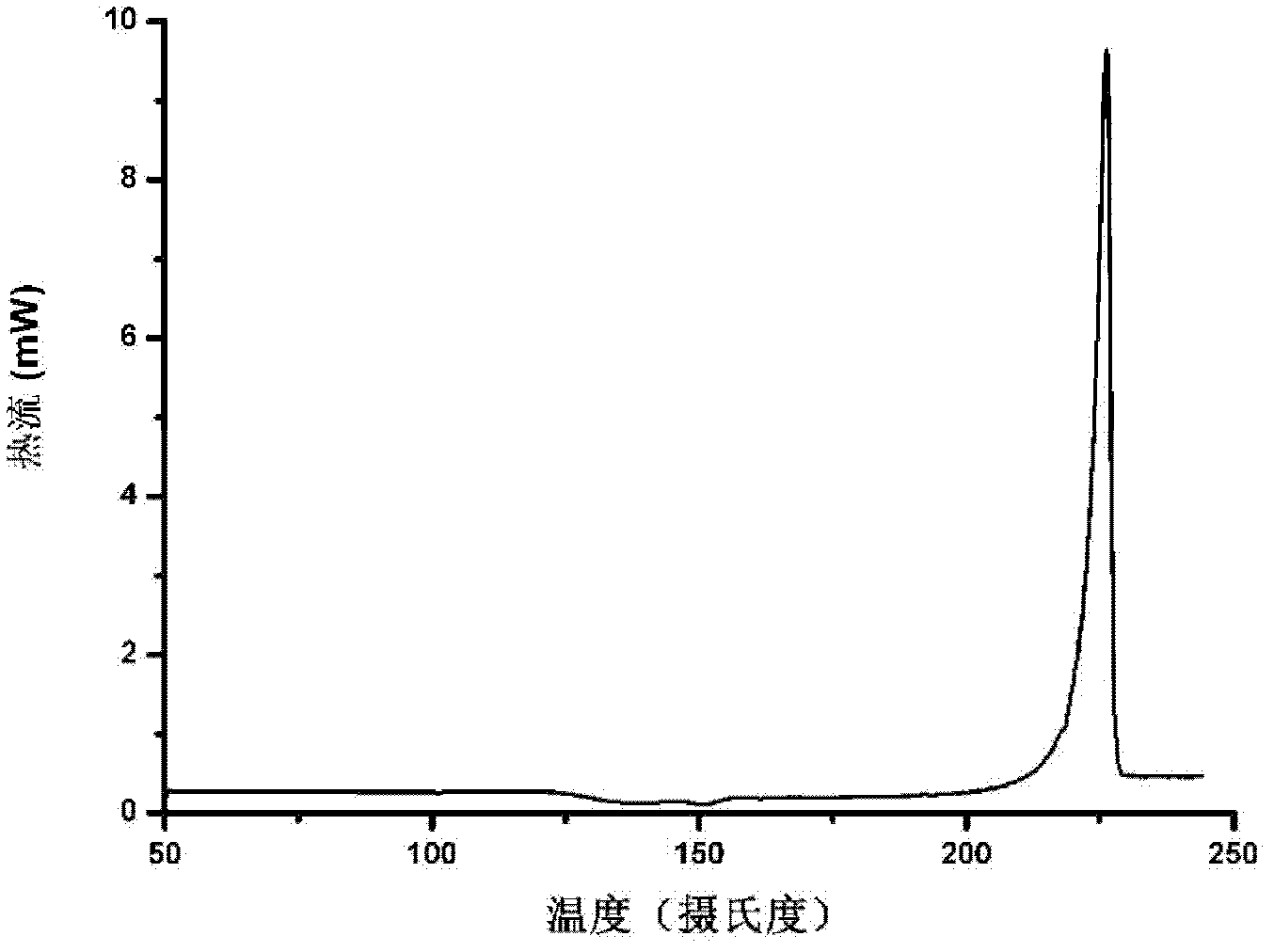
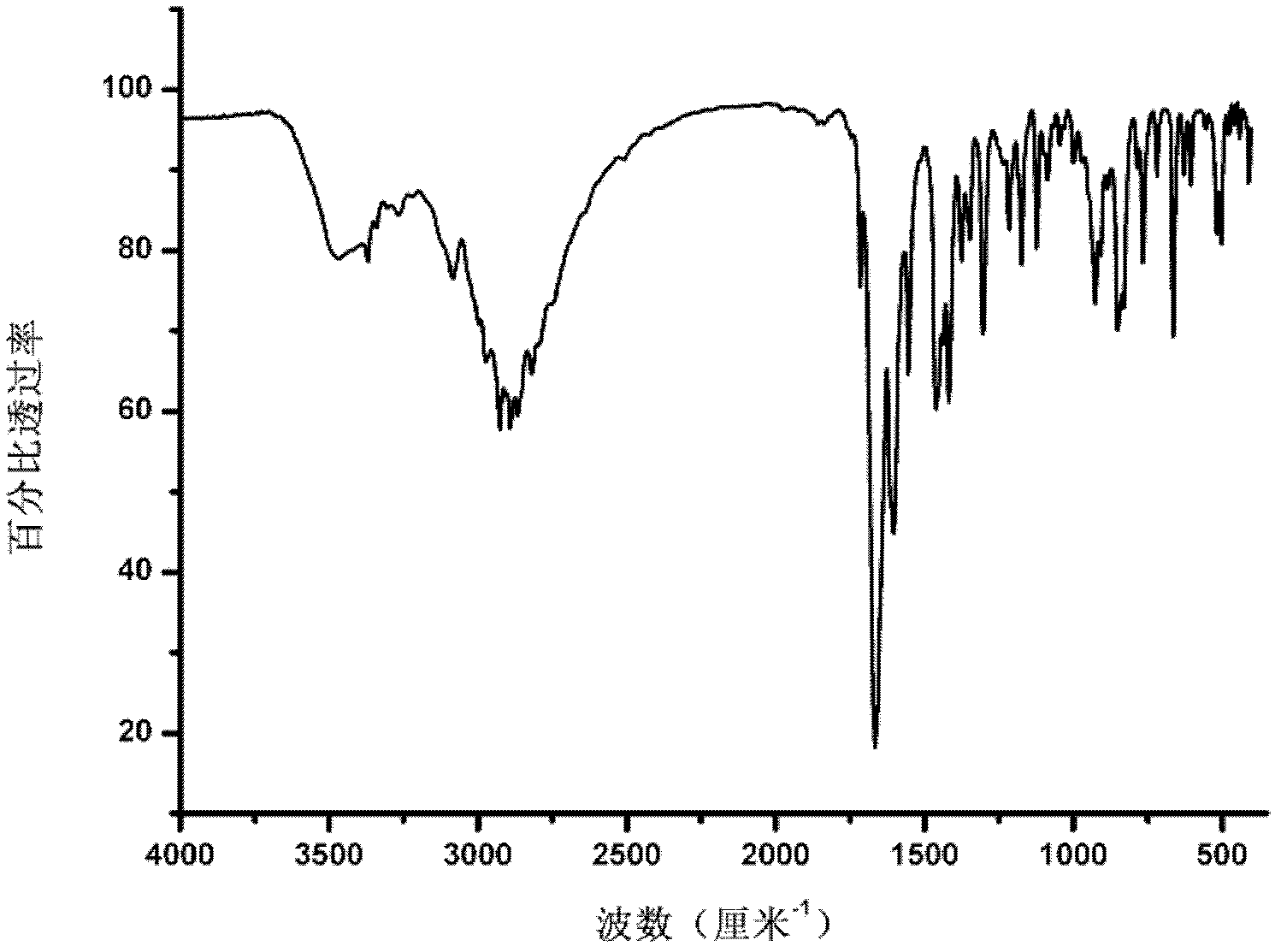
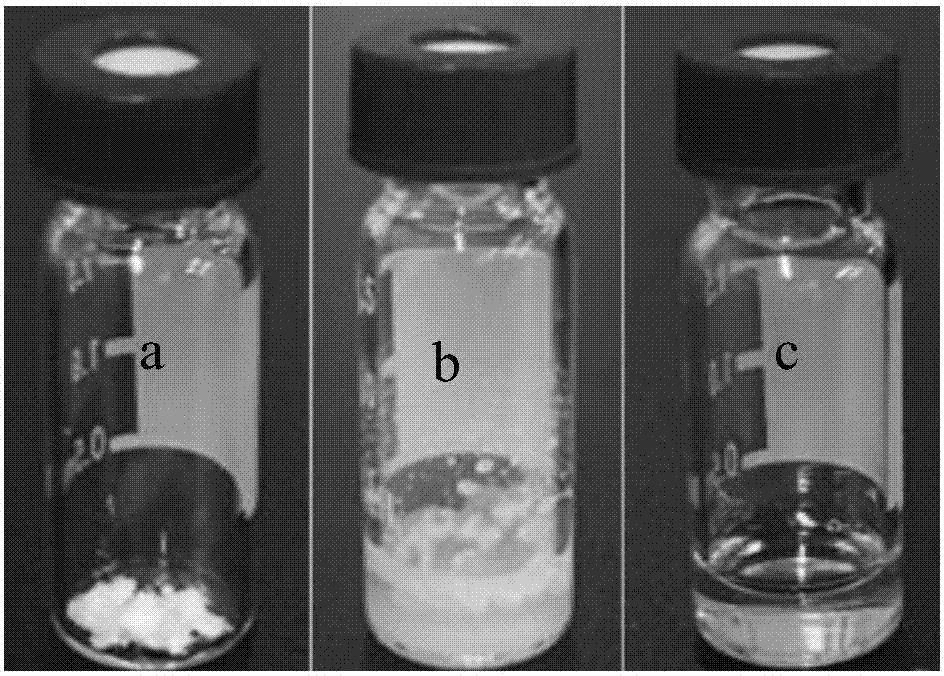
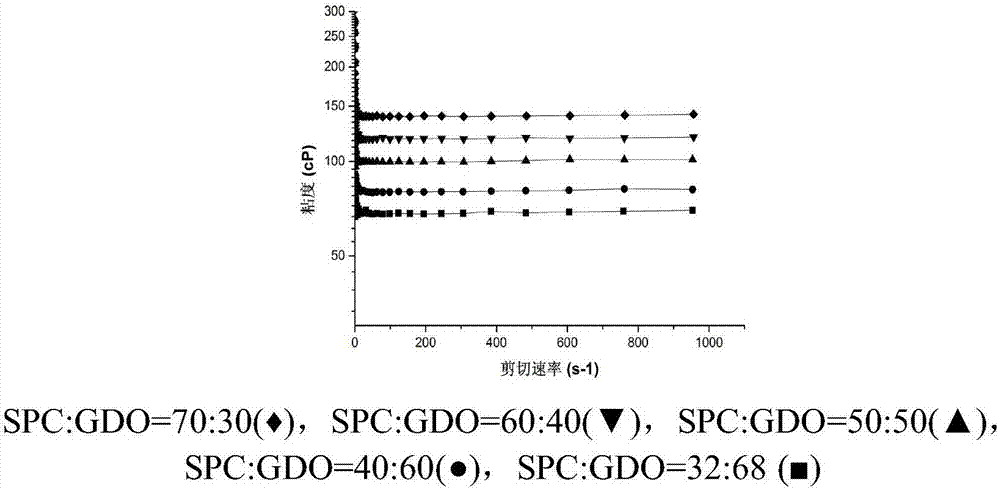
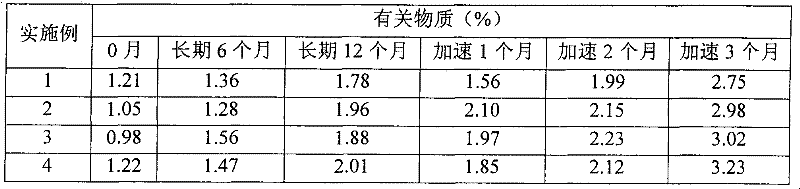
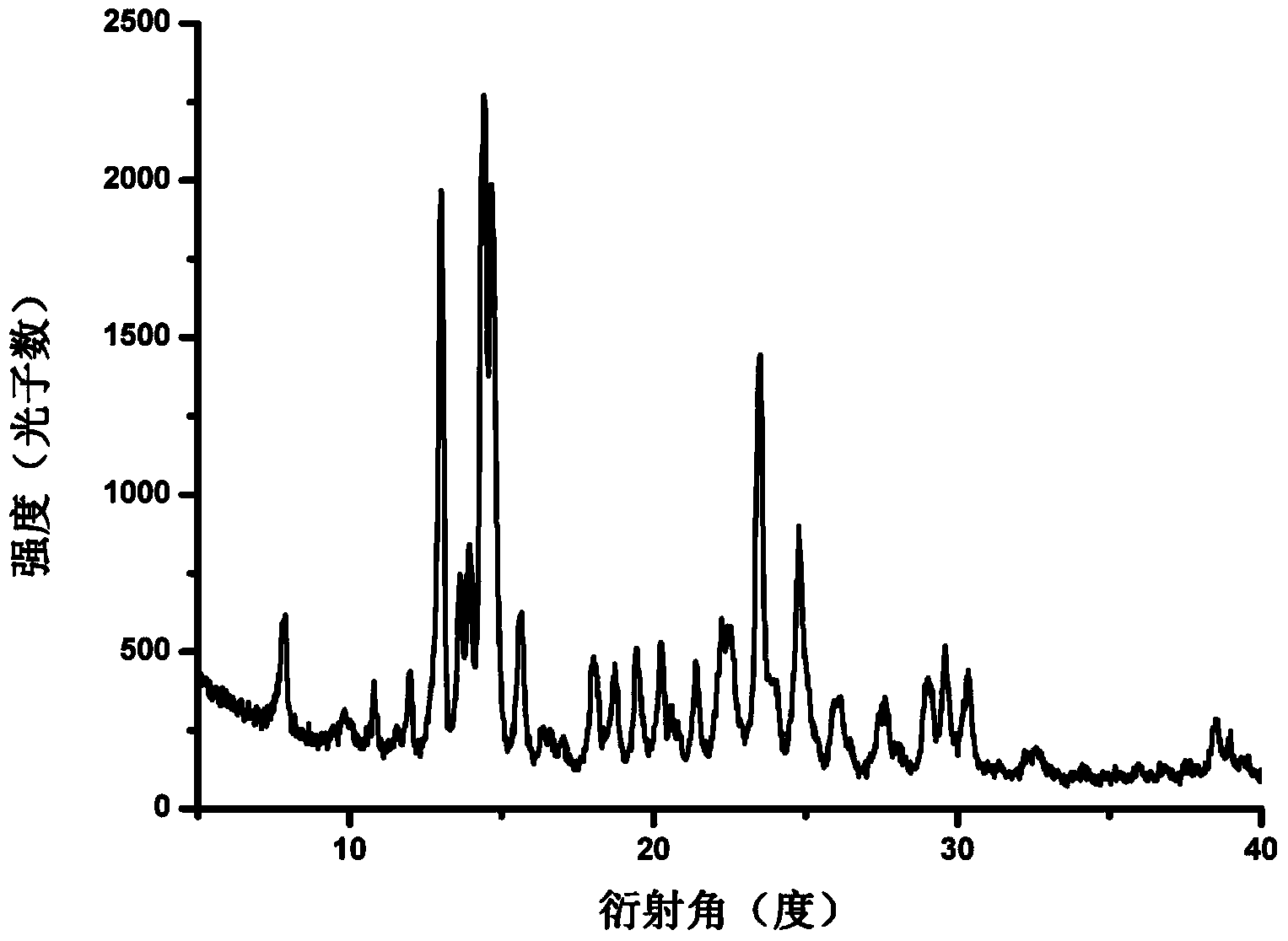
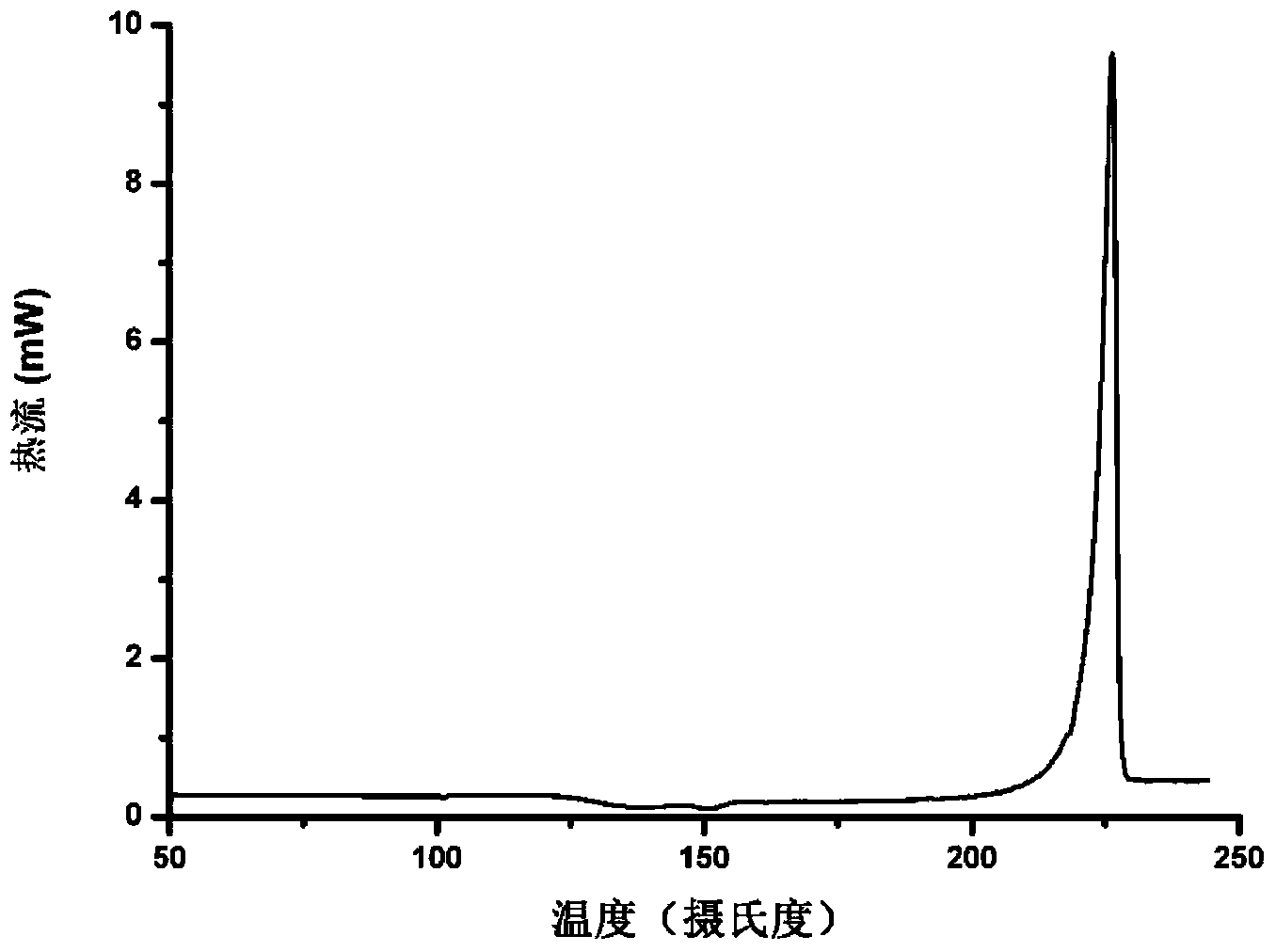
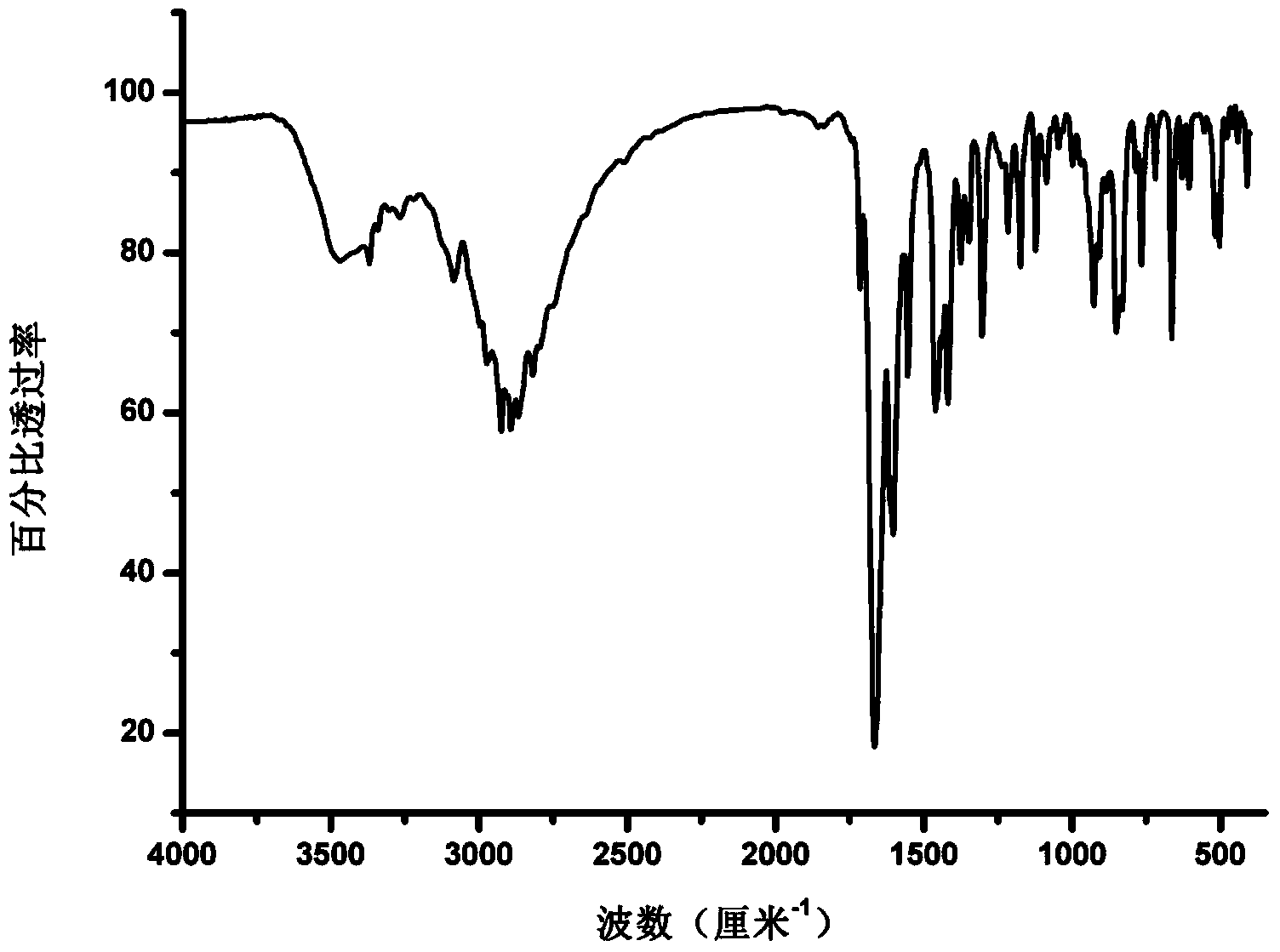
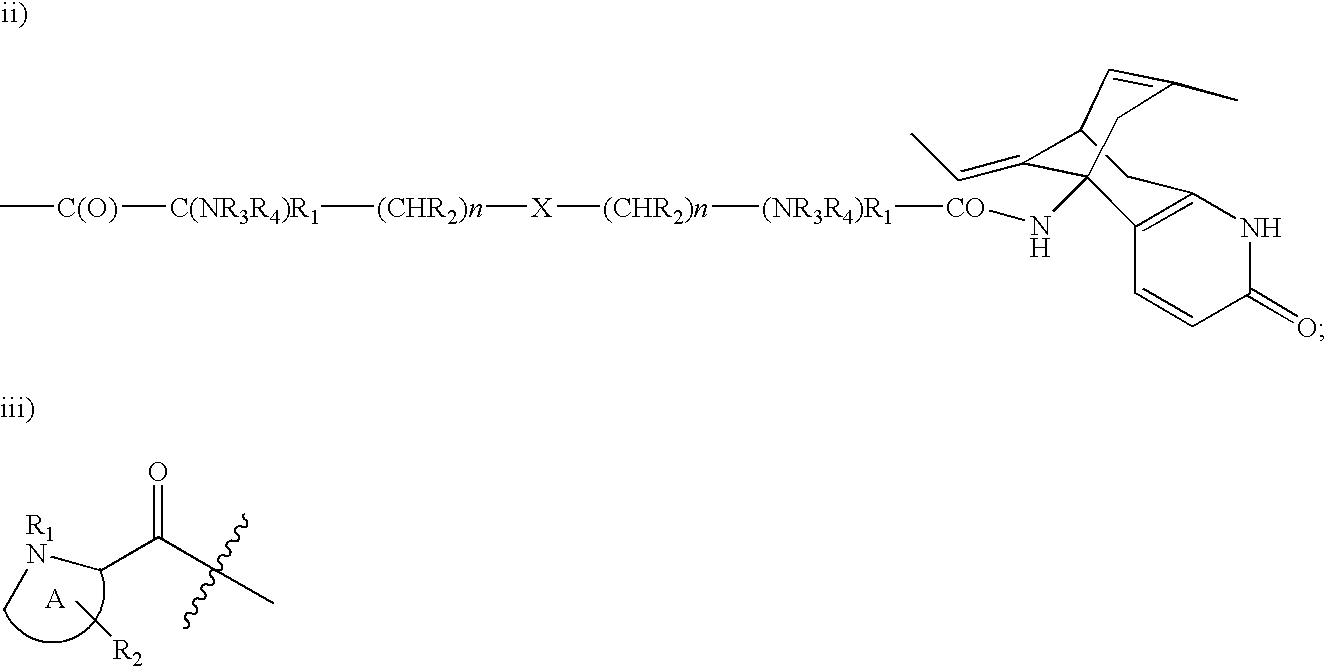Patents
Literature
77 results about "A huperzine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Transdermal administration of huperzine
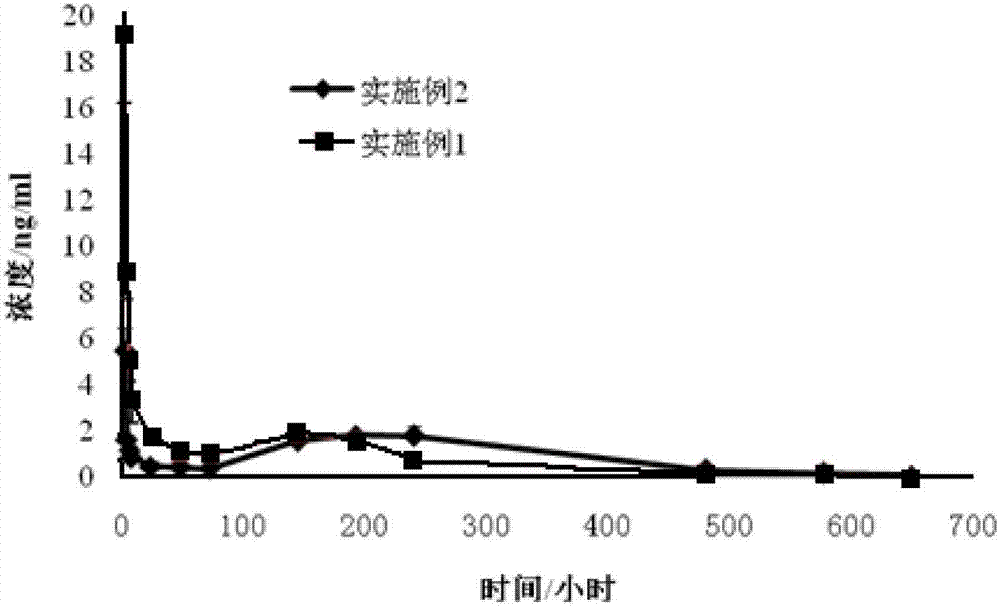
The present invention provides a composition of transdermally administered huperzine for improving memory and cognitive function. In one aspect, huperzine is delivered in a sufficient amount to achieve and maintain a blood plasma Huperzine level of about 0.5 ng / mL to about 30 ng / mL. Huperzine may be delivered by itself, or in combination with other elements, such as additional cholinesterase inhibitors, drugs or treatment agents, or positive health promoting substances. Various formulations for the transdermal delivery of huperzine are disclosed, and may include selected penetration enhancers.
