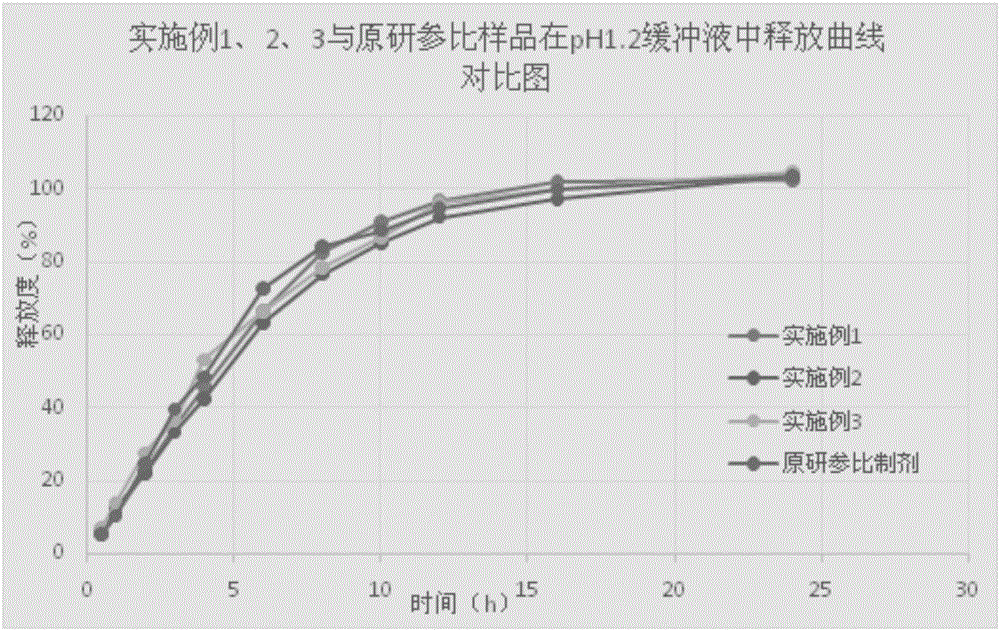
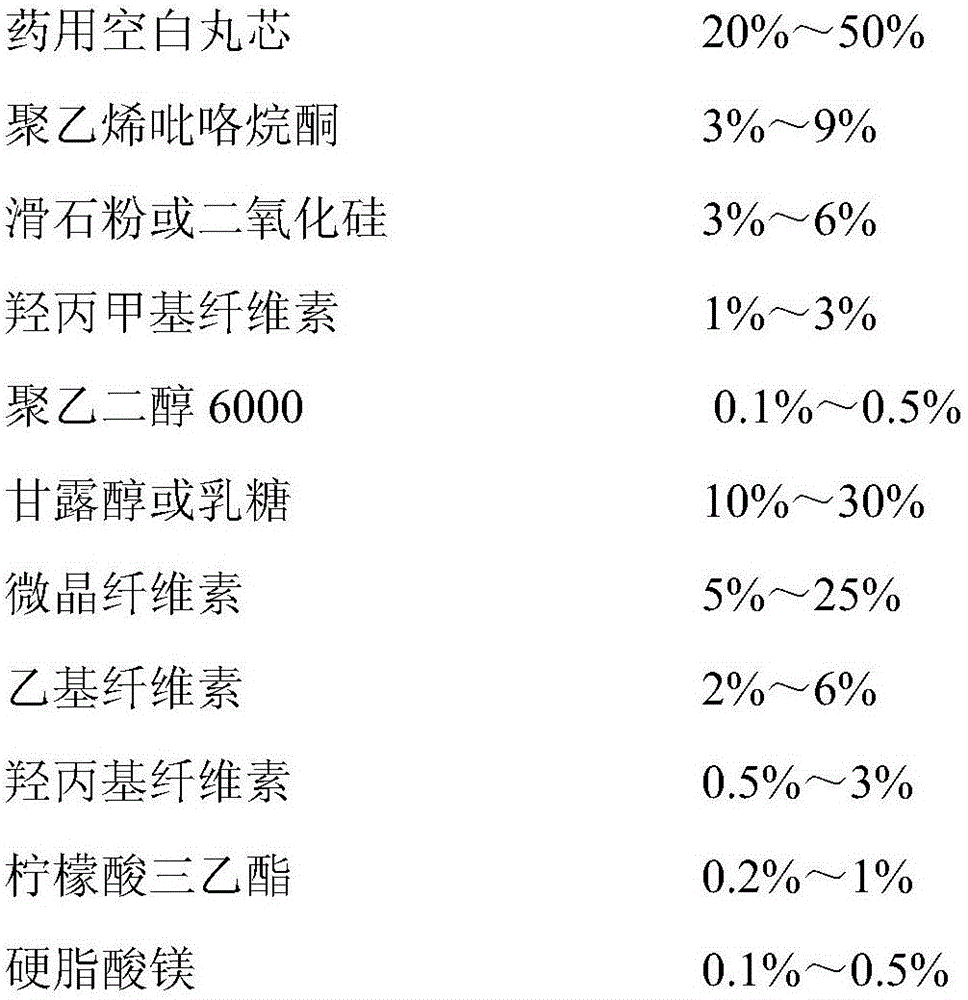
Memantine hydrochloride slow release-donepezil quick release compound capsule
A technology of donepezil hydrochloride and memantine hydrochloride, applied in the field of memantine hydrochloride sustained-release-donepezil immediate-release compound capsules and its preparation, can solve the problems of inability to reach donepezil, increase the burden of accompanying staff, and difficulty in ensuring patient compliance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] The preparation method of memantine hydrochloride sustained-release-donepezil quick-release compound capsule comprises the following steps:
[0080] Step 1: Preparation of memantine hydrochloride sustained-release pellets
[0081] (1) Preparation of drug-containing pellets:
[0082] The drug-containing pellets include the following raw materials in parts by weight: 62 parts of blank ball cores, 20 parts of memantine hydrochloride, 10 parts of povidone, and 8 parts of talcum powder;
[0083] Add the binder into 95% ethanol under stirring, add the memantine hydrochloride and the lubricant in turn after the dissolution is complete, stir evenly to obtain the drug solution, and spray the drug solution on the blank pills On the core, after drying, the drug-containing pellets are obtained;
[0084] (2) Preparation of isolated pellets:
[0085] The drug-containing pellets include the following raw materials in parts by weight: 92 parts of drug-containing pellets, 3 parts of ...
Embodiment 2
[0096] The preparation method of memantine hydrochloride sustained-release-donepezil quick-release compound capsule comprises the following steps:
[0097] Step 1: Preparation of memantine hydrochloride sustained-release pellets
