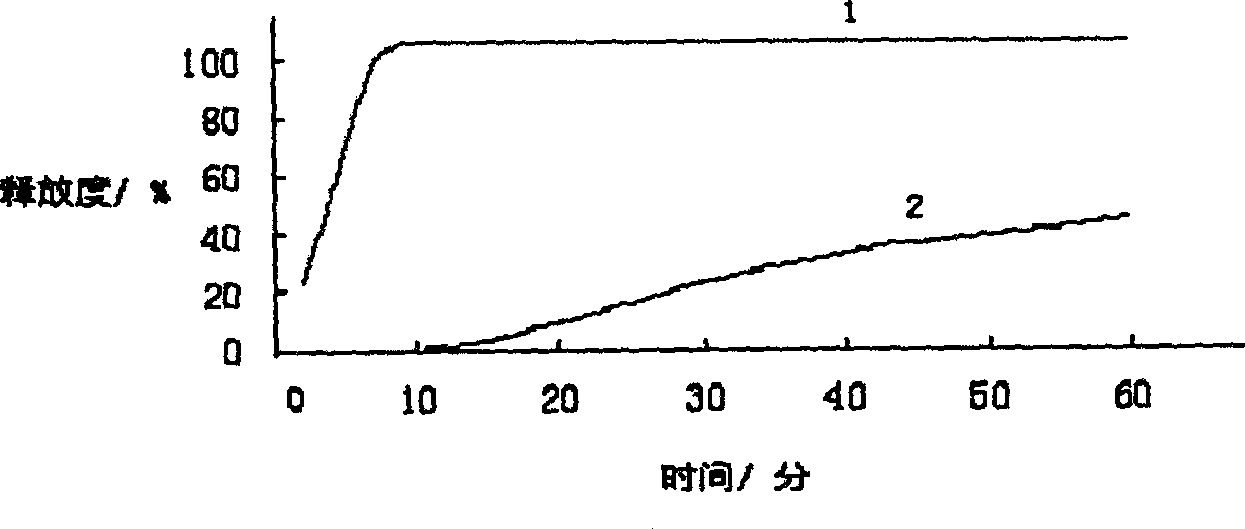
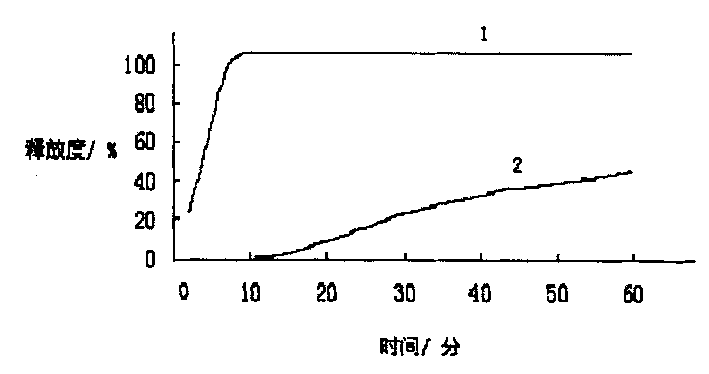
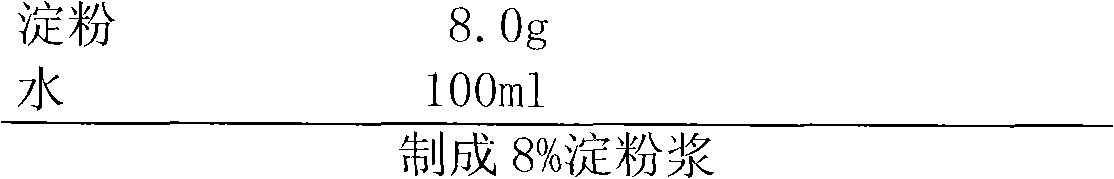
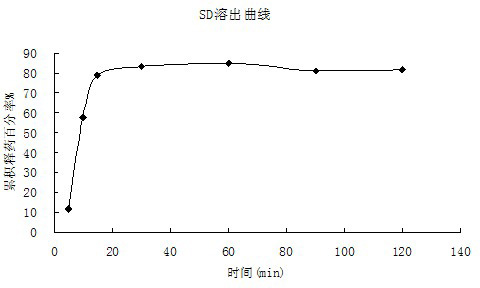
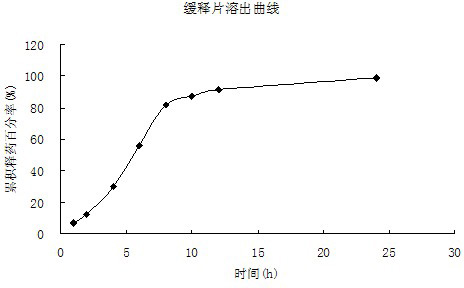
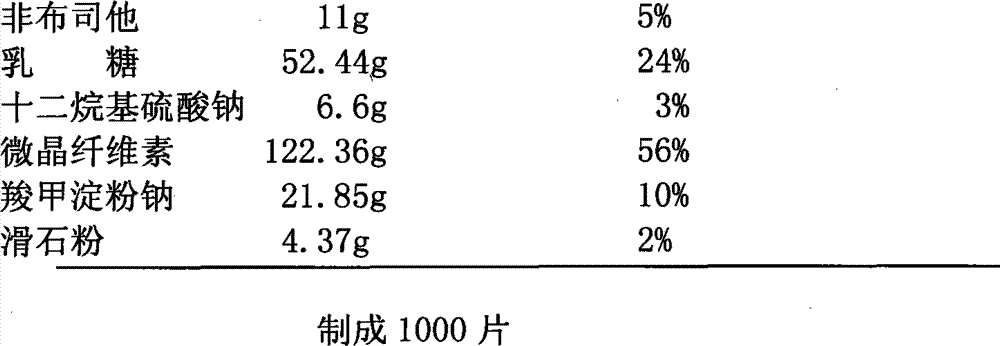
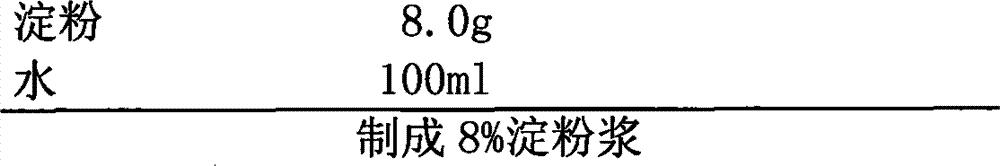
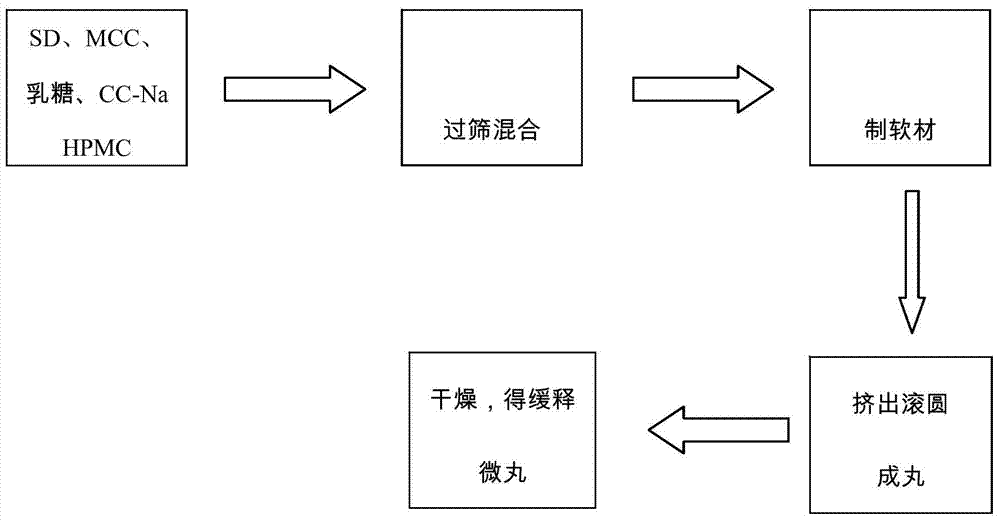
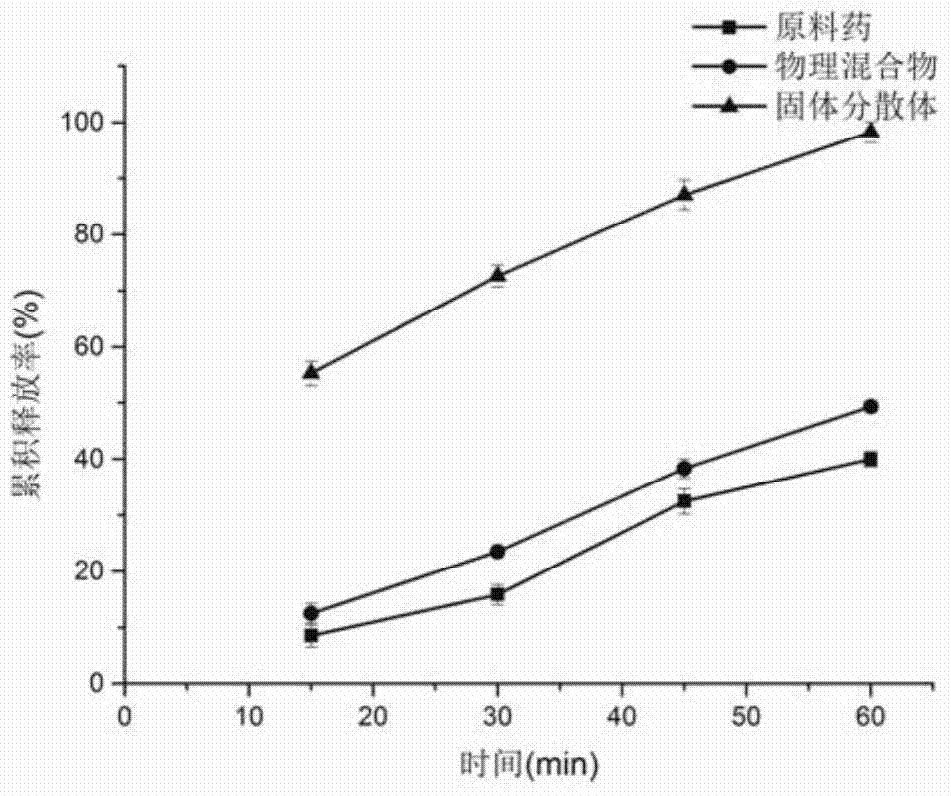
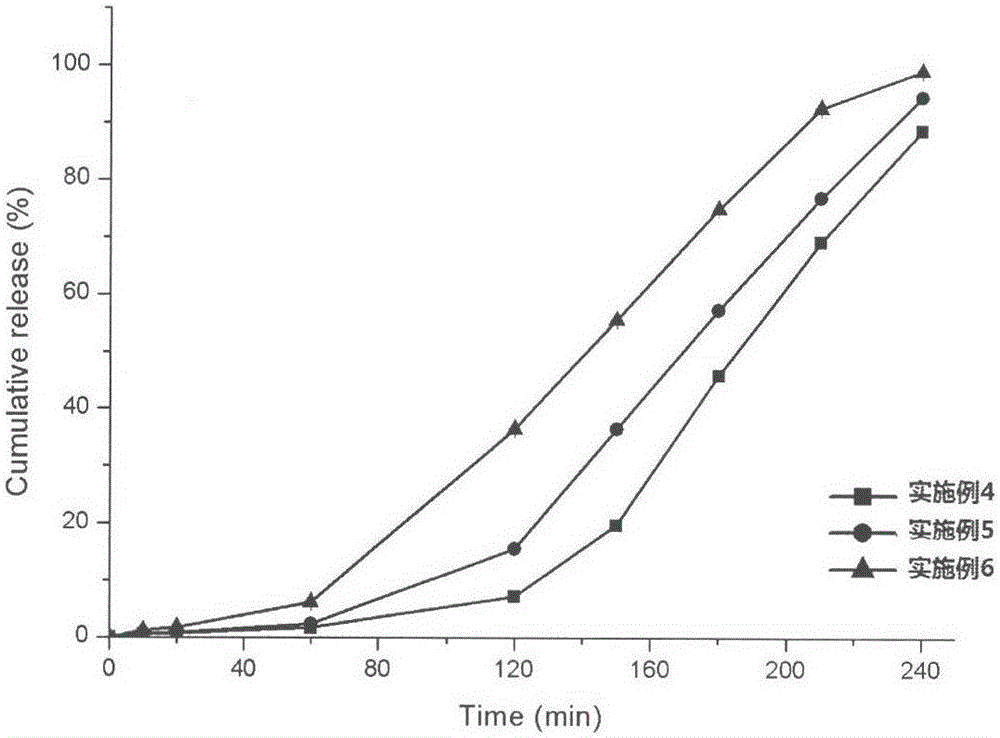
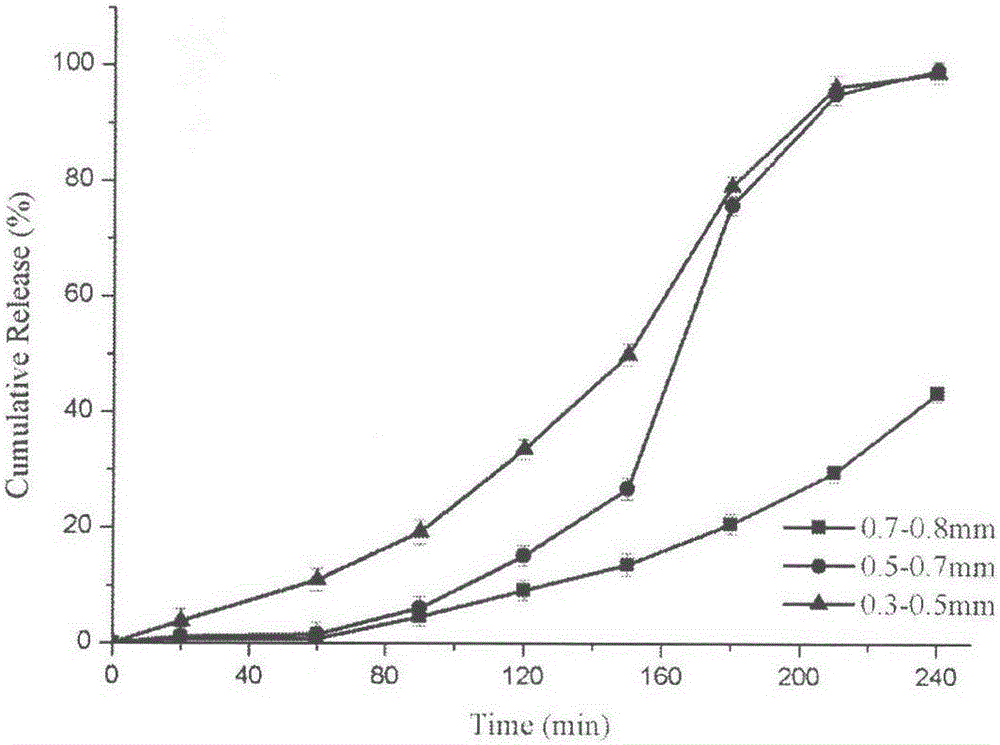
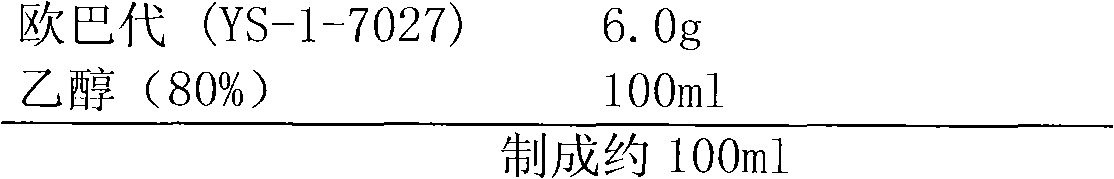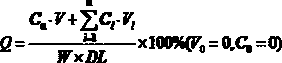Patents
Literature
58results about How to "Excipients are easy to get" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Production method of artificial isaria cicadae miq
