Huperzine A mono-layer osmotic pump controlled release tablets
A single-layer osmotic pump and huperzine A technology, applied in the field of medicines containing huperzine A, can solve problems such as pain, fast drug release, complicated preparation process, etc., achieve stable drug release rate, and reduce the number of medications , The effect of constant blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
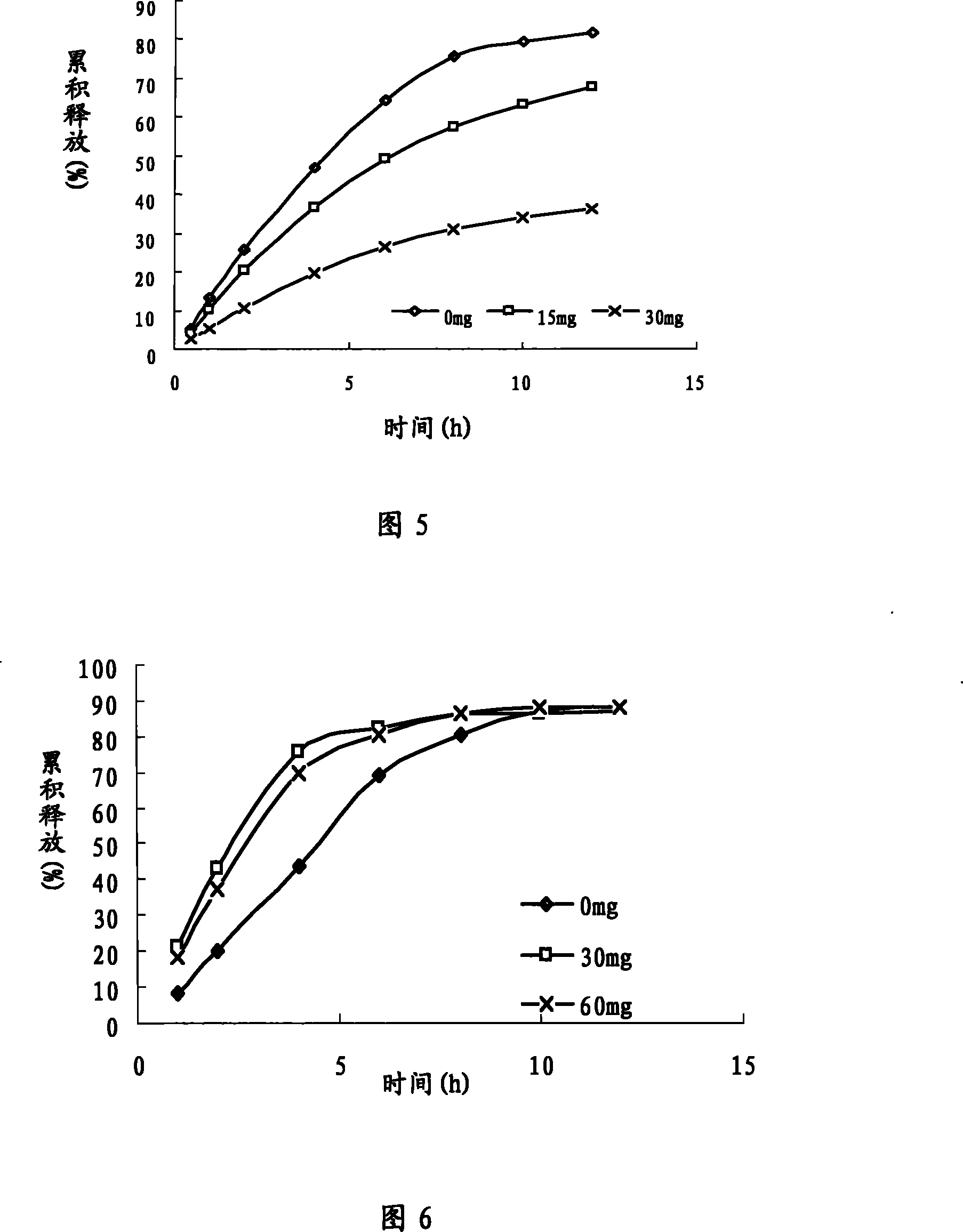
[0109] prescription:
[0110] Drug-containing layer of tablet core Huperzine A 0.2g
[0111] Yellow iron oxide 0.5g
[0113] Mannitol 127.5g
[0114] Povidone K30 10.0g
[0115] Magnesium Stearate Appropriate amount
[0116] 150.0mg per tablet
[0117] A total of 1000 pieces
[0118] Coating liquid cellulose acetate 25.0g
[0119] Polyethylene glycol 4.125g
[0120] Acetone 850mL
