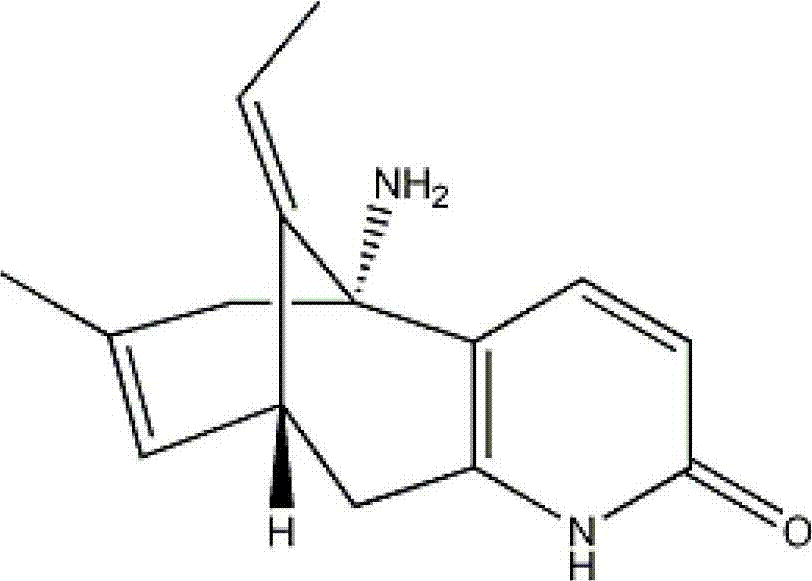
Huperzine-A particle long-acting injection and preparation method thereof
A technology of huperzine A and injection, applied in the field of huperzine A microparticle long-acting injection, can solve problems such as low dose and burst release effect, and achieve the effects of dose control, avoidance of side effects and small gaps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
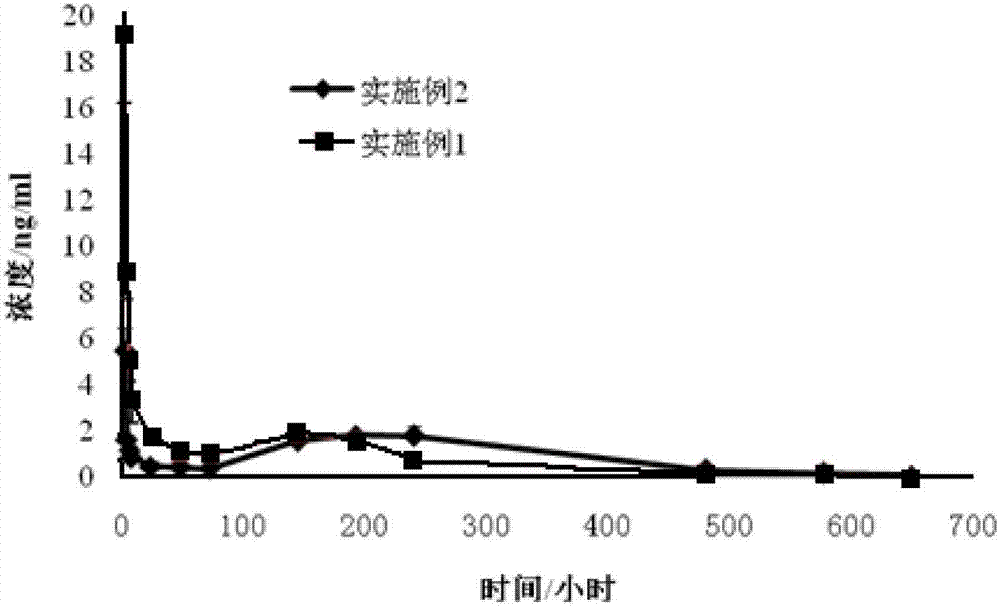
Embodiment 1
[0034] Dissolve 420mg of poly-L-lactic acid in 5ml of acetonitrile, add 180mg of huperzine A, and stir to dissolve.