[0098] (1) Preparation of drug-containing pellets:
[0099] The drug-containing pellets include the following raw materials in parts by weight: 60 parts of blank core, 20 parts of memantine hydrochloride, 11 parts of povidone, and 10 parts of talcum powder;
[0100] Add the binder into 95% ethanol under stirring, add the memantine hydrochloride and the lubricant in turn after the dissolution is complete, stir evenly to obtain the drug solution, and spray the drug solution on the blank pills On the core, after drying, the drug-containing pellets are obtained;
[0101] (2) Preparation of isolated pellets:
[0102] The drug-containing pellets include the following raw materials in parts by weight: 98 parts of drug-containing pellets, 5 parts of hydro...
Embodiment 3
[0113] The preparation method of memantine hydrochloride sustained-release-donepezil quick-release compound capsule comprises the following steps:
[0114] Step 1: Preparation of memantine hydrochloride sustained-release pellets
[0115] (1) Preparation of drug-containing pellets:
[0116] The drug-containing pellets include the following raw materials in parts by weight: 53 parts of blank ball cores, 25 parts of memantine hydrochloride, 15 parts of povidone, and 10 parts of talcum powder;
[0117] Add the binder into 95% ethanol under stirring, add the memantine hydrochloride and the lubricant in turn after the dissolution is complete, stir evenly to obtain the drug solution, and spray the drug solution on the blank pills On the core, after drying, the drug-containing pellets are obtained;
[0118] (2) Preparation of isolated pellets:
[0119] The drug-containing pellets include the following raw materials in parts by weight: 88 parts of drug-containing pellets, 2 parts of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com