[0121] Ethanol 150mL
[0122] Preparation Process:
[0123] Including the following steps:
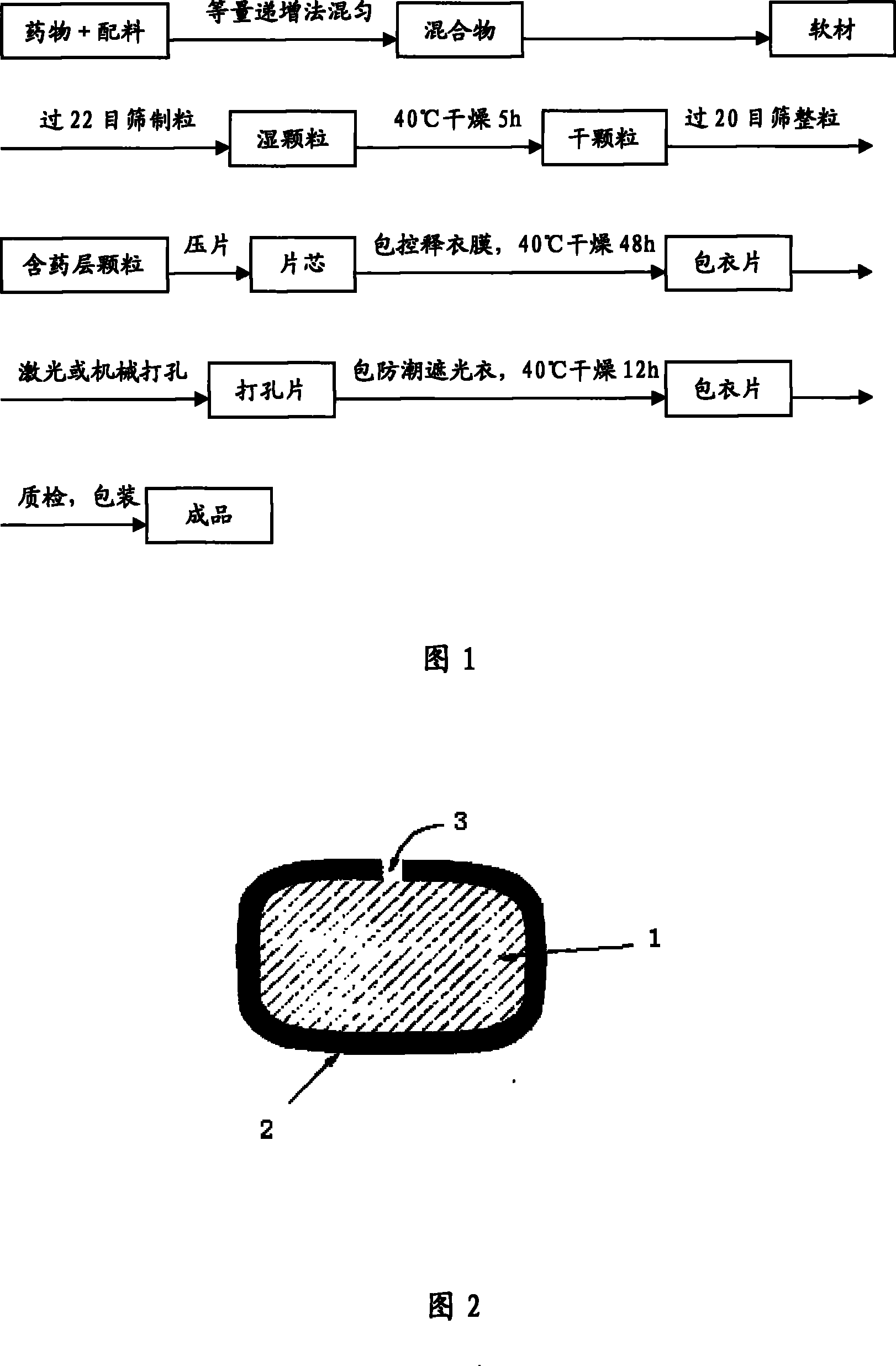
[0124] a. Mix huperzine A and other pharmaceutical excipients according to the method of equal increase to make a mixture;
[0125]b. Mix the mixture with 10% povidone K30 ethanol solution to make a soft material, the amount of the added ethanol solution is to make the soft mater...
Embodiment 2
[0131] prescription:
[0132] Drug-containing layer of tablet core Huperzine A 0.015g
[0133] Yellow iron oxide 0.5g
[0135] Lactose 75.96g
[0136] Povidone K30 12.0g
[0137] Magnesium stearate 1.525g
[0138] 150.0mg per tablet
[0139] A total of 1000 pieces
[0140] Coating liquid cellulose acetate 25.0g
[0141] Polyethylene glycol 1.25g
[0142] Acetone 850mL
[0143] Ethanol 150mL
[0144] Preparation process: with embodiment 1.
