A double-coated cyclosporin A sustained-release pellet preparation and preparation method thereof
A technology of sustained-release pellets and cyclosporine, which is applied in the direction of pill delivery, sugar-coated pills, and medical preparations of non-active ingredients. It can solve problems that are not suitable for industrial production and improve drug compliance and reproducibility. Good, easy to get the effect of excipients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
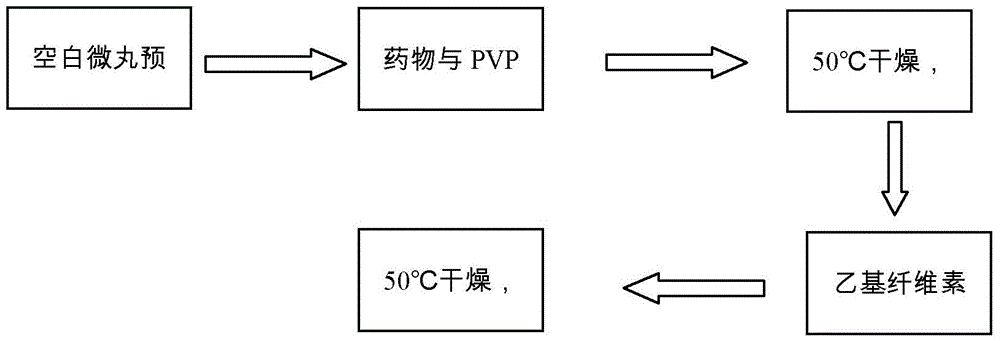
[0033] Weigh 80 g of blank sugar pills, place them in a Mini250 extrusion spheroid fluidized coating machine, and preheat for 30 minutes.
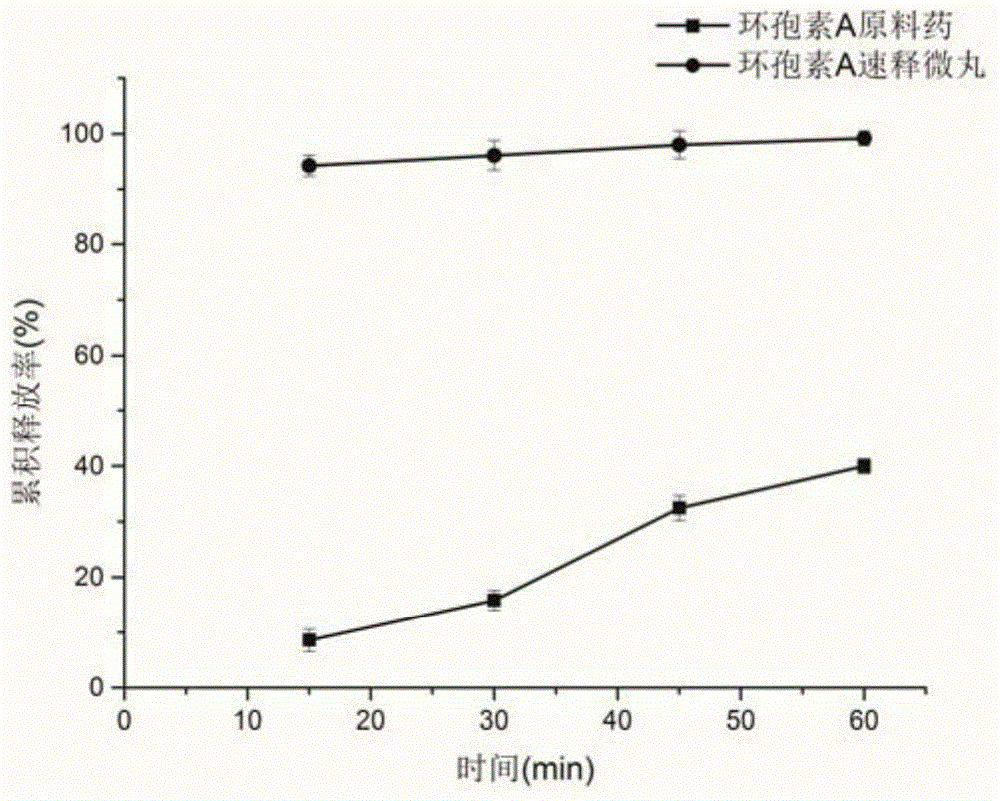
[0034] Weigh 4g of cyclosporin A, 4g of polyvinylpyrrolidone k30, 0.8g of polyethylene glycol 400, 1880.8g of poloxamer, 1g of micropowder silica gel, and magnetically stir with 100ml of 40% (v / v, the same below) ethanol aqueous solution Dissolve, coat, take out after coating, and dry at 50°C for 2 hours to obtain cyclosporine A immediate-release pellets. The cyclosporine A quick-release pellets were carried out in vitro drug release test, the results are shown in figure 2 .
