Synthesis method for preparing huperzine A intermediate
A synthesis method and technology of huperzine A are applied in the field of intermediate synthesis, can solve the problems of large amount of methyl propiolate, harsh synthesis method conditions, and high synthesis reaction cost, and achieve convenient purification, few reaction steps, and memory. Barrier improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
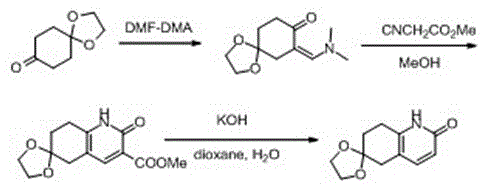
[0024] The preparation process of 7-((dimethylamino)methenyl)-1,4-cyclohexanedione monoethylene glycol ketal is:
[0025] Add the compound 1,4-cyclohexanedione monoethylene ketal (15.6 g, 0.1 mol) and N,N-dimethylformamide dimethyl acetal (50 mL, 0.38 mol) to the In a 100ml three-necked bottle. The reaction solution was stirred at 100°C for 24 hours. The reaction liquid was directly spin-dried to obtain compound 7-((dimethylamino)methenyl)-1,4-cyclohexanedione monoethylene glycol ketal (20 g, yield 95%) as a brown liquid, Used directly in the next step.
[0026] The reaction equation is: .
[0027] 1 H NMR (400 MHz, CDCl 3 ) δ 7.52 (s, 1H), 4.03 – 4.01 (m, 4H), 3.09 (s, 6H), 2.94 (s, 2H), 2.55-2.51 (t, J = 7.0 Hz, 2H), 1.99-1.95 ( t, J = 7.0 Hz, 2H).
Embodiment 2
[0029] The preparation process of 2-carbonyl-6-ethylene ketal-5,7,8-dihydro-3-quinolinecarboxylic acid methyl ester is:
[0030] Mix 7-((dimethylamino)methenyl)-1,4-cyclohexanedione monoethylene glycol ketal (10.6 g, 0.05 mol) and methyl cyanoacetate (24.8 g, 0.25 mol) at room temperature , Methanol (80 mL) was added to a 250-mL three-necked flask, protected by nitrogen. The reaction solution was stirred for 24 hours under reflux conditions. Rotate directly to dry, pass through the column (dichloromethane (v) / methanol (v) = 15:1) to obtain the compound 2-carbonyl-6-ethylene ketal-5,7,8-dihydro-3-quinoline Yellow solid of methyl carboxylate (12 g, 91% yield).
[0031] The reaction equation is: .
[0032] m / z: 266 (M+H) + ; 1 H NMR (400 MHz, CDCl 3 ) δ 7.99 (s, 1H), 4.05-4.04 (m, 4H), 3.92 (s, 3H), 3.02-2.99 (t, J = 6.6 Hz, 2H), 2.81 (s, 2H), 2.00-1.97 ( t, J = 6.6 Hz, 2H).
Embodiment 3
[0034] The preparation method of 2-carbonyl-6-ethylene ketal-5,7,8-dihydro-quinoline:
[0035] Combine 2-carbonyl-6-ethylene ketal-5,7,8-dihydro-3-quinolinecarboxylic acid methyl ester (5 g, 0.019 mol) and potassium hydroxide (3.2 g, 0.057 mol) at room temperature , Dioxane (50 mL) and water (10 mL) were added to a 100 mL three-necked flask, protected by nitrogen. The reaction solution was stirred for 24 hours under reflux conditions. After cooling, ethyl acetate (40 mL) was added to the reaction system. The organic phase was dried with anhydrous sodium sulfate, spin-dried, and passed through a column (dichloromethane (v) / methanol (v) = 15:1) to obtain the compound 2-carbonyl-6-ethylene ketal-5,7,8 -Dihydro-quinoline (3 g, 77% yield) as a white solid.
[0036] The reaction equation is: .
[0037] m / z: 208 (M+H) + ; 1 H NMR (400 MHz, DMSO-d 6 ) δ 11.42 (brs, 1H), 7.12 (d, J = 9.2 Hz, 1H), 6.12 (d, J = 9.2 Hz, 1H), 3.92-3.91 (m, 4H), 2.62-2.57 (m, 4H) , 1.82-1.79 (t, J = 6.6 Hz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com