Huperzine osmotic-pump controlled release tablet
A technology of osmotic pump controlled release and huperzine A, which is applied in the field of medicine, can solve the problems of complex process, difficult to control quality stability, unstable drug release, etc., achieve simple composition and preparation process, prolong drug action time, reduce The effect of the number of doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
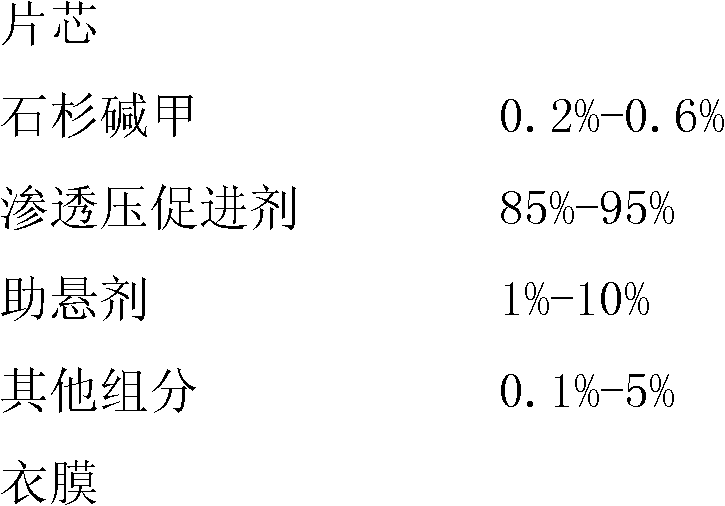
[0038] (1) Prescription
[0039]
[0040]
[0041] (2) Preparation process
[0042] Tablet core preparation: mix huperzine A, sodium chloride, HPMC K4M, magnesium stearate, and colloidal silicon dioxide evenly, press into tablets, φ6 punched tablets, pressure control 40-70N, tablet weight 100mg.
[0043] Coating: Dissolve polyethylene glycol 4000 in 5ml of water, add 95ml of acetone, add cellulose acetate and triethyl citrate, and stir for 4 hours. The tablet cores were coated in a coating pan with a rotating speed of 22rpm and a hot air temperature of 40°C. Up to a coating weight gain of 12%.
[0044]Hole punching: dry the coated tablet at 45°C for 24 hours, take it out, and punch holes with laser (φ=0.6mm).
[0045] (3) Preparation of control drug
[0046] Control drug ①
[0047] Prepare according to CN101485640A pilot test prescription:
[0048]
[0049] Process:
[0050] a. Mix huperzine A and other pharmaceutical excipients according to the method of equal...
Embodiment 2
[0068] (1) Prescription
[0069]
[0070]
[0071] (2) Preparation process
[0072] Tablet core preparation: Mix huperzine A, sodium chloride, and carboxymethyl starch sodium evenly, add appropriate amount of water to make soft material, granulate through a 30-mesh sieve, dry at 60°C, granulate at 30 mesh, add magnesium stearate and mix Uniform, tablet pressing, φ6.0 stamping tablet, pressure control 40-70N, tablet weight 100mg.
[0073] Coating: Dissolve polyethylene glycol 4000 in 5ml of water, add 95ml of acetone, add cellulose acetate and triethyl citrate, and stir for 4 hours. The tablet cores were coated in a coating pan with a rotating speed of 22rpm and a hot air temperature of 40°C. Up to a coating weight gain of 12%.
[0074] Hole punching: dry the coated tablet at 45°C for 24 hours, take it out, and punch holes with laser (φ=0.6mm).
