Huperzine a lyotropic liquid crystal preparation and preparation method thereof
A technology of huperzine A and lyotropic liquid crystal, which is applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. , the effect of slowing down the speed and reducing the frequency of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The present invention also provides a preparation method of the huperzine A lyotropic liquid crystal preparation described in the technical scheme, comprising: mixing the huperzine A, an organic solvent, phospholipid and oil to obtain a huperzine A lyotropic liquid crystal preparation .
[0037] In the present invention, the mixing is preferably: (1) mixing the huperzine A with an organic solvent to obtain a huperzine A solution; (2) mixing the huperzine A solution with the phospholipid and oil .
[0038] In the present invention, the huperzine A is mixed with an organic solvent to obtain a huperzine A solution, and then mixed with phospholipids and oils, which helps the composition to be uniformly dispersed inside when used as a preparation and improves the uniformity of the preparation.
[0039] The present invention has no special requirements on the mixing method of the huperzine A and the organic solvent, and the mixing method known to those skilled in the art can...
Embodiment 1
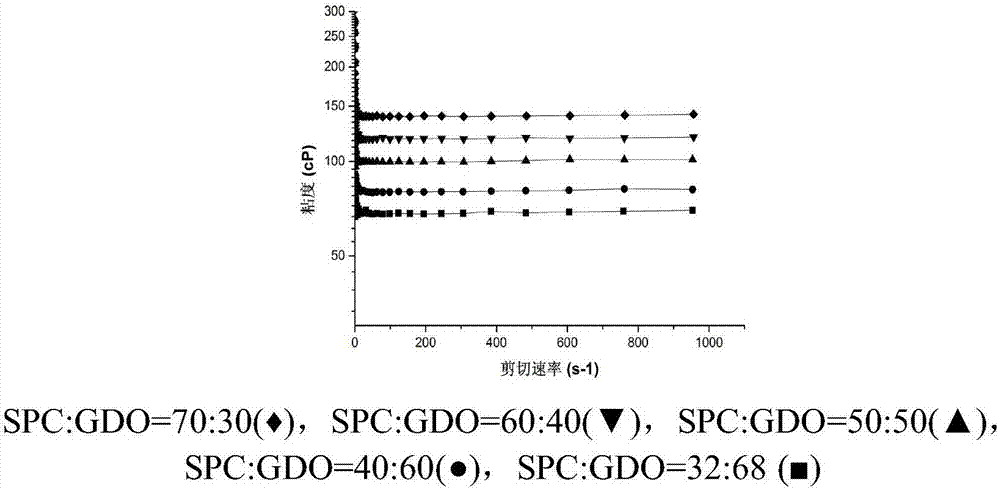
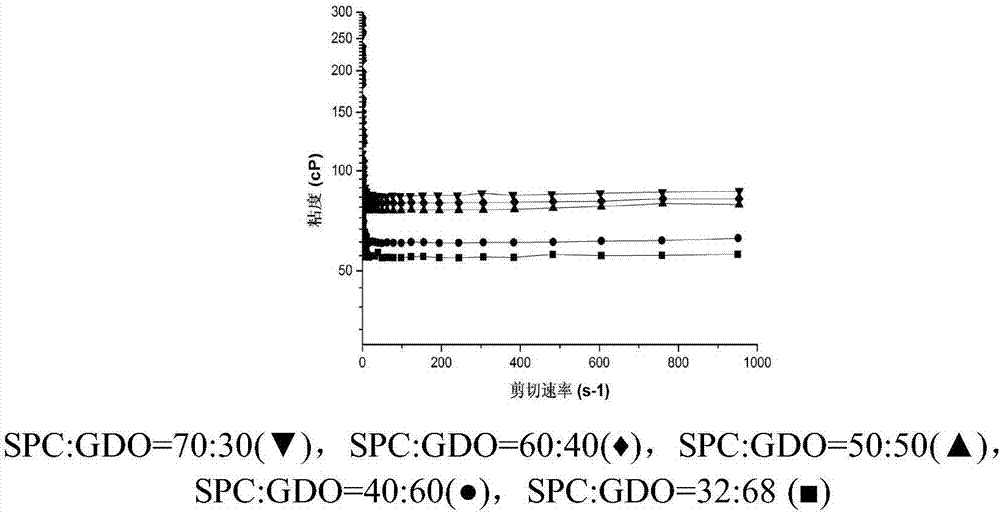
[0045] Weigh 3 mg of huperzine A, 0.075 g of absolute ethanol, 0.2975 g of soybean lecithin and 0.1275 g of glyceryl dioleate.
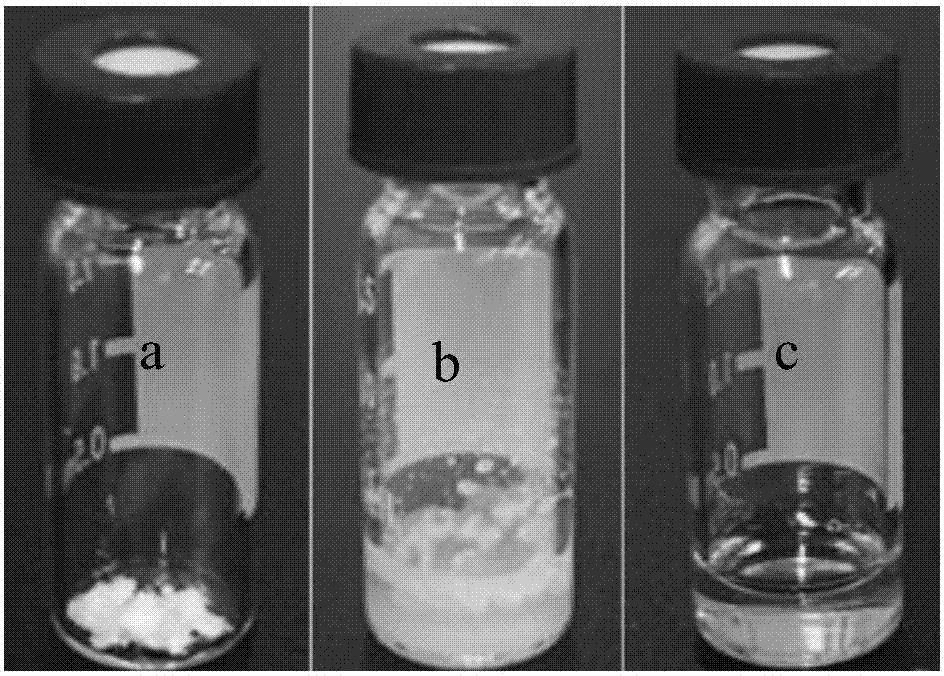
[0046] Above-mentioned huperzine A is placed in the non-transparent glass vial of 5ml black cover, then add 0.075g dehydrated alcohol to dissolve, then add 0.2975g soybean lecithin (SPC) and 0.1275g glyceryl dioleate (GDO) successively, in Wrap the glass vial with aluminum foil at room temperature and rotate it on a rotational rheometer at a speed of 15 rpm for 12 hours to obtain the huperzine A lyotropic liquid crystal preparation.
Embodiment 2
[0048] Prepare huperzine A lyotropic liquid crystal according to the mode of embodiment 1, the difference with embodiment 1 is that the quality of huperzine A is 3mg, the quality of dehydrated alcohol is 0.075g, the quality of soybean lecithin is 0.255g, two The mass of glyceryl oleate is 0.17g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| cumulative release rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com