Owner:XEL HERBACEUTICALS INC
Combination of huperzine and nicotinic compounds as a neuroprotective agent
InactiveUS6369052B1Enhance memoryPrevent and reverse memory declineBiocideNervous disorderRisk strokeDisease cause
The present invention provides compositions and methods for treating, preventing, or reversing neuronal dysfunction including cognitive decline, such as cognitive decline associated with aging and minimal cognitive impairment; severe neurodegenerative disorders, such as Alzheimer's disease; and neuronal dysfunction associated with loss of motor skills, such as Parkinson's disease and amyotrophic lateral sclerosis. The compositions and methods of the invention can also treat or prevent neuronal dysfunction resulting from CNS injury, such as stroke, spinal-cord injury, and peripheral-nerve injury. The compositions of the invention comprises a huperzine compound and a nicotinic compound.
Owner:GEORGETOWN UNIV
Huperzine A solid lipid nano particle and preparation method thereof
ActiveCN101658494AImprove stabilityImprove bioavailabilityPowder deliveryOrganic active ingredientsLipid formationOrganic solvent
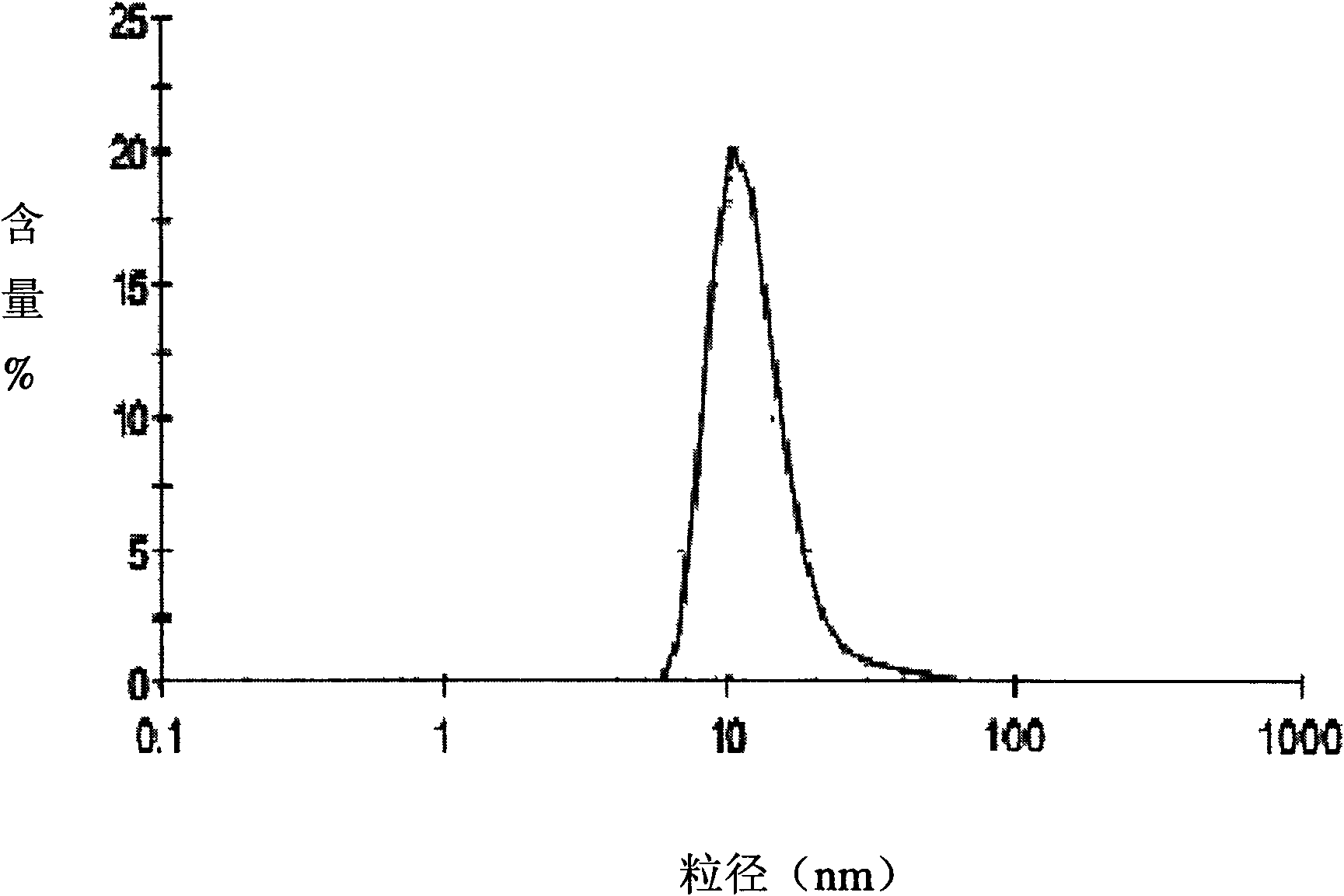
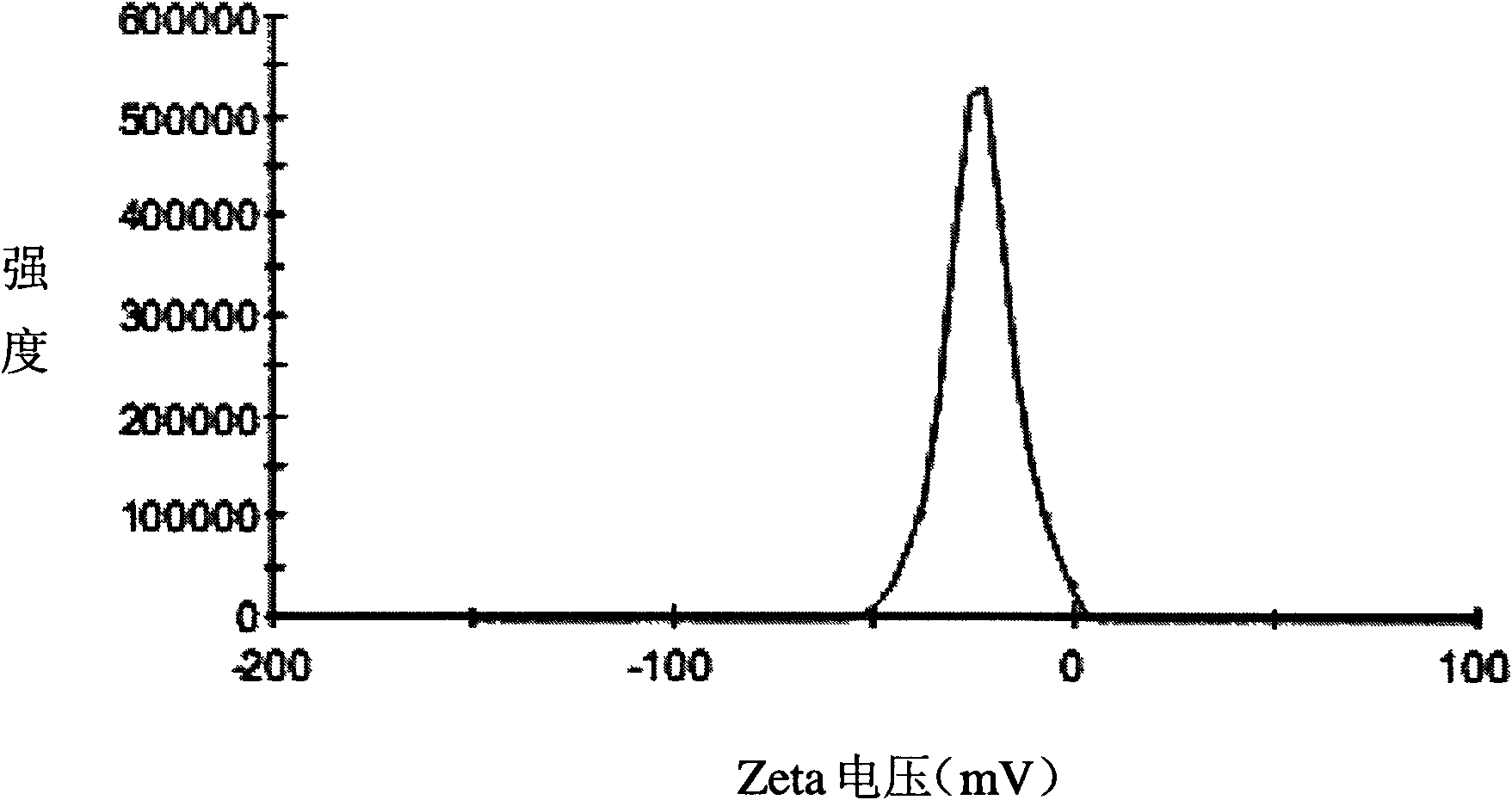
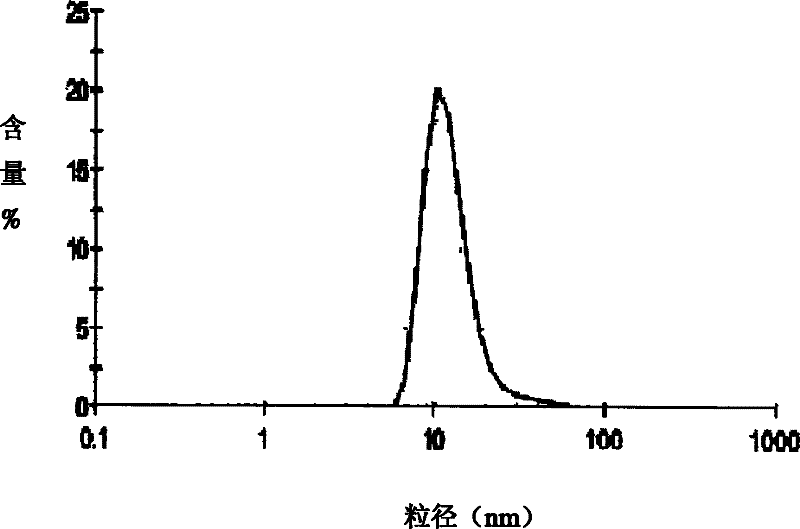
The invention relates to the field of pharmaceutical preparations, which discloses a huperzine A solid lipid nano particle and a preparation method thereof. The huperzine A solid lipid nano particle is prepared from the following components in weight percent: 0.05-1% of huperzine A, 3-10% of lipid material, 2-10% of emulsifying agent and the balance water. The preparation method adopts a high-pressure even emulsification method to coat the huperzine A into a solid lipid nano particle so as to prepare the huperzine A solid lipid nano particle. The particle diameter of the huperzine A solid lipid nano particle prepared by the invention is 10-100nm, the medicine envelopment rate and the medicine-carrying quantity are high, and the stability is good; simultaneously, the use of an addition agent and an organic solvent which are harmful to a human body is avoided; and the bioavailability is enhanced, the brain targeting property is increased, and the dose and the toxic or side effect are small.