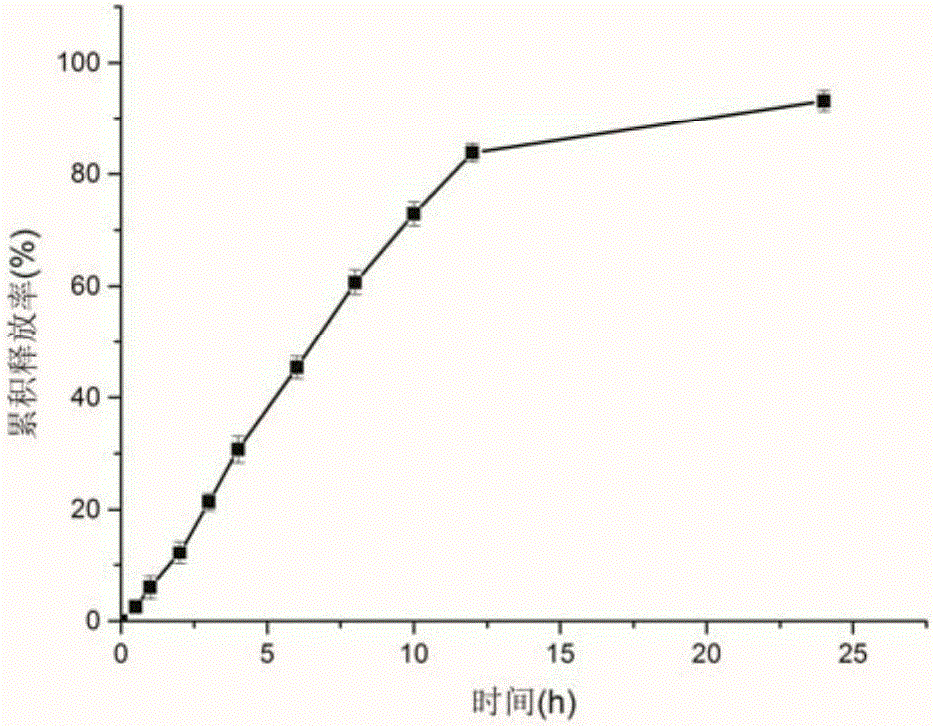
[0035] Weigh 3 g of ethyl cellulose, 0.6 g of diethyl phthalate, 0.9 g of polyethylene glycol 4000, and 3 g of micropowder silica gel, and dissolve them with 150 ml of 60% ethanol aqueous solution with magnetic stirring, and take the cyclosporin A prepared above. Release the pellets for coating, take them out after coating, and dry at 50°C for 2 hours to obtain cy...
Embodiment 2
[0037] Weigh 90 g of blank sugar pills, place them in a Mini250 extrusion spheronizing fluidized coating machine, and preheat for 30 minutes.
[0038] Weigh 6 g of cyclosporine A, 4 g of polyvinylpyrrolidone k30, 1.2 g of polyethylene glycol 400, 1880.9 g of poloxamer, and 1.5 g of micropowder silica gel, and dissolve them with 100 ml of 60% ethanol aqueous solution with magnetic stirring, and coat them. After the coating is completed, take it out and dry at 50°C for 2 hours to obtain cyclosporine A immediate-release pellets. The cyclosporine A quick-release pellets were carried out in vitro drug release test, the results are as follows: figure 2 .
[0039] Weigh 8 g of ethyl cellulose, 0.8 g of diethyl phthalate, 0.8 g of polyethylene glycol 4000, and 3 g of micropowder silica gel, and dissolve them with 200 ml of 40% ethanol aqueous solution with magnetic stirring, and take the cyclosporin A prepared above. Release the pellets for coating, take them out after coating, and...
Embodiment 3
[0041] Weigh 100 g of blank microcrystalline cellulose pellets, place them in a Mini250 extrusion spheronizing fluidized coating machine, and preheat for 30 minutes.
[0042] Weigh 10g of cyclosporine A, 10g of polyvinylpyrrolidone k30, 1g of polyethylene glycol 400, 1.8g of Tween 80, and 3g of talcum powder, and dissolve them with 200ml of 60% ethanol aqueous solution with magnetic stirring, and then coat them. Take it out and dry at 50°C for 2 hours to obtain cyclosporin A immediate-release pellets. The cyclosporine A quick-release pellets were carried out in vitro drug release test, the results are as follows: figure 2 .
[0043] Weigh 5 g of cellulose acetate, 1 g of polyethylene glycol 4000, and 2 g of micropowdered silica gel, and dissolve them with 250 ml of absolute ethanol magnetic stirring, take the cyclosporine A quick-release pellets prepared above for coating, and take out after coating is completed, Dry at 50°C for 2 hours to obtain cyclosporine A sustained-re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com