[0035] Heat to 60°C to gradually evaporate the organic solvent to dryness while stirring to form granules.
[0036] Divide the above granules into 6 parts, add 0.5 g of pure water to each grinding tube, and grind the granules into fine particles with a multifunctional sample homogenizer;
[0037] The rotation speed of the multifunctional sample homogenizer is 5500rpm, the grinding time is 40s, and the grinding time is 4 times with an interval of 30s between each time;
[0038] Centrifuge the formed particles, wash with pure water, collect the particles on the filter membrane under reduced pressure, dry the particles in a vacuum oven at 40°C, and pass through a 120-mesh sieve to obtain long-acting huperzine A for injection with a particle size of less than 120 μm. particle.
Embodiment 2
[0040] Dissolve 546mg of poly-L-lactic acid (poly-L-lactide, PLLA) in 2ml of chloroform, transfer it to a glass mortar, add 54mg of huperzine A to dissolve, keep grinding until the solvent evaporates, and obtain particles with uniform particle size .
[0041] Divide the above granules into 6 parts, add 0.5ml of pure water to each grinding tube, and use a multifunctional sample homogenizer to grind the granules into fine particles;
[0042] The rotation speed of the multifunctional sample homogenizer is 6500rpm, the grinding time is 30s, and the grinding time is 3 times, with an interval of 25s between each time, and repeated 3 times;
[0043] Other operations are the same as Example 1. Long-acting microparticles for huperzine A injection with a particle size of less than 120 μm are obtained.
Embodiment 3
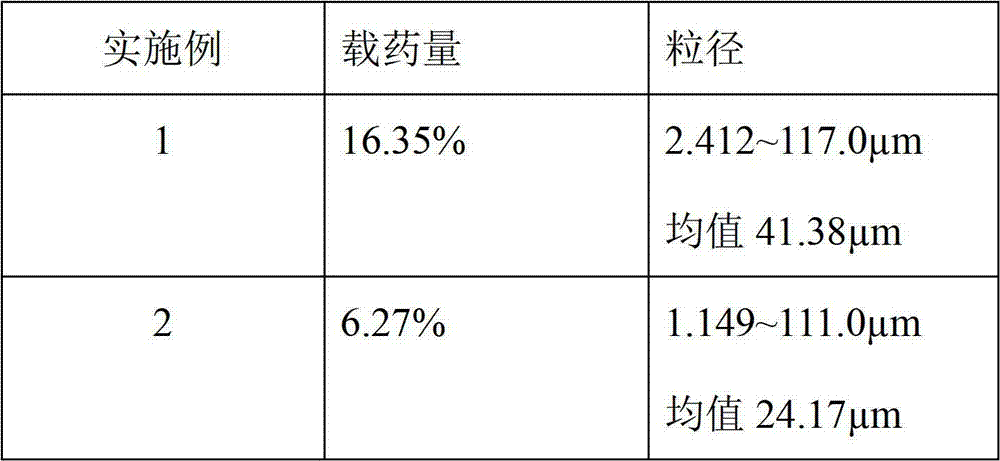
[0045] The drug loading and particle size of Huperzine A microparticles are as follows:
[0046]
[0047] Drug loading detection method:
[0048] The drug loading refers to the weight percentage of the drug contained in the microparticles.
[0049] Weigh huperzine A microparticles, add 50 times the weight of huperzine A microparticles for ultrasonic dissolution in chloroform, dilute with methanol to the final measured concentration, shake well, centrifuge, take the supernatant to determine huperzine A by HPLC method concentration to calculate the drug loading.
[0050] Particle size detection method: Take the huperzine A microparticle suspension, and measure its particle size and distribution with a LS-230 laser scattering particle size analyzer.
[0051] The mixing ratio of the suspension is as follows: 1% of long-acting microparticles for huperzine A injection, 99% of normal saline containing Tween 80, and 0.1% by weight of Tween 80 in the normal saline.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com