Owner:GUANGDONG PHARMA UNIV
Huperzine A polymorph, its preparation method, medicinal composition containing huperzine A polymorph and its application
The invention discloses a huperzine A polymorph, its preparation method, a medicinal composition containing huperzine A polymorph and its application. Part of the huperzine A polymorph has better dissolvability than currently saled medicinal crystal forms, thus being more conducive to medicine absorption. Also, part of the huperzine A polymorph has substantially lower hygroscopicity than saled medicinal crystal forms, thus being more conducive to preparation and storage of medicinal preparations.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Huperzine a lyotropic liquid crystal preparation and preparation method thereof
ActiveCN106924172AImprove solubilityOvercome the disadvantages of insoluble in waterOrganic active ingredientsNervous disorderTO-18Organic solvent
The invention provides a huperzine a lyotropic liquid crystal preparation and a preparation method thereof. The huperzine a lyotropic liquid crystal preparation is prepared from the following components in parts by weight: 0.12 to 0.7 part of huperzine a, 10 to 18 parts of organic solvent, 12 to 70 parts of phospholipid, and 12 to 59.5 parts of grease. According to the huperzine a lyotropic liquid crystal preparation provided by the invention, the phospholipid, the grease and the organic solvent are used as carriers of the huperzine a, so that the solubleness of the huperzine a is effectively improved, the defect that the huperzine a is insoluble in water is overcome, the pharmacological function of the huperzine a is played favorably; meanwhile, under the condition of the limited component proportion, the huperzine a lyotropic liquid crystal preparation encounters water to form a lyotropic liquid crystal after being injected into a human body, so that the adhesion of a medicine and the preparation is improved, the medicine diffusion speed is retarded, the administration period is increased, the administration times are reduced, and thus the compliance of a patient is greatly improved.
Owner:武汉百纳礼康生物制药有限公司
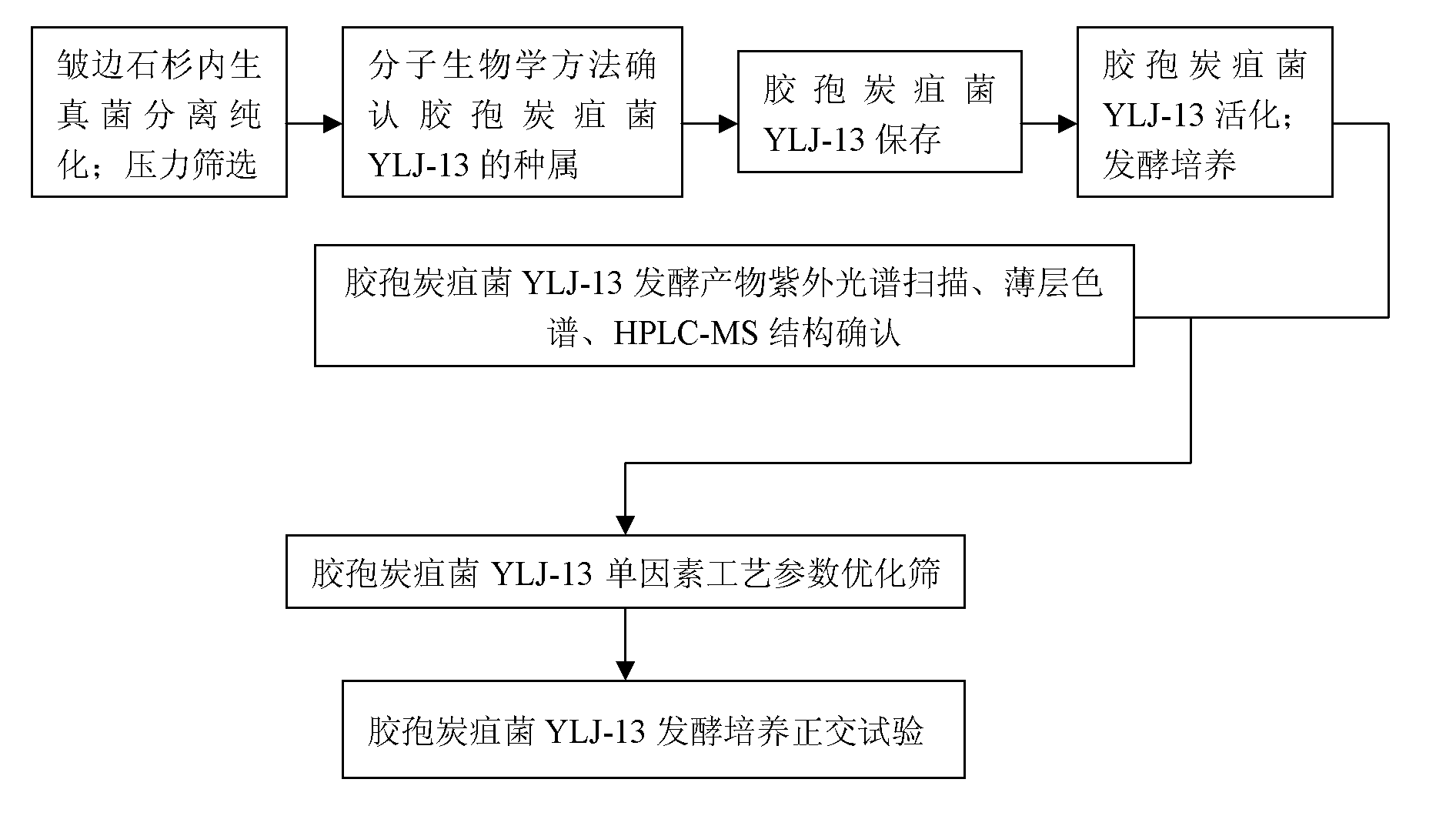
Huperzia serrata endophytic fungi and its use in production of huperzine a
ActiveCN103103134ASolve bottlenecksSave natural resourcesFungiMicroorganism based processesBiotechnologyNatural resource
The invention relates to the technical field of microbes and especially relates to huperzia serrata endophytic fungi and its use in production of huperzine a. The huperzia serrata endophytic fungi are named as Colletotrichum sp. SY-1, are preserved in the China center for type culture collection (CCTCC) on August 19, 2011, and have the preservation number CCTCC M2011293. Through fermentation, the huperzia serrata endophytic fungi can produce a huperzine a compound. Through utilization of characteristics of microbe-based huperzine a production and the modern fermentation breeding technology, huperzine a industrial production is realized; bottleneck problems of huperzine a exploitation are solved; and the endangered huperzine a natural resource is protected.
Owner:吴水生
Nasal cavity administrated huperzine prepn
InactiveCN1383824AEnhance memoryImprove learning effectNervous disorderHeterocyclic compound active ingredientsNasal cavityMedicine
The nasal cavity administrated huperzine preparation consists of huperzine as effective medicine component, lauryl azatroketone and other infiltration promoter and other assistants. It is used for treating senile dementia and memory dysfunction and raising memory and learning capacity. It has high mucous membrane penetrating property and high brain targeting property and each administration can lead 80-500 microgram of huperzine into body.
Owner:ZHEJIANG ACAD OF MEDICAL SCI