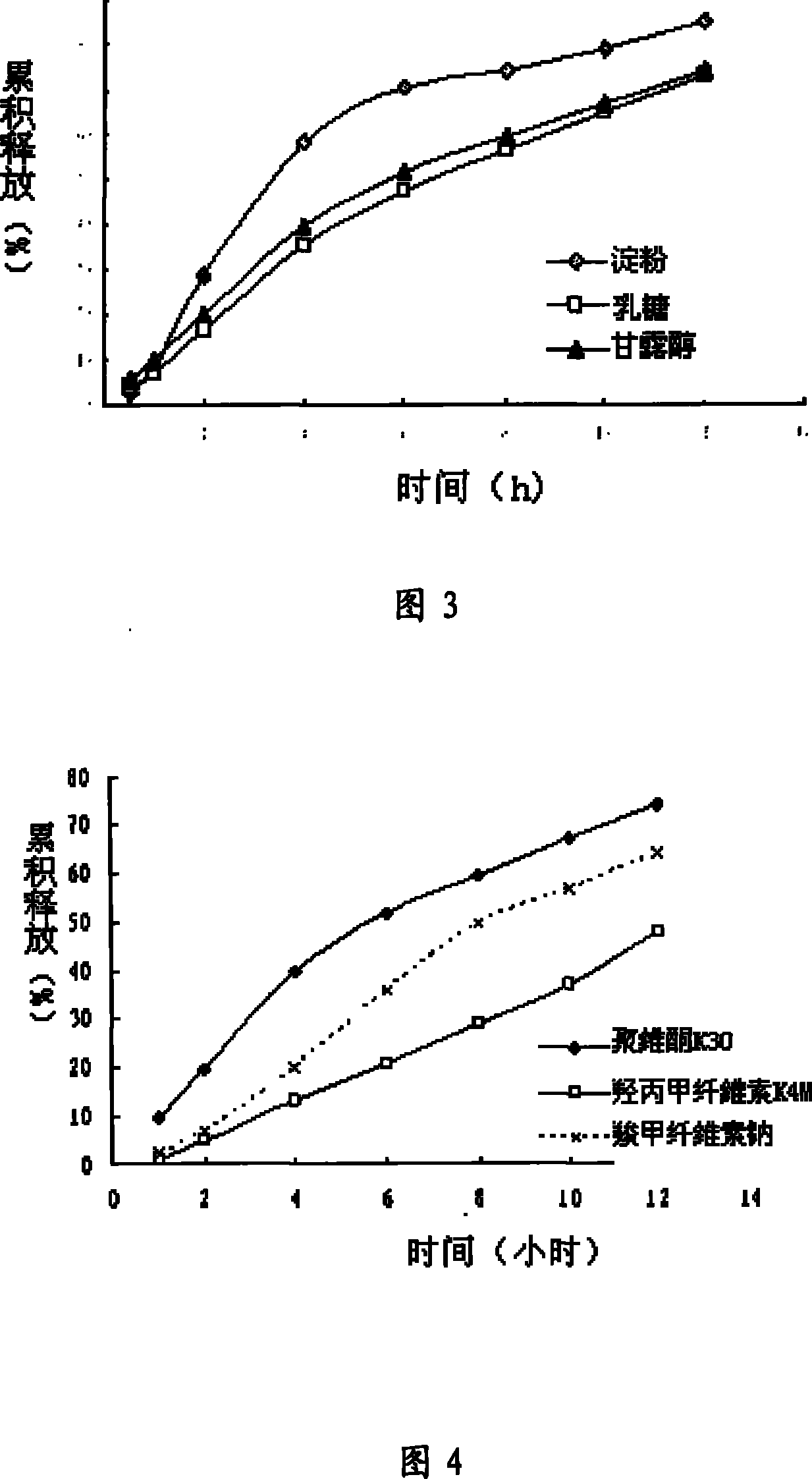
[0145] Film weight gain: 19mg per tablet.
Embodiment 3
[0147] prescription:
[0148] Drug-containing layer of tablet core Huperzine A 0.75g
[0149] Yellow iron oxide 0.5g
[0151] Starch 60g
[0152] Tartaric acid 12g
[0153] Povidone K30 15.0g
[0154] Magnesium Stearate 1.25g
[0155] 150.0mg per tablet
[0156] A total of 1000 pieces
[0157] Coating liquid cellulose acetate 25.0g
[0158] Polyethylene glycol 6.25g
[0159] Acetone 850mL
[0160] Ethanol 150mL
[0161] Preparation process: with embodiment 1.
[0162] Film weight gain: 17mg per tablet.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com