Ligustrazine film coating agent and preparation method thereof
A technology of ligustrazine and film-coating agents, which is applied in the direction of anti-toxic agents, anti-inflammatory agents, and pharmaceutical formulations, can solve the problems of cumbersome preparation process, large coating area, and inconvenient use, and reduce toxic and side effects and adhesion Strong, reduce the effect of water evaporation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
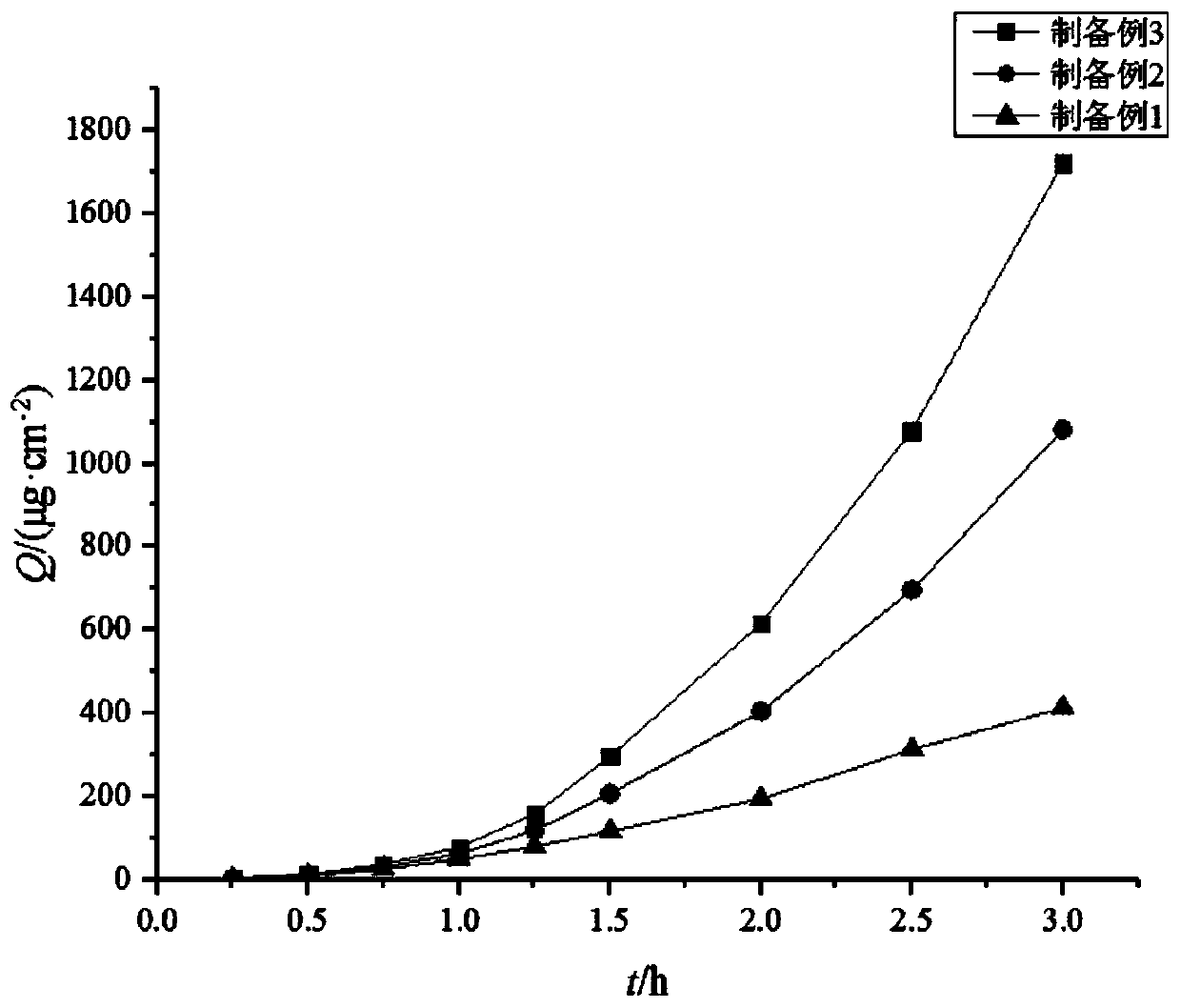
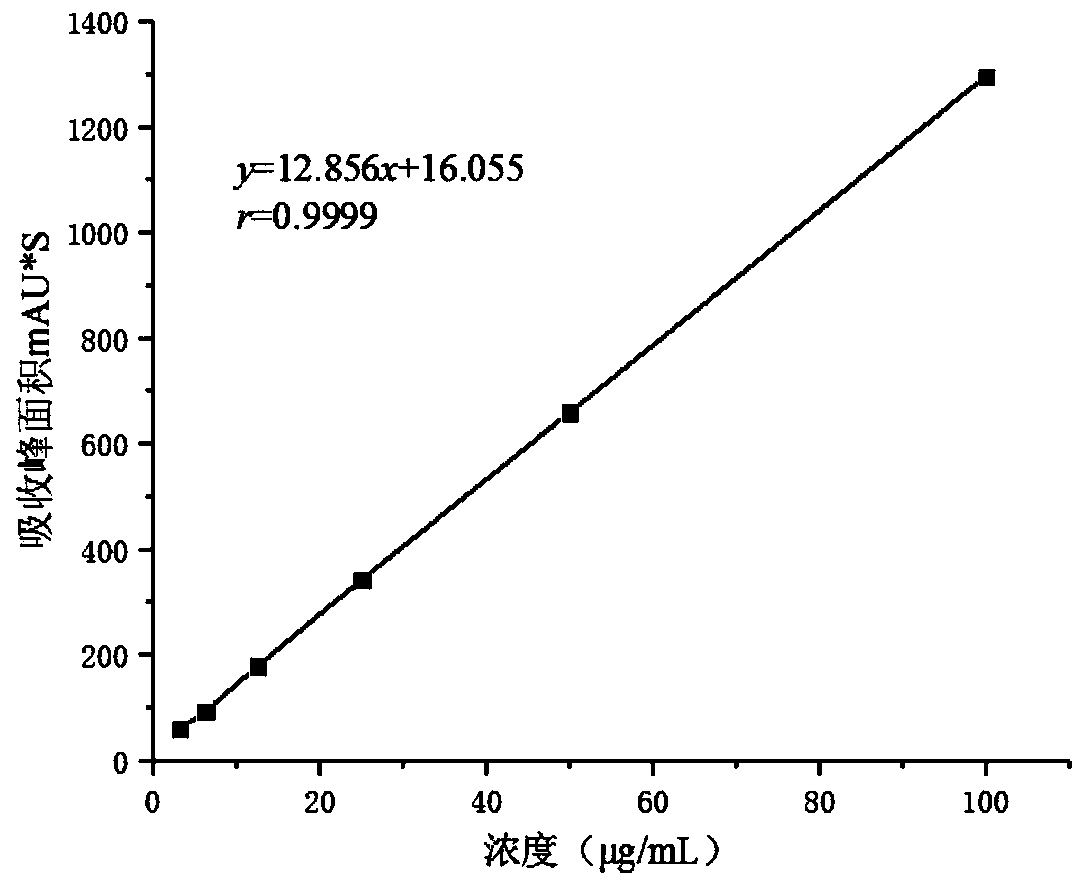
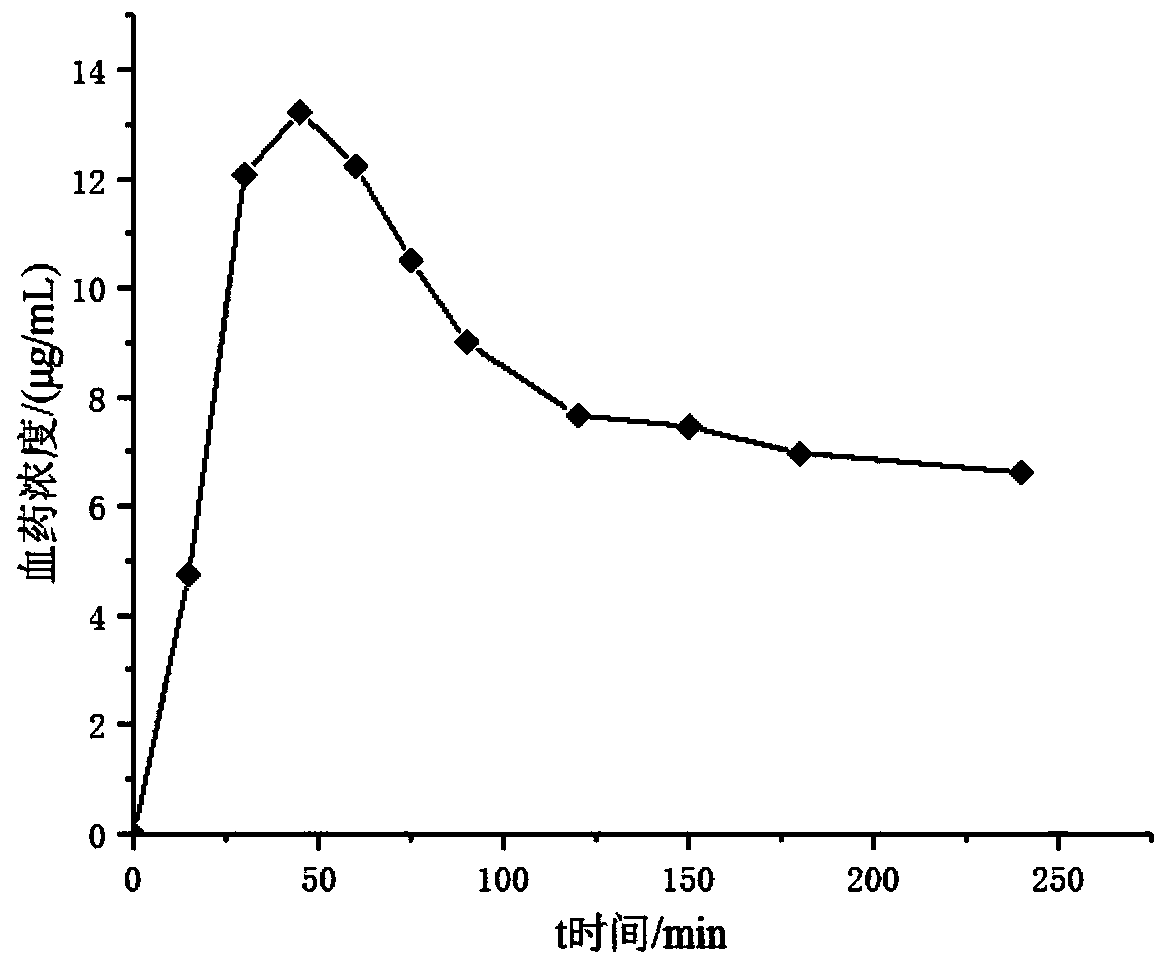
Image
Examples
preparation example Construction
[0035] The invention provides the preparation method of the ligustrazine coating agent, comprising the following steps: 1) mixing water with a film-forming material, sealing, soaking and swelling, and swelling in a water bath to obtain a film-forming matrix; 2) mixing plasticizer, The organic solvent, ligustrazine, surfactant and penetration enhancer are mixed with the film-forming matrix prepared in step 1) to obtain ligustrazine coating agent.