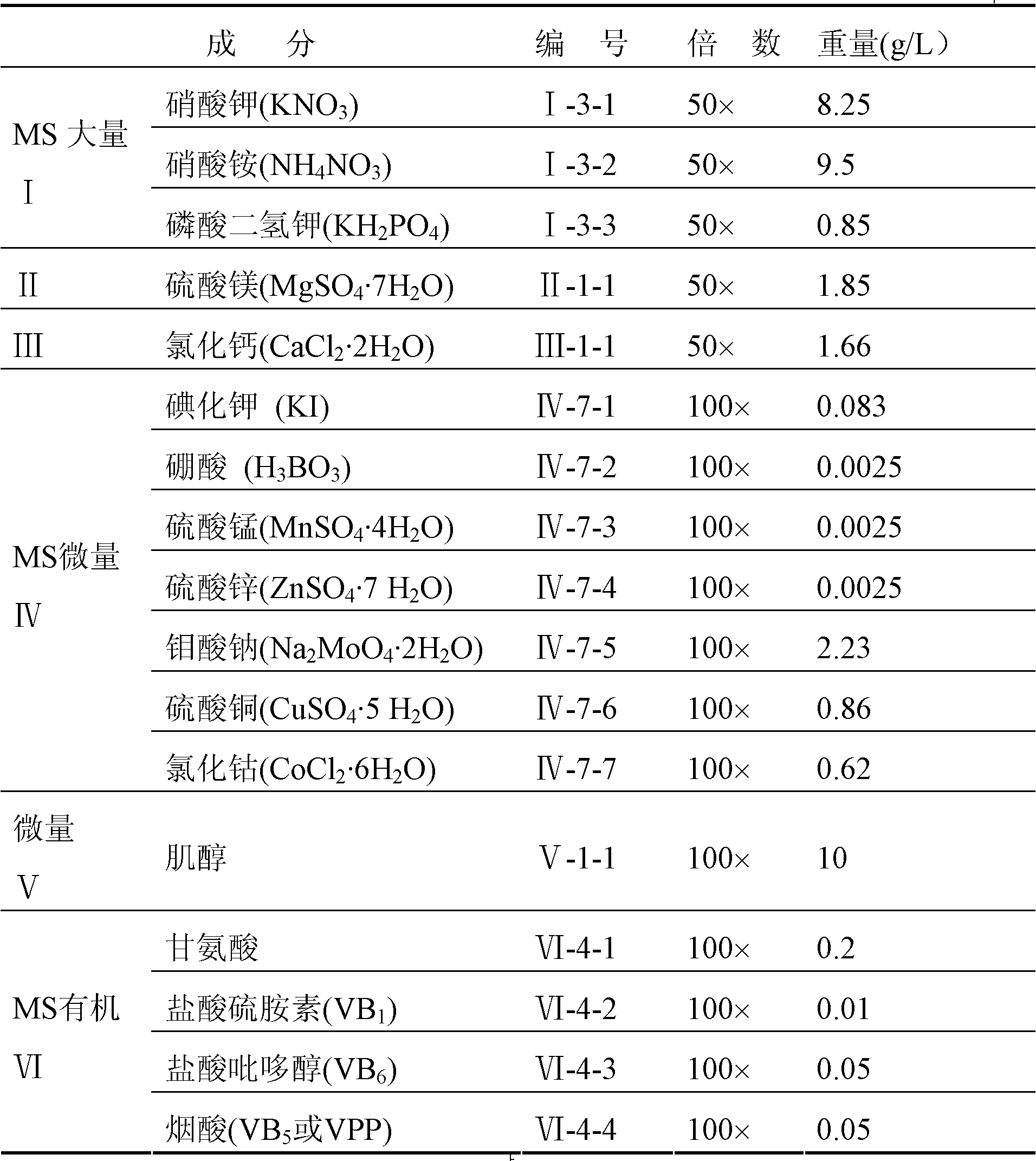
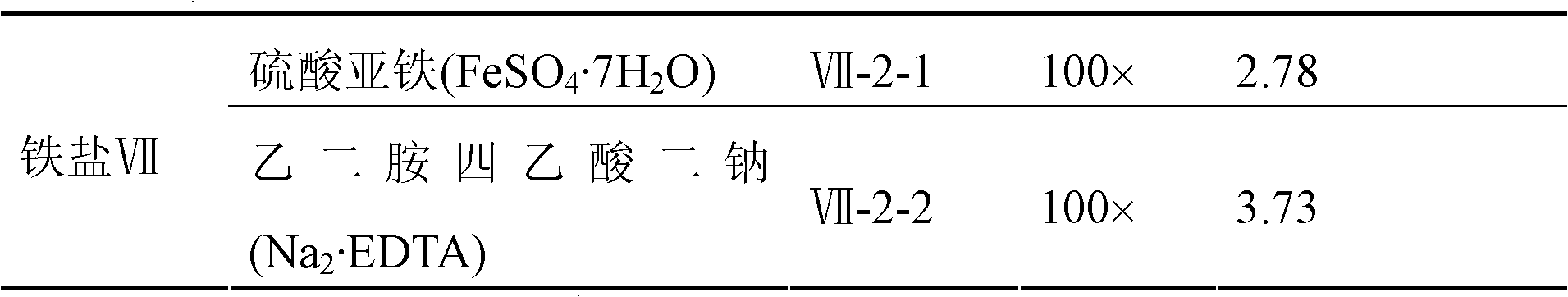
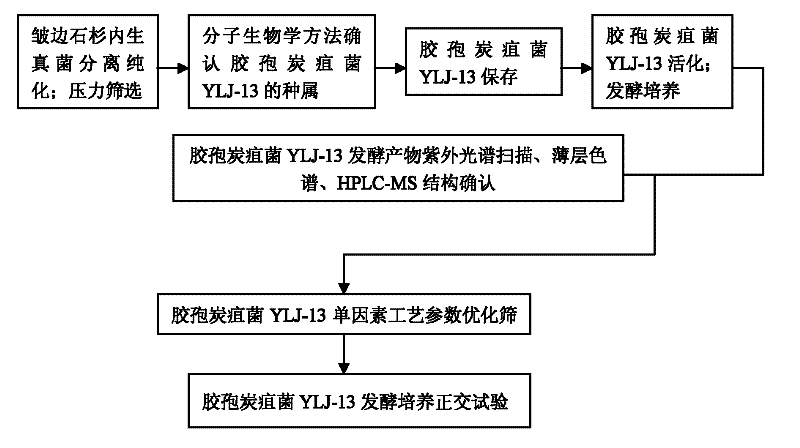
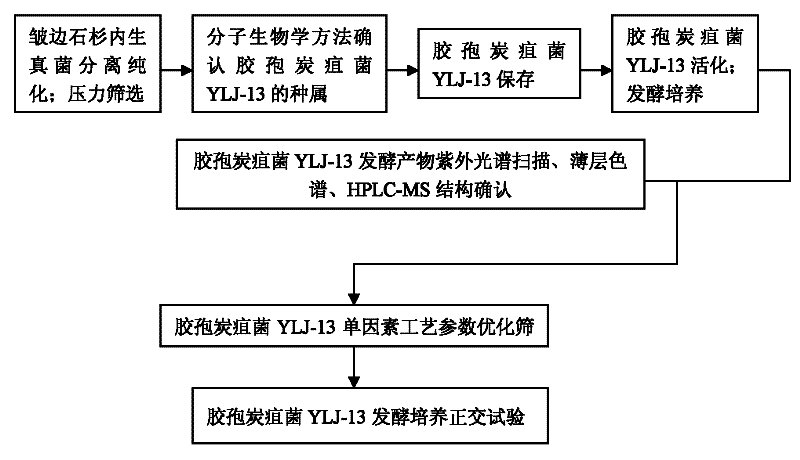
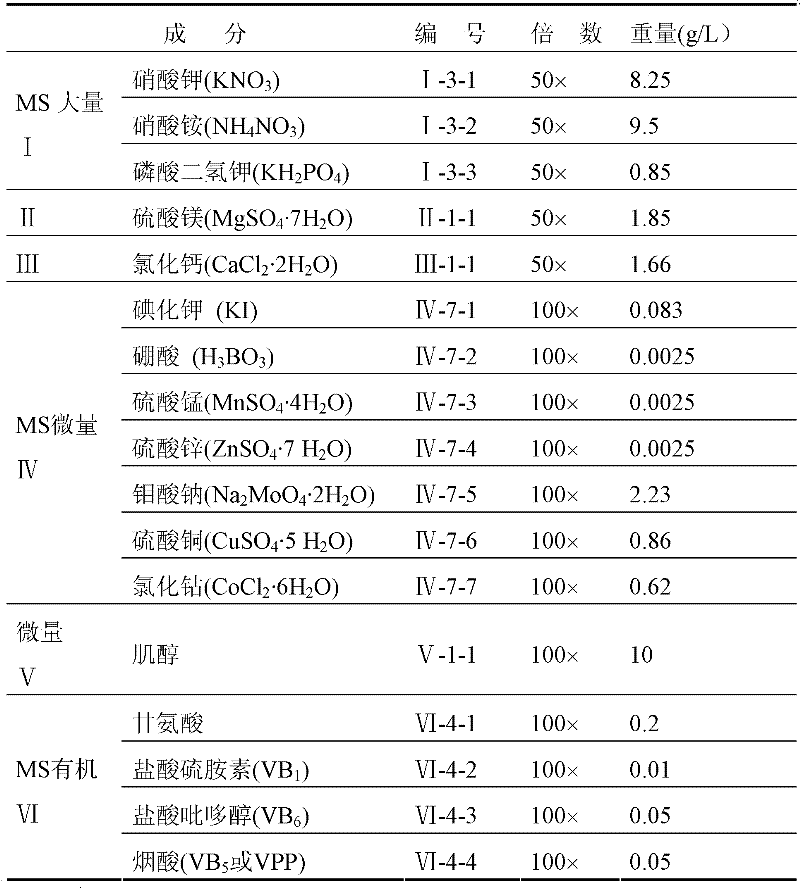
Huperzine A high-producing strain and method for producing huperzine A by fermenting same
The invention discloses a huperzine A high-producing strain and a method for producing huperzine A by fermenting the same, belonging to the technical field of biology. The huperzine A high-producing strain is Colletotrichum gloeosporioidesisolate YLJ-13 preserved in the China Center for Type Culture Collection on July 17, 2010, and the preservation number is CCTCC No. M2010181. The strain provided by the invention is separated and purified from huperzia crispate through pressure screening, and the strain is identified as a new variety of Colletotrichum gloeosporioidesisolate through a molecular biological means. The strain is fermented in an improved potato liquid culture medium, and huperzine A is obtained from the fermentation liquid. Huperzine A obtained through fermentation production can be secreted to be out of cell, the improved potato liquid culture medium is used as a precursor of biological transformation, and up to 199.6 mg / L huperzine A can be obtained from each liter of culture system. The strain provided by the invention is used for producing huperzine A. The existing rare medicinal huperziaceae plant resources can be protected, but also the shortage situation of huperzine A medication demand can be alleviated.
Owner:HUNAN AGRICULTURAL UNIV
Huperzine a prodrugs and uses thereof
Disclosed are huperzine A prodrugs and method of synthesis thereof. The invention further relates to methods of treating, preventing or reversing neurodegenerative diseases, such as, Alzheimer's Disease and neuronal dysfunctions, such as, memory impairment using a pharmaceutical composition comprising a huperzine A prodrug as disclosed herein.
Owner:PAN BAI CHUAN
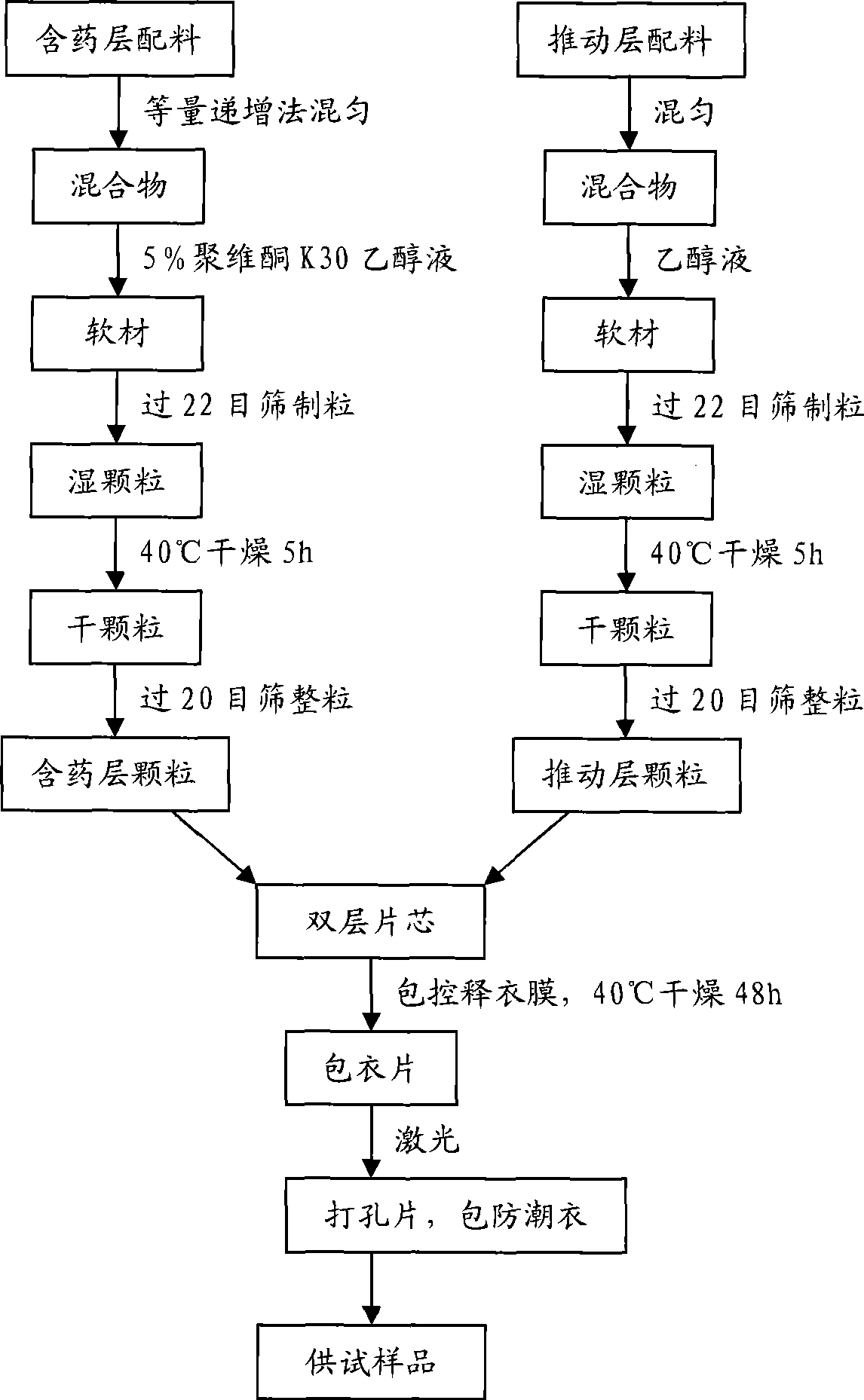
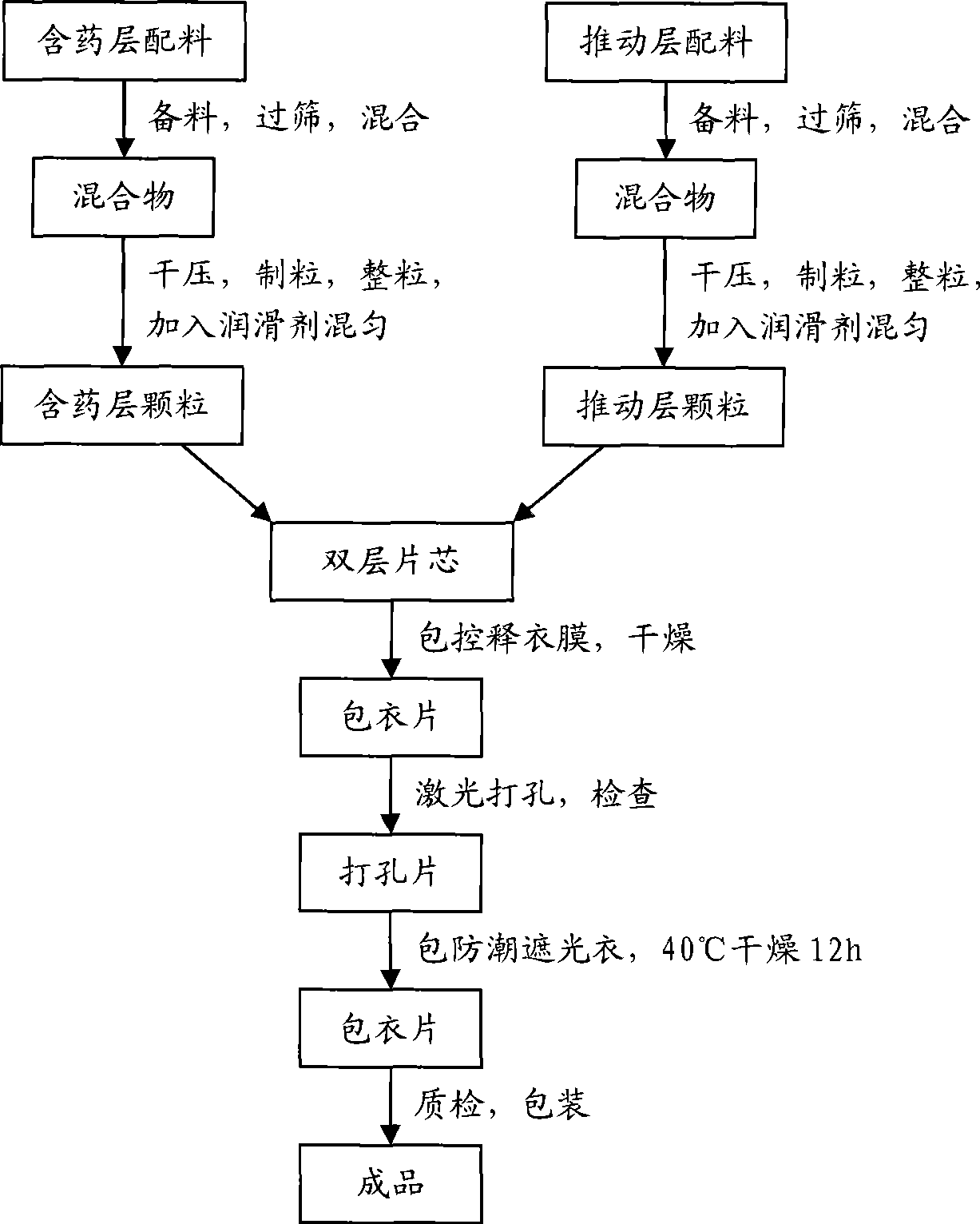
Huperzine A double-layer osmotic pump controlled release tablets and preparation method thereof
InactiveCN101485639AStable blood concentrationReduce the number of dosesOrganic active ingredientsNervous disorderDrug release ratePolyethylene glycol
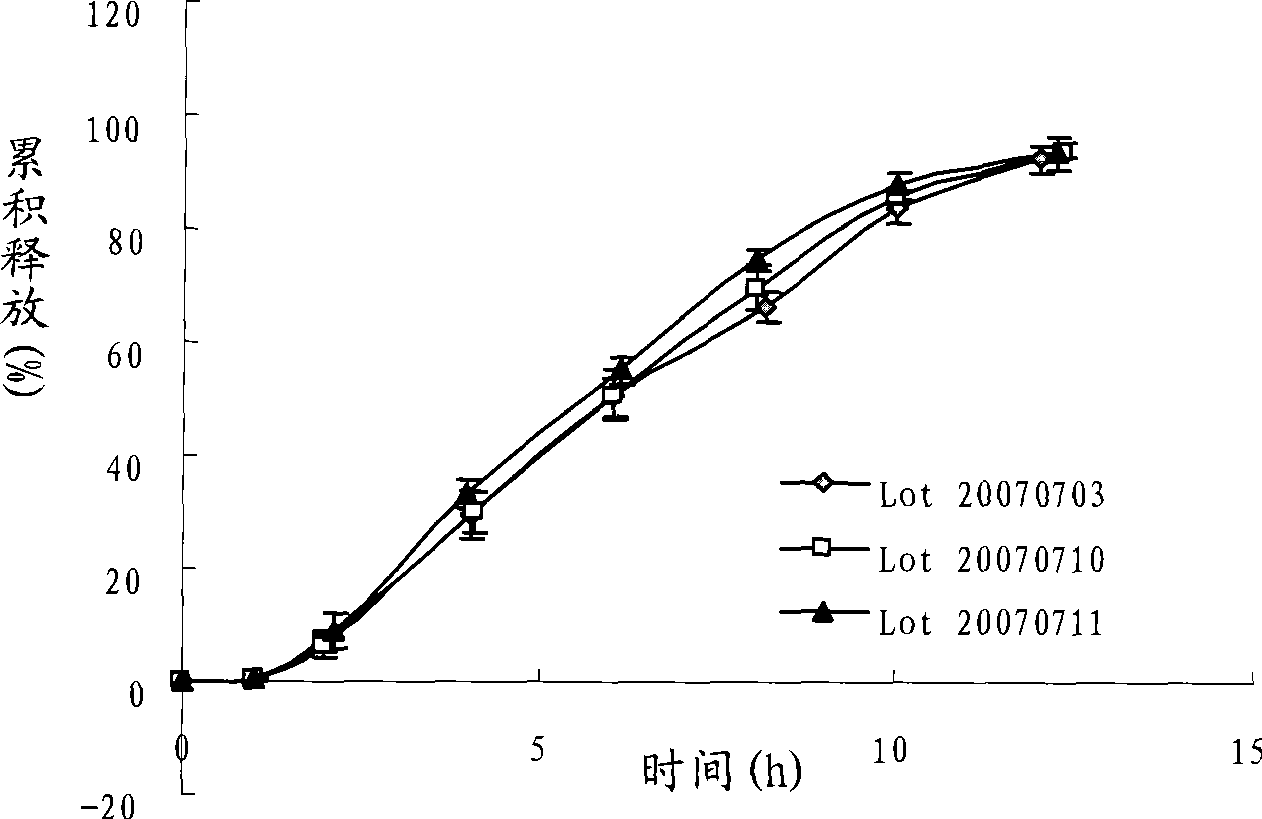
The invention discloses a huperzine A double-layer osmotic pump controlled-release tablet. The tablet comprises a double-layer tablet core consisting of a drug containing layer and a pushing layer, and a semipermeable coating membrane coating the double-layer tablet core, wherein one side of the drug containing layer of the coating membrane is provided with a drug release hole; the drug containing layer of the tablet core contains huperzine, a suspending agent, osmotic pressure active matters and pharmaceutical excipients which are all in effective dosage; the pushing layer contains an expanding agent, a permeation enhancer, and a binder; and the coating membrane contains polyethylene glycol used as a plasticizer, the weight gain of the coating membrane against the weight of the tablet core is between 8 and 20 percent, and the aperture of the drug release hole on the surface of the drug containing layer of the coating membrane is between 0.2 and 2.0 millimeters. The invention also discloses a method for preparing the controlled-release tablet. After patients take the controlled-release tablet, the blood concentration of the patients is relatively stable; the accumulated drug release rate in 12 hours reaches more than 90 percent, thereby greatly reducing the drug taking frequency of the patients; and the invention provides a novel formulation which is safer, more effective, stable, controllable and convenient for application in clinically treating senile dementia.
