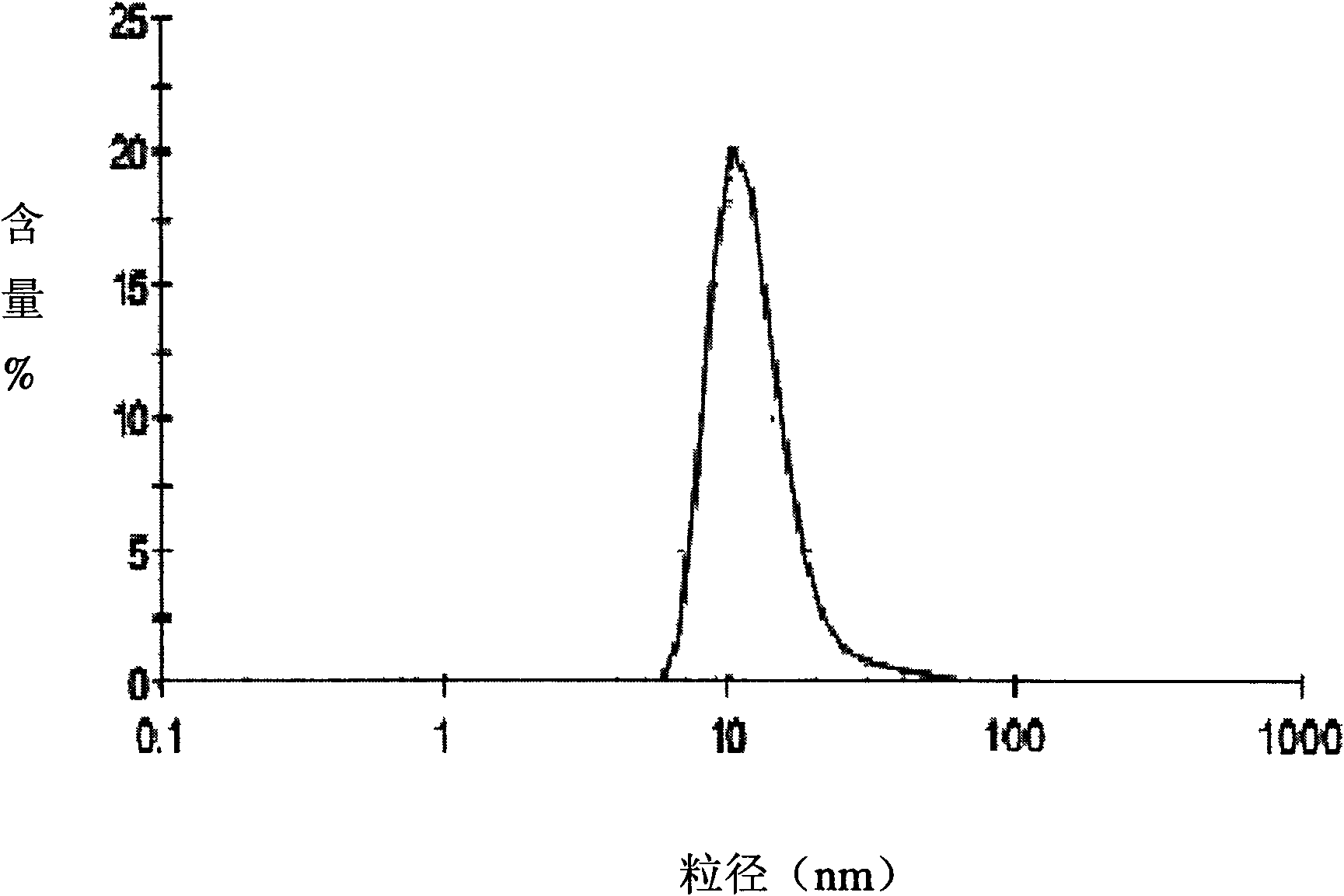
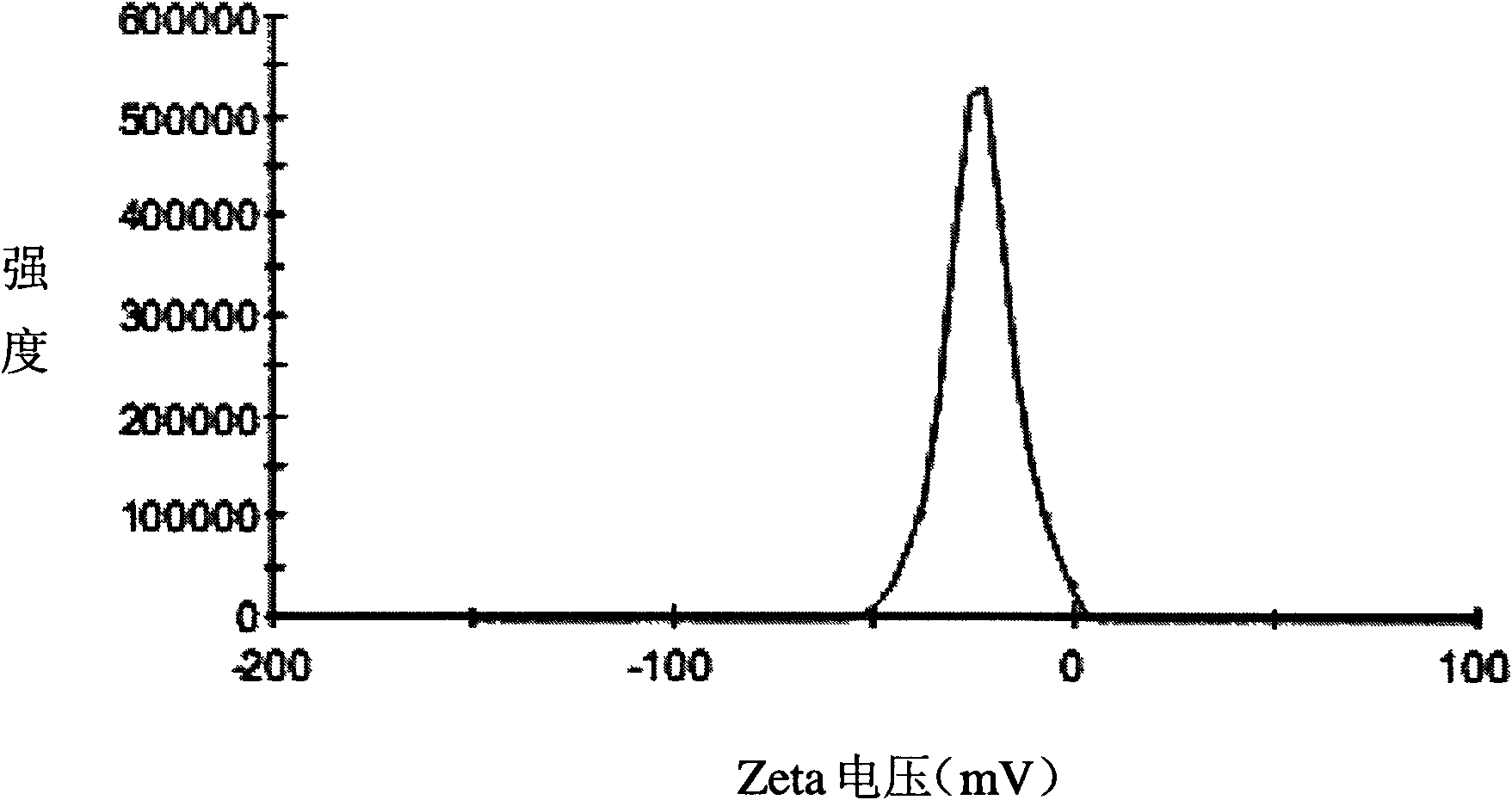
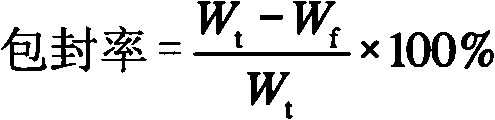Huperzine A solid lipid nano particle and preparation method thereof
A technology of solid lipid nano and huperzine A, which is applied in the direction of pharmaceutical formula, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of solid lipid nano Particles, restrictions on the application of SLN pharmaceutical carriers, etc., to achieve the effects of increasing brain targeting, improving bioavailability, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Prepare huperzine A solid lipid nanoparticles (the percentage in the prescription is the weight percentage that this component occupies in the whole prescription, and the following embodiments are identical):
[0043] Prescription: Huperzine A 30mg
[0044] Glyceryl monostearate 1.5g
[0045] Poloxamer 188 0.5g
[0046] Tween-80 0.5g
[0047] Distilled water 50mL
[0048] Preparation:
[0049] Step 1: Weigh Poloxamer 188 and Tween-80, add appropriate amount of distilled water and ultrasonically disperse until completely dissolved to form an aqueous phase; mix and melt huperzine A and glyceryl monostearate to form an oil phase;
[0050] Step 2: Heat the water phase and the oil phase in water baths to 65°C respectively. After the oil phase is completely dissolved into a clear hot body, add the water phase to the oil phase dropwise under constant temperature magnetic stirring conditions. The stirring speed is 1500rpm, and keep stirring at a cons...
Embodiment 2
[0054] Preparation of huperzine A solid lipid nanoparticles:
[0055] Prescription: Huperzine A 80mg
[0056] Glyceryl monostearate 2g
[0057] Poloxamer 188 1g
[0058] Egg yolk phospholipids 0.5g
[0059] Distilled water 50mL