[0036]In the present invention, water is mixed with polyvinyl alcohol, and after sealing, soaking and swelling, the water bath is swollen to obtain a film-forming matrix. In the present invention, the time for the sealing infiltration and swelling is preferably 20-28 hours, more preferably 22-26 hours, most preferably 24 hours; the sealing infiltration and swelling is natural swelling, and the temperature of the sealing infiltration and swelling is not limited. Room temperature is fine. In the present invention, after the seal is in...
preparation example 1
[0040] The composition of raw materials is as follows:
[0041]
[0042] The preparation method comprises the following steps:
[0043] (1) Take 80g of ultrapure water, seal and infiltrate 5g of polyvinyl alcohol for 24 hours, let it swell naturally, swell in a water bath at 90°C for 2 hours to gel, take it out and let it cool for later use, and obtain a film-forming matrix;
[0044] (2) Add 5.78g ligustrazine to the film-forming matrix in step (1) while stirring, then slowly add 11.046g ethanol solution, 3.132g glycerin, 1.02g Tween-80, 1.15g azone and 8.545g Ultrapure water, stirred to disperse evenly to obtain 114.845 mL of ligustrazine coating agent.
preparation example 2
[0046] The composition of raw materials is as follows:
[0047]
[0048] The preparation method comprises the following steps:
[0049] (1) Take 60g of ultrapure water, seal and infiltrate 13.5g of polyvinyl alcohol for 24 hours, let it swell naturally, swell in a water bath at 90°C for 2 hours to gel, take it out and let it cool for later use, and obtain a film-forming matrix;
[0050] (2) Add 8.68g ligustrazine to the film-forming matrix in step (1) while stirring, then slowly add 23.25g ethanol solution, 5.24g glycerol, 0.69g Tween-80, 0.46g azone and 3.853g Ultrapure water, stirred to disperse evenly to obtain 117.703 mL of ligustrazine coating agent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com