Owner:普尔药物科技开发(深圳)有限公司
Huperzine A oral formulation and a preparation method thereof
InactiveCN101732312AImprove complianceFast absorptionOrganic active ingredientsNervous disorderWater insolubleDrug product
The invention relates to a huperzine A oral formulation and a preparation method thereof. The formulation is prepared from huperzine A and officinal auxiliary materials, wherein the officinal auxiliary materials comprise water-insoluble excipient, disintegrant, adhesive and lubricant. The formulation is prepared from the following components by weight percentage: 0.005-1% of huperzine A, 0.1-99% of water-insoluble excipient, 0.1-10% of adhesive, 0.1-80% of disintegrant and 0.1-10% of lubricant. The invention improves the compliance of patients on the medicament and enhances the bioavailability of the medicament.
Owner:武汉朗欧科技有限公司
Method for extracting huperzine A from serrate clubmoss herb
ActiveCN104262251AEasy to operateSuitable for industrial productionOrganic chemistryAcid dissolutionHuperzine U
The invention discloses a method for extracting huperzine A from a serrate clubmoss herb. The method comprises the following steps: (1) pre-treatment; (2) ultrasonic countercurrent extraction: carrying out ultrasonic countercurrent extraction on the serrate clubmoss herb and filtering to obtain leach liquor; (3) membrane separation and purification: centrifuging, and carrying out ultrafiltration and nanofiltration on the leach liquor to obtain trapped fluid; (4) concentration, namely concentrating the trapped fluid into paste; (5) acid dissolution, dissolving the paste by using acid liquid, filtering, and adjusting pH to alkalinity; (6) extraction: extracting by using chloroform and collecting extract liquor; (7) concentration: concentrating the extract liquor to obtain dry powder; (8) crystallization and recrystallization: dissolving to obtain the dry powder, crystallizing and recrystallizing to obtain white crystals; and (9) drying, namely drying the white crystal to obtain a huperzine A product. According to the method disclosed by the invention, the purity of the huperzine A extracted from the serrate clubmoss herb is greater than or equal to 99%, the product yield is greater than or equal to 0.026%, the final yield is 50-55%, and the yield, yield coefficient and purity of huperzine A is high, the extraction time is short, and the cost is low; therefore, the method for extracting huperzine A from a serrate clubmoss herb is suitable for industrial production.
Owner:HUNAN HUACHENG BIOTECH
Huperzine A high yield strain TCM-01
InactiveCN101914452AIncrease productionLow costFungiMicroorganism based processesBiotechnologyA huperzine
The invention relates to a huperzine A high yield strain TCM-01 of which classified nomenclature is Endophyte sp.TCM-01, conservation address is the China center for type culture collection, conservation date is April 16, 2010 and CCTCC NO is M2010086. The high yield strain uses Penicillium chrysogenum Thom as parent strain and is prepared through microwave mutagenesis-screening. The invention further optimizes the condition of fermentation culture of the strain; and under the condition of culture, the yield of huperzine A is up to 4.761mu g / ml. Under the same condition of fermentation, the yield of the product is greatly increased, and the cost can be largely saved.
Owner:洪亚辉 +1
Huperzine A preparation for treating schizophrenia and nervous function damage and preparation method thereof
InactiveCN101804038AImprove bioavailabilitySmooth releaseOrganic active ingredientsNervous disorderApoptosisSolvent
The invention relates to a huperzine A preparation for treating schizophrenia and nervous function damage and a preparation method thereof. The preparation method comprises the following steps: using a solvent dispersing method and a solid-liquid phase dispersing and melting method to properly dissolve huperzine A by solvent, preparing stable huperzine A hydrochloride solution, spraying the huperzine A hydrochloride solution in an auxiliary material by dispersion, or preparing solid dispersoid mixture with uniform phase dispersion, crushing, mixing and stirring uniformly, directly adding a lubricating agent for tabletting, also being capable of adding a binding agent, granulating, drying, adding the lubricating agent, and tabletting. The phase-dispersing mixture of