ActiveCN103598014AQuality improvementShort training daysHorticultureBiotechnologyAgricultural science
The invention discloses a production method of artificial isaria cicadae miq. The production method has the advantages that the technical parameters, cultivation medium conditions and the like of the method are obtained after years of optimization, each parameter, cultivation medium proportion and production conditions are quite scientific and precise, and tests show that high-quality artificial isaria cicadae miq can be obtained by the method; the method is short in cultivation time, the cultivation medium is rice, wheat or mixture of wheat and yeast, the auxiliary materials are easy to get and low in cost, and massive industrial production can be performed; the artificial isaria cicadae miq produced by the method can be used as food, healthcare product, and medicine, or as the auxiliary materials of foods, healthcare products and medicines.
Owner:湖州新驰医药科技有限公司
Hiliezdum fast-release tablet and preparing method thereof
InactiveCN1454600AGreat tasteHigh strengthOrganic active ingredientsNervous disorderCross-linkCellulose
The invention is a kind of quick-released tablet of shenshuai fruit elements and the manufacturing method. The tablet contains 10-30 % shenshuai fruit element extraction material, 2-5 % sputtering agent, 1.5-3%, the other is filter. The sputtering agent is cross-linked sodium cellulose glycolate, sodium carboxy methyl starch or the compound of them. The additive includes lubricant, flow aid, deodorizing agent. The filler is the compound of alditol and cellulose. The method used polyketone as adhesive, used alcohol or the fixed liquid of alcohol and water as humectant. The sputtering agent uses inner adding method or outer adding method, wet process to produce particles. The character of the invention lies in fast dilution, the medicine can be dissolved entirely in then minutes, it needn't sugar-coat, the troche has good taste, it has no obvious bitterness.
Owner:TSINGHUA UNIV
Febuxostat tablet and preparation method thereof
ActiveCN102488665ALess prescription ingredientsExcipients are easy to getOrganic active ingredientsSkeletal disorderActive agentCombinatorial chemistry