[0060] Step 1: Weigh poloxamer 188 and egg yolk phospholipid, add appropriate amount of distilled water and ultrasonically disperse until completely dissolved to form an aqueous phase; mix and melt huperzine A and glyceryl monostearate to form an oil phase;
[0061] Step 2: Heat the water phase and the oil phase in a water bath to 85°C. After the oil phase is completely dissolved into a clear hot body, add the water phase to the oil phase dropwise under the condition of constant temperature magnetic stirring. The stirring speed is 800rpm, and the constant temperature is maintained. Stir for 30 minutes; then remove the water bath and continue to stir to room temperature, making it a milky white emulsion and forming colostrum...
Embodiment 3
[0065] Preparation of huperzine A solid lipid nanoparticles:
[0066] Prescription: Huperzine A 180mg
[0067] Glyceryl monostearate 3g
[0068] Poloxamer 188 1.5g
[0069] Tween-80 1.5g
[0070] Distilled water 50mL
[0071] Preparation:
[0072] Step 1: Weigh Poloxamer 188 and Tween-80, add appropriate amount of distilled water and ultrasonically disperse until completely dissolved to form an aqueous phase; mix and melt huperzine A and glyceryl monostearate to form an oil phase;
[0073] Step 2: Heat the water phase and the oil phase to 70°C in a water bath, and after the oil phase is completely dissolved into a clear hot body, add the water phase to the oil phase dropwise under the condition of constant temperature magnetic stirring, the stirring speed is 1000rpm, and the constant temperature is maintained Stir for 18 minutes; then remove the water bath and continue stirring to room temperature, making it a milky white emulsion and forming colos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com