huperzine A also can be added with the lubricating agent with proper amount for mixing, and then filled into a capsule. The preparation is used for treating the symptoms of all the periods of schizophrenia, and has the advantages of having the effects of protecting oxidative stress toxicity of nerve cells generated by resisting Beta amyloid peptide and inducing cell apoptosis, promoting the growth of nerve growth factor, being effective for treating inflammation in cerebral hemorrhage and inflammation of cerebral ischemic injury, having long action time of drugs, being convenient to application of oral administration / containing administration and the like. The invention has high content of huperzine A preparation, stable preparation quality and good bioavailability and can improve the medication compliance of patients with schizophrenia and senile dementia.
Owner:赵守明 +1
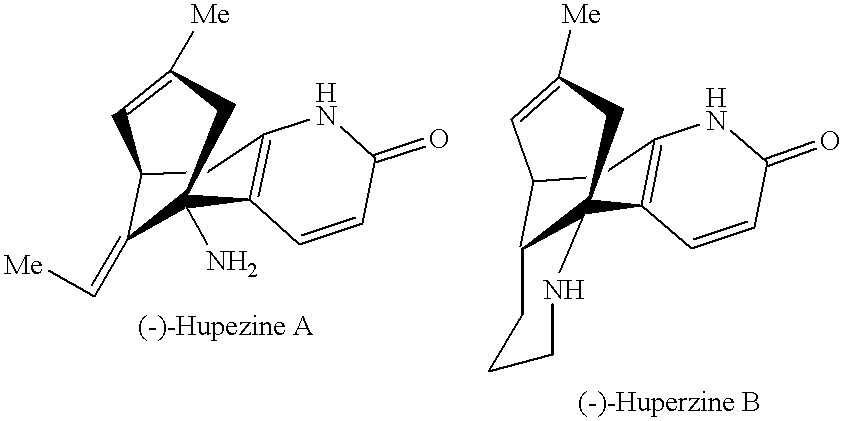
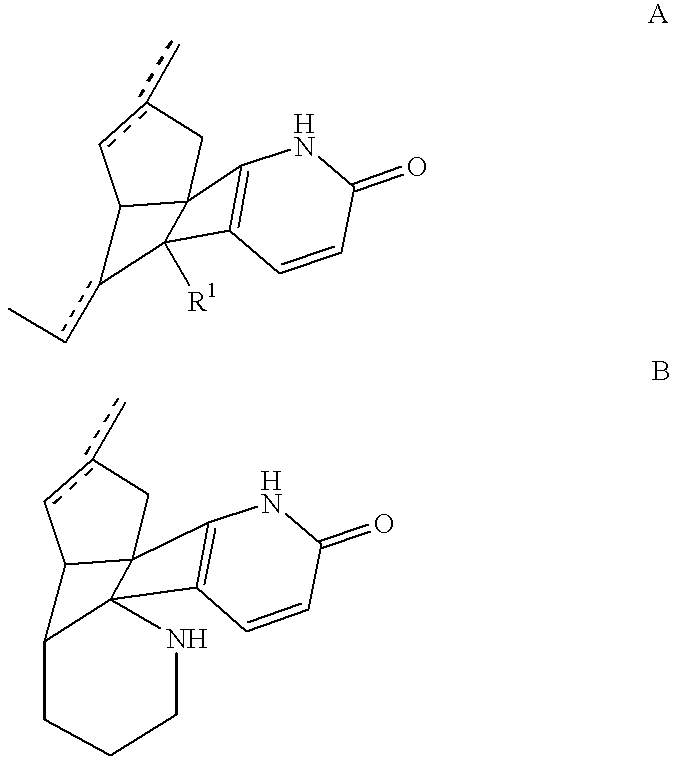
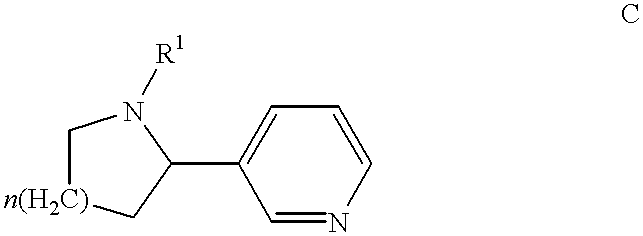
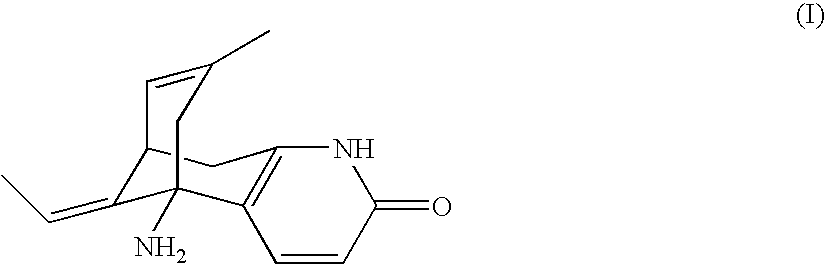
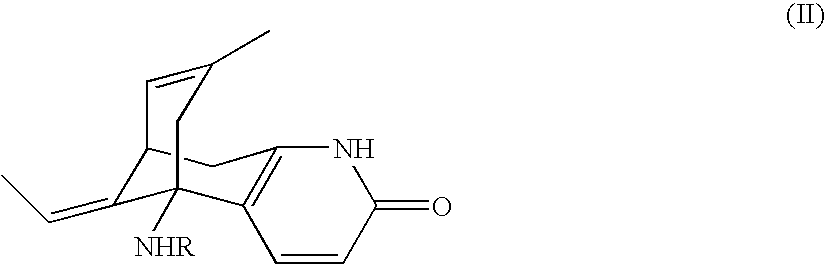
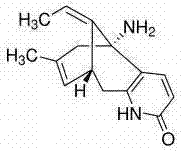
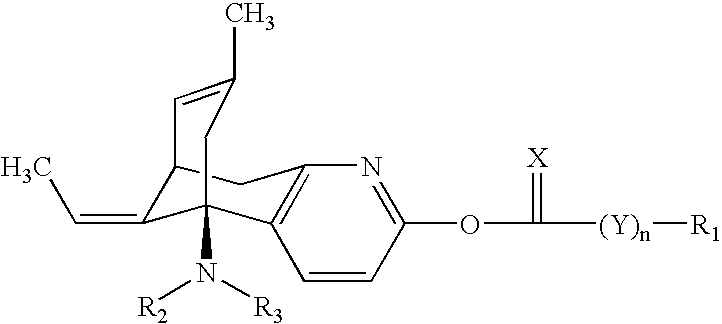
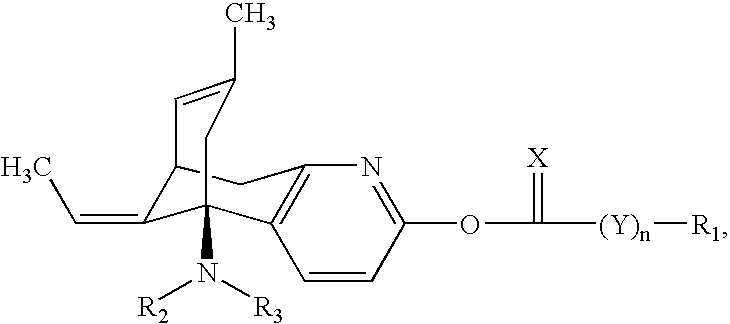
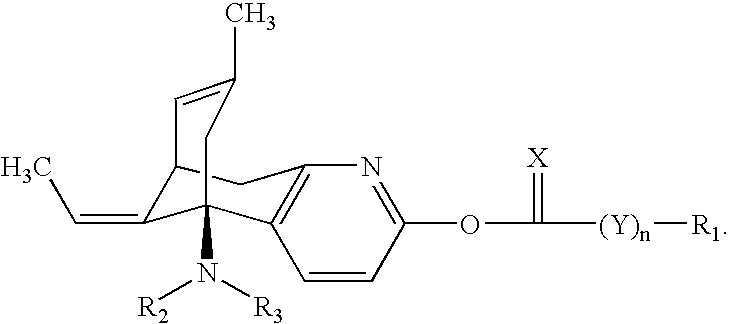
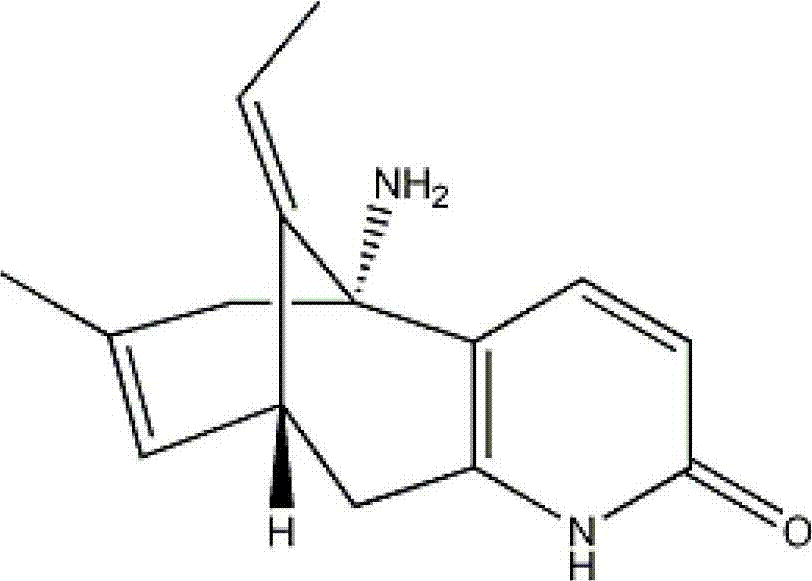
Huperzine a compound for treatment of Alzheimer's disease
A huperzine A compound is provided. The huperzine A compound has following formula: wherein X comprises O or S, Y comprises —O—, —S—, —CH(R4)—, —C(R4)(R5)—, —C(R4)═C(R5)—, —C≡C—, —NH— or —N(R4)—, n is 0, 1 or 2, R3 is C(═X)—(Y)n-R1 provided that R2 is H or R2 and R3 are combined to form ═CH—Ar, wherein R1, R4 and R5 independently comprise hydrogen, C1-C32 alkyl, C1-C32 alkenyl, C1-C32 alkynyl, C1-C32 aryl or C1-C32 heteroaryl, in which alkyl, alkenyl, alkynyl, aryl or heteroaryl with one or more substituents comprising halogen, hydroxyl, alkoxy, aryloxyl, amino, alkylamino, arylamino, dialkylamino, diarylamino, imino, alkylimino, arylimino, acylamido, diacylamido, acylimido, cyano, nitro, mercapto, carbamido, carbamoyl, carboxyl, thioureido, thiocyanato, sulfonamido, thio, sulfonyl or sulfinyl, and Ar comprises aryl or heteroaryl.
Owner:IND TECH RES INST
Huperzine A tablet and preparation method thereof
InactiveCN104352467AHigh dissolution rateImprove bioavailabilityOrganic active ingredientsNervous disorderFiller ExcipientAdhesive
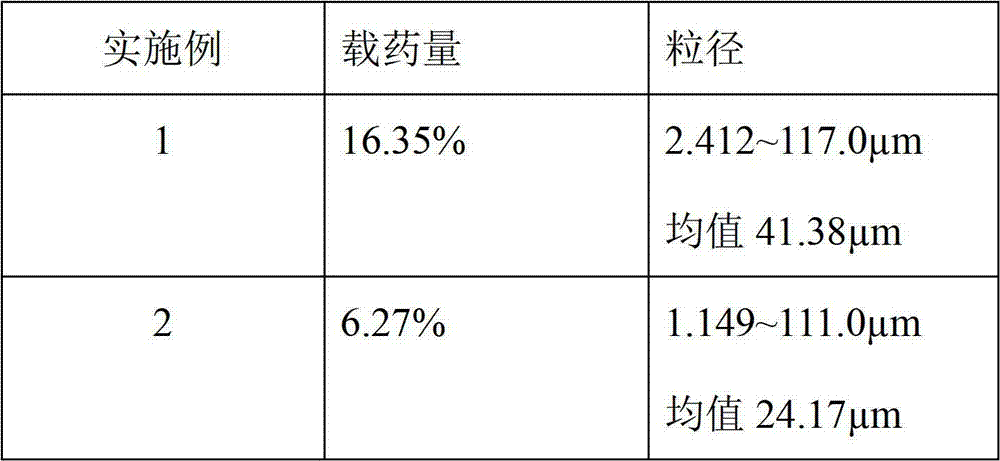
The invention belongs to the technical field of drug preparation, and particularly relates to a huperzine A tablet and a preparation method thereof. The huperzine A tablet comprises the following components in parts by mass: 0.5 parts of huperzine A, 500-700 parts of a filler, 5-15 parts of an adhesive, 20-50 parts of a disintegrating agent, and 1-5 parts of a lubricant, wherein the average particle size of huperzine A is controlled at 110-120 mu m. Compared with the prior art, by carrying out micronization treatment on raw materials, the particle size of huperzine A is controlled at 110-120 mu m, so that the disintegrating speed of the tablet can be increased, and the dissolution of huperzine A can be improved, thereby improving the bioavailability and stability of the huperzine A tablet.
Owner:CISEN PHARMA
Huperzine osmotic-pump controlled release tablet
InactiveCN102512395AProlong the action timeStable effective therapeutic concentrationOrganic active ingredientsNervous disorderCellulose acetateSuspending Agents
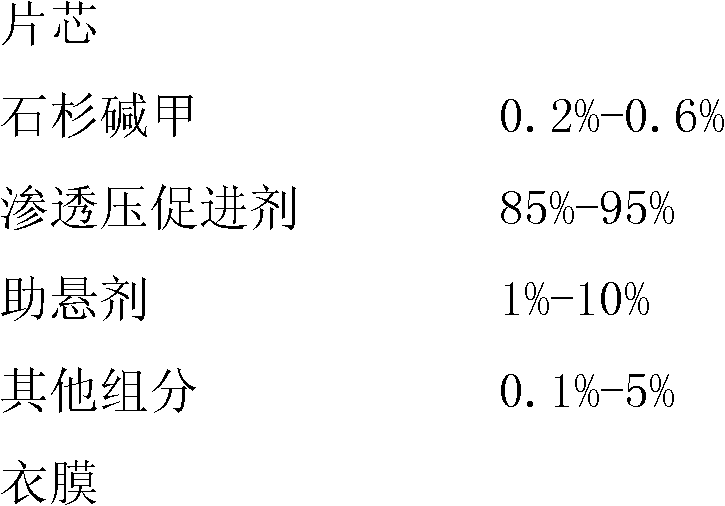
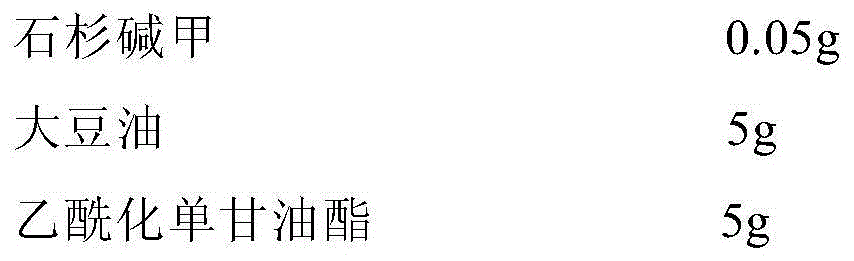
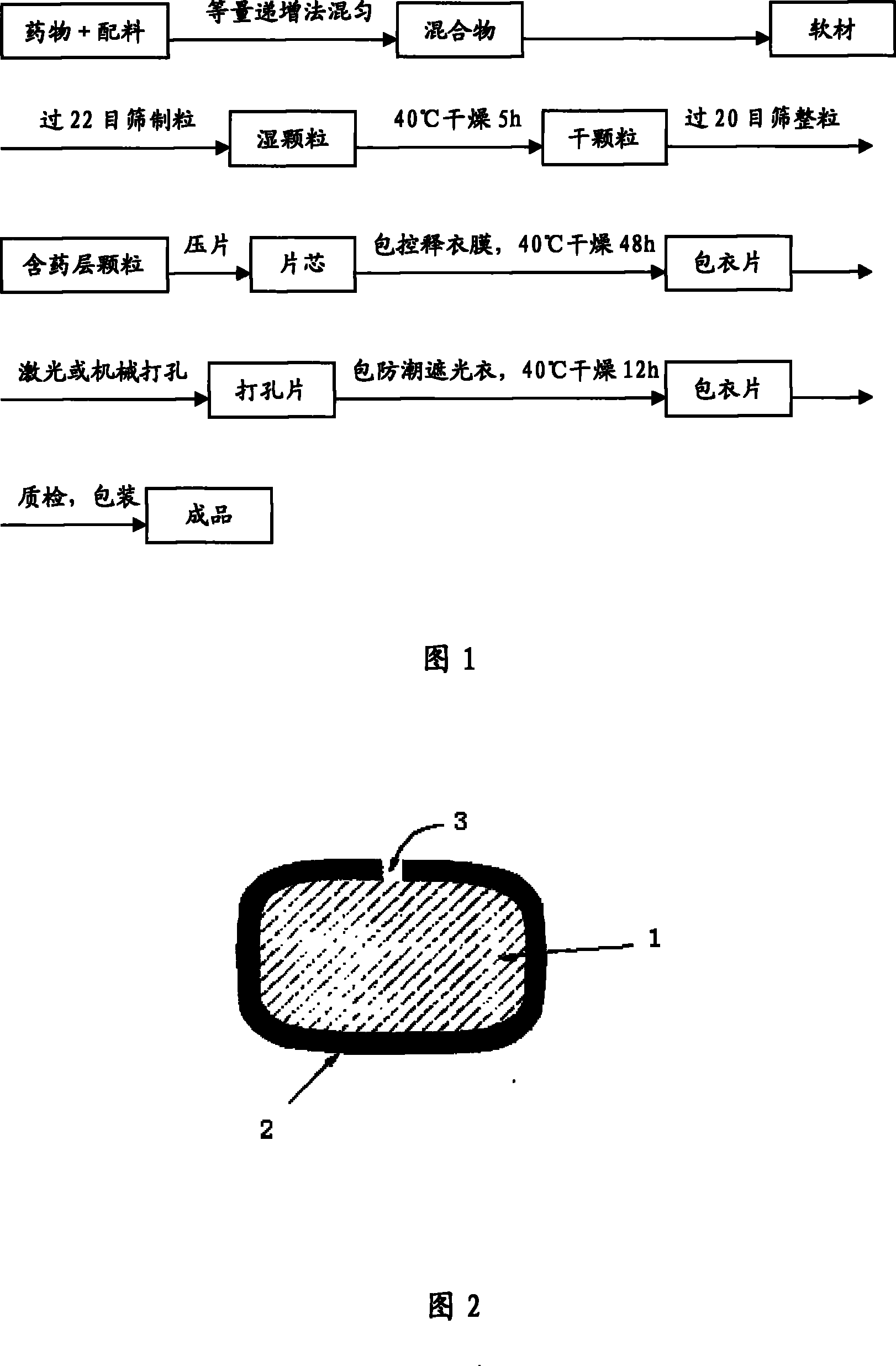
The invention relates to a huperzine osmotic-pump controlled release tablet, which is composed of tablet core containing huperzine and semipermeable film coating with small holes. The huperzine osmotic-pump controlled release preparation is characterized in that the preparation comprises the following components by the weight percent: 0.2-0.6 percent of huperzine in the tablet core, 85-95 percent of osmotic pressure accelerating agent, 1-10 percent of suspending agent and o.1-5 percent of other components. A coating film contains cellulose acetate, PEG (polyethyleneglycol) 4000 and a plasticizing agent, and the proportion of the cellulose acetate, the PEG4000 and the plasticizing agent is 1: (0.3-0.4) : (0.1-0.2). The weight of the coating is increased by 11-13 percent.