[0075] (3) Determination of release rate
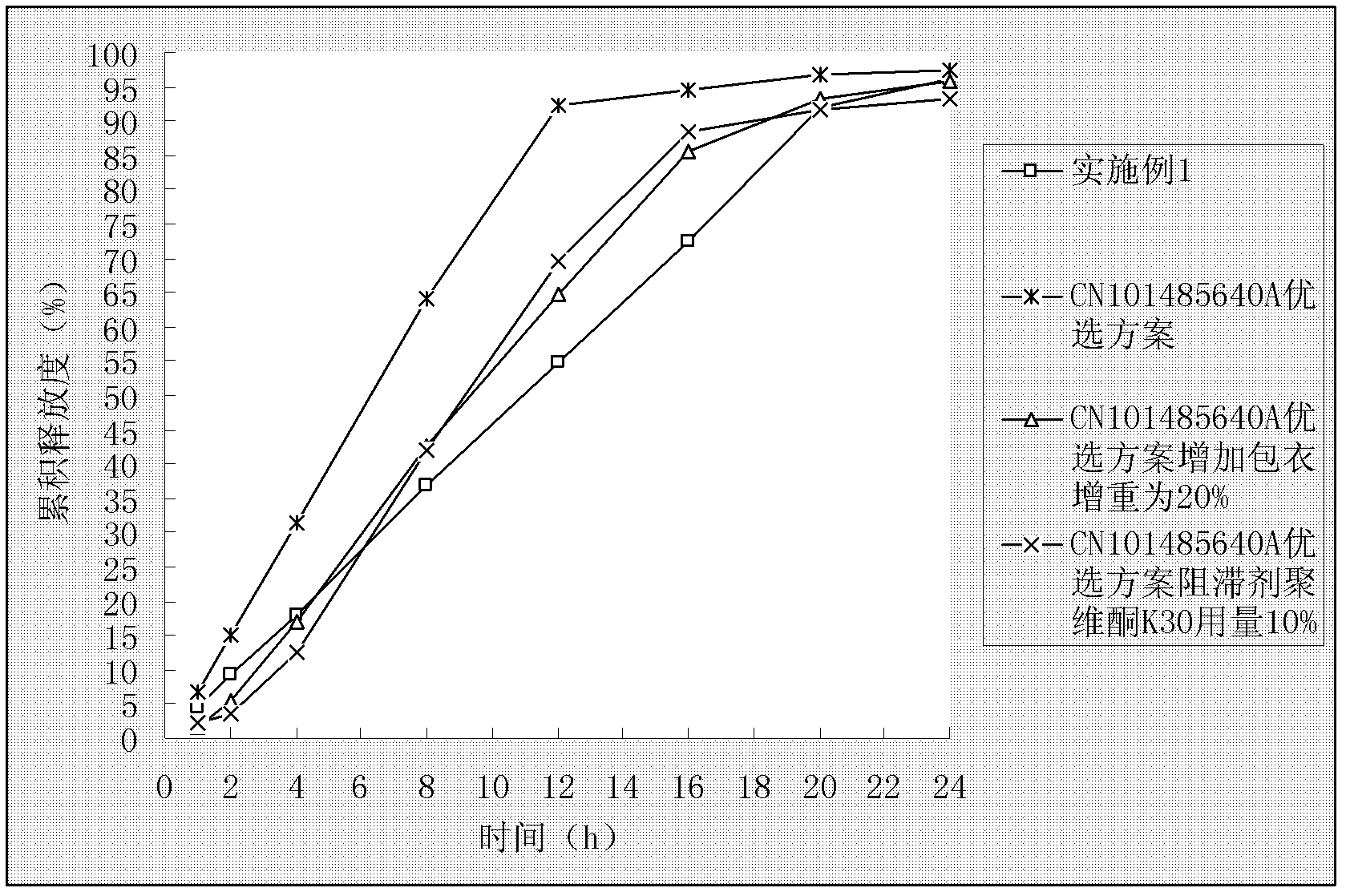
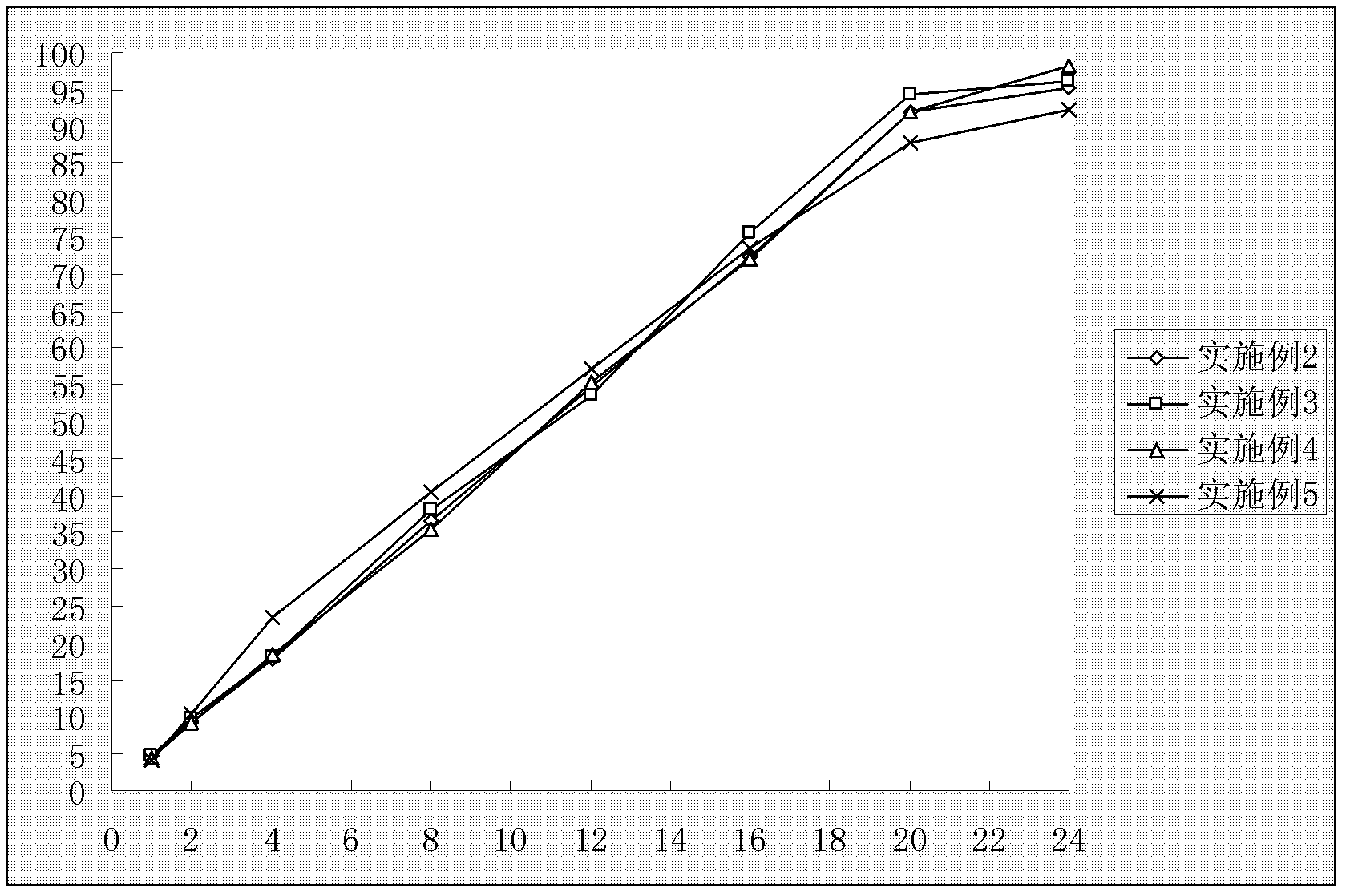
[0076] With embodiment 1, measurement result is as figure 2 shown.
Embodiment 3
[0078] (1) Prescription
[0079]
[0080] (2) Preparation process
[0081] Tablet core preparation: Mix huperzine A, mannitol, and hypromellose evenly, add appropriate amount of water to make soft material, granulate through a 30-mesh sieve, dry at 60°C, granulate at 30 mesh, add magnesium stearate and mix Uniform, tablet pressing, φ6.0 stamping tablet, pressure control 40-70N, tablet weight 100mg.
[0082] Coating: Dissolve polyethylene glycol 4000 in 5ml of water, add 95ml of acetone, add cellulose acetate and methyl phthalate, and stir for 4 hours. The tablet cores were coated in a coating pan with a rotating speed of 22rpm and a hot air temperature of 40°C. The weight gain to the coating was 11%.
[0083] Hole punching: dry the coated tablet at 45°C for 24 hours, take it out, and punch holes with laser (φ=0.6mm).
[0084] (3) Determination of release rate
[0085] With embodiment 1, measurement result is as figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com