Owner:北京振东生物科技有限公司
Huperzine-a lipid micro-sphere preparation
InactiveCN104958267AImprove solubilityImprove stabilityOrganic active ingredientsPowder deliveryLipid formationIrritation
The invention discloses a huperzine-a lipid micro-sphere preparation. The huperzine-a lipid micro-sphere preparation is mainly made of huperzine-a, oil for injection, emulsifiers and isotonic agents. The huperzine-a lipid micro-sphere preparation has the advantages that perfect treatment effects can be realized, problems of insolubilization of huperzine-a in water and adverse reaction under high-dosage conditions can be solved, the stability of medicine can be improved, irritation and toxic reaction can be reduced, the quality of preparation products can be improved, and the huperzine-a lipid micro-sphere preparation is applicable to industrial large-scale production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Huperzine-A solid dispersion and method for preparing tablet thereof
InactiveCN101697960AGood water solubilityHigh dissolution ratePowder deliveryOrganic active ingredientsSolubilityPolymer science
The invention discloses a huperzine-A solid dispersion and a method for preparing a tablet thereof. The huperzine-A solid dispersion comprises huperzine-A and a carrier used as a dispersant; the weight ratio range of the carrier to the huperzine-A is 1-25:1; the carrier material may be one or more of polyvinylpyrrolidone, polyethylene glycol 4000, polyethylene glycol 6000, Poloxamer 188, mannitol, lactose or microcrystalline cellulose; and the huperzine-A solid dispersion is prepared by adopting a grinding method, and the obtained solid dispersion can be prepared into huperzine-A tablets by adopting a wet granulating and tabletting method. The huperzine-A solid dispersion can remarkably increase the solubility of a medicament and promote the absorption so as to greatly improve the dissolution and bioavailability of the medicament.
Owner:HENAN TALOPH PHARMACEUTICAL STOCK CO LTD
Huperzine A mono-layer osmotic pump controlled release tablets
InactiveCN101485640BStable release rateConstant plasma concentrationOrganic active ingredientsNervous disorderDrug release rateCellulose acetate
The invention discloses a huperzine A single-layer osmotic pump controlled-release tablet. The controlled-release tablet comprises a tablet core containing huperzine A and pharmaceutic adjuvants in effective dosage, and a semipermeable coating membrane coating the tablet core, wherein the coating membrane is provided with a drug release hole. The controlled-release tablet is characterized in that: the tablet core contains the following components in percentage by weight: 0.01 to 1.0 percent of huperzine A, 1.0 to 10 percent of retarder and 1.0 to 40 percent of permeation enhancer; and the weight ratio of polyethylene glycol 6,000 contained in the coating liquid to cellulose acetate is (0.05-0.25):1, and the weight gain of the coating membrane against the weight of the tablet core is between 5 and 25 percent. The controlled-release tablet has the advantages that the drug release rate is stable, the blood consentration of patients is relatively constant after the patients take the controlled-release tablet, and the drug taking frequency of the patients is reduced, so that the controlled-release tablet is a novel drug formulation which is safer, more effective, stable, controllable and convenient for application in clinically treating senile dementia.
Owner:普尔药物科技开发(深圳)有限公司
Huperzine A solid composition and preparation method thereof
ActiveCN101721416ASolve the problem of easy exceeding the standardQuality improvementOrganic active ingredientsNervous disorderFiller ExcipientA huperzine
The invention provides a huperzine A solid composition, which comprises huperzine A, filler, a pH regulator and a lubricant. The invention also provides a method for preparing the composition, which comprises the following steps: dissolving the pH regulator in water; adding the huperzine A into the pH regulator solution; mixing and stirring the solution and the filler after the huperzine A is dissolved; pelletizing, drying and granulating the mixture; mixing the grains and the lubricant; and filling the grains into capsules or tabletting the grains.
Owner:YAOPHARMA CO LTD
Huperzine dropping pills and preparation method thereof
InactiveCN101176729ADisintegrates quicklyHigh dissolution rateOrganic active ingredientsNervous disorderSolventA huperzine
The invention relates to a huperzine A dropping pill and a preparation method, which is characterized in that: the huperzine A is dissolved through proper solvent and then added into the matrix by the dispersion dropping method, is agitated evenly and condensed to be the pill.
Owner:ZHEJIANG WANBANG PHARMA
Mixed endophyte and method for preparing huperzine A by using the endophyte
The present invention provides a mixing endophyte, especially an endophyte separated from plants. The invention also relates to the use of the endophyte. The mixing endophyte of the invention is separated from the plant huperzia serrata. The invention also provides a method of huperzine a prepared by the obtained endophyte. The invention provides a separating and culturing endophyte from the huperzia serrata plant for the first time, a huperzine a compound is extracted from the broth and the mycelium when the mixing endophyte is cultured and fermented. The invention provides a simple path for the compound preparation, having a low cost, the rare plant is not damaged.
Owner:洪亚辉
Huperzine A solid lipid nano particle and preparation method thereof
ActiveCN101658494BImprove stabilityImprove bioavailabilityPowder deliveryOrganic active ingredientsLipid formationOrganic solvent
The invention relates to the field of pharmaceutical preparations, which discloses a huperzine A solid lipid nano particle and a preparation method thereof. The huperzine A solid lipid nano particle is prepared from the following components in weight percent: 0.05-1% of huperzine A, 3-10% of lipid material, 2-10% of emulsifying agent and the balance water. The preparation method adopts a high-pressure even emulsification method to coat the huperzine A into a solid lipid nano particle so as to prepare the huperzine A solid lipid nano particle. The particle diameter of the huperzine A solid lipid nano particle prepared by the invention is 10-100nm, the medicine envelopment rate and the medicine-carrying quantity are high, and the stability is good; simultaneously, the use of an addition agent and an organic solvent which are harmful to a human body is avoided; and the bioavailability is enhanced, the brain targeting property is increased, and the dose and the toxic or side effect are small.
Owner:GUANGDONG PHARMA UNIV
Huperzine A high-producing strain and method for producing huperzine A by fermenting same
Owner:HUNAN AGRICULTURAL UNIV
Huperzine A solid composition and preparation method thereof
ActiveCN101721416BSolve the problem of easy exceeding the standardQuality improvementOrganic active ingredientsNervous disorderFiller ExcipientA huperzine
The invention provides a huperzine A solid composition, which comprises huperzine A, filler, a pH regulator and a lubricant. The invention also provides a method for preparing the composition, which comprises the following steps: dissolving the pH regulator in water; adding the huperzine A into the pH regulator solution; mixing and stirring the solution and the filler after the huperzine A is dissolved; pelletizing, drying and granulating the mixture; mixing the grains and the lubricant; and filling the grains into capsules or tabletting the grains.
Owner:YAOPHARMA CO LTD
Huperzine A polymorphism body, preparation method thereof, pharmaceutical composition comprising polymorphism body and application thereof
The invention discloses a huperzine A polymorphism body, a preparation method thereof, a pharmaceutical composition comprising the polymorphism body and an application thereof. Partial of the huperzine A polymorphism body has better solubility than that of a commercially available medicinal crystal form and is more beneficial to absorption of medicine. Partial of the huperzine A polymorphism body has much lower hygroscopicity than that of the commercially available medicinal crystal form and is more beneficial to preparation and storage of medicine preparations.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
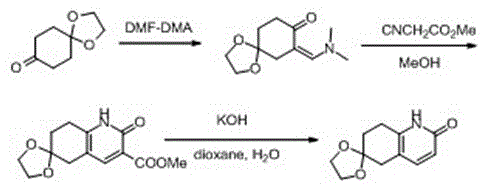
Synthesis method for preparing huperzine A intermediate
The invention discloses a synthesis method for preparing a huperzine A intermediate, which comprises the following steps: carrying out substitution at the alpha site of the carbonylic group by using 1,4-cyclohexyl diketone ethylene glycol and N,N-dimethylformamide dimethyl acetal as raw materials to obtain a substitution product, cyclizing the substitution product under the action of methyl cyanoacetate to obtain a cyclization product, and decarboxylating the cyclization product under the action of a strong base to obtain 2-carbonyl-6-ethylene ketal-5,7,8-dihydro-quinoline. The method has the advantages of fewer synthesis reaction steps, mild reaction conditions, low requirements for operating personnel and low environmental pollution, greatly lowers the raw material cost, and is simple to operate and convenient for refinement; and the obtained 2-carbonyl-6-ethylene ketal-5,7,8-dihydro-quinoline has high quality and high yield.
Owner:ZHEJIANG WANBANG PHARMA
Huperzine-A particle long-acting injection and preparation method thereof
ActiveCN103505413BReduce voidsSlow drug releaseOrganic active ingredientsNervous disorderSide effectBlood concentration
The invention discloses a huperzine-A particle long-acting injection and a preparation method thereof. The huperzine-A particle long-acting injection is composed of huperzine-A and a biodegradable polymer, wherein the percentage by weight of huperzine-A is 9-30%. An emulsification step is not required in the preparation method disclosed by the invention, and the preparation method is simple in preparation process and low in requirements on equipment. The prepared huperzine-A microspheres have not obvious burst release effect and can release medicine for 30 days, thus avoiding the side effects caused by a blood concentration peak-valley phenomenon occurring in case of multiple dosing; a curative effect can be kept for half a month to a month by one-time dosing, thus being beneficial to treatment for patients with dementia; moreover, the huperzine-A microspheres has the characteristic of convenience in dosing.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
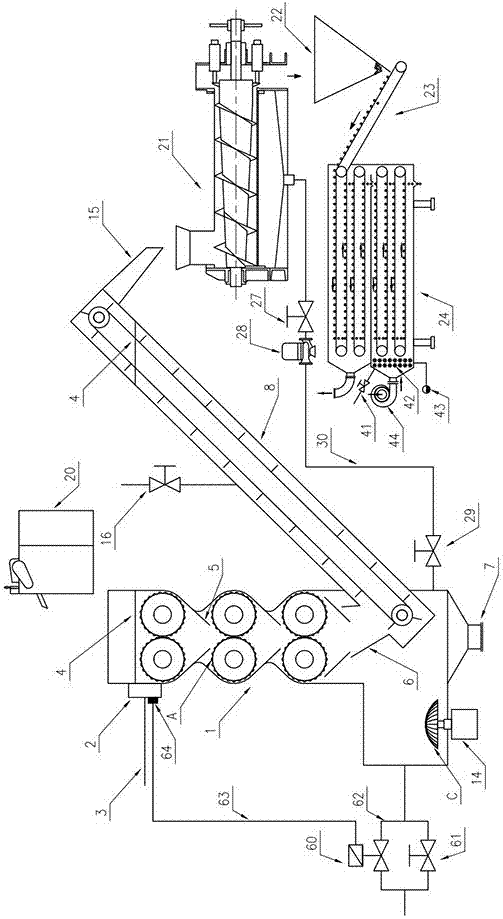
Mechanical ultrasonic type huperzine A leaching device with cutting machine and drying machine
InactiveCN107362574AContinuous and reliable biteExtrusion compression ratio is largeOrganic chemistryCounter-current extractionAutomatic controlThree stage
The invention discloses a mechanical ultrasonic type huperzine A leaching device with a cutting machine and a drying machine. The cutting machine is arranged above a machine body, the machine body is internally provided with three stages of contrarotating spline rollers, a contrarotating spline roller surface is a continuous spline plane, a huperzine serrate guiding slot is formed below each stage of contrarotating spline roller, the final stage of guiding slot is connected to a hopper of a huperzine serrate elevator, and a discharging slot of the elevator is connected to the drying machine through a spiral extrusion dehydrating machine and a slag hopper; the elevator is provided with a liquid inlet valve, and the upper part of the machine body is provided with a filter and a liquid discharge pipe; a mechanical ultrasonic vibration exciter is arranged at the left lower corner of the machine body; and one side of the machine body is connected to an automatic control steam valve and a manual steam valve through steam pipelines. The three stages of contrarotating spline rollers and the mechanical ultrasonic vibration exciter are of distinct characteristics respectively, leaching and extraction of huperzine A can be promoted cooperatively, the device has working principles and technical effects hardly unforeseeable for technical persons in the field, and the device has relatively high practical application values.
Owner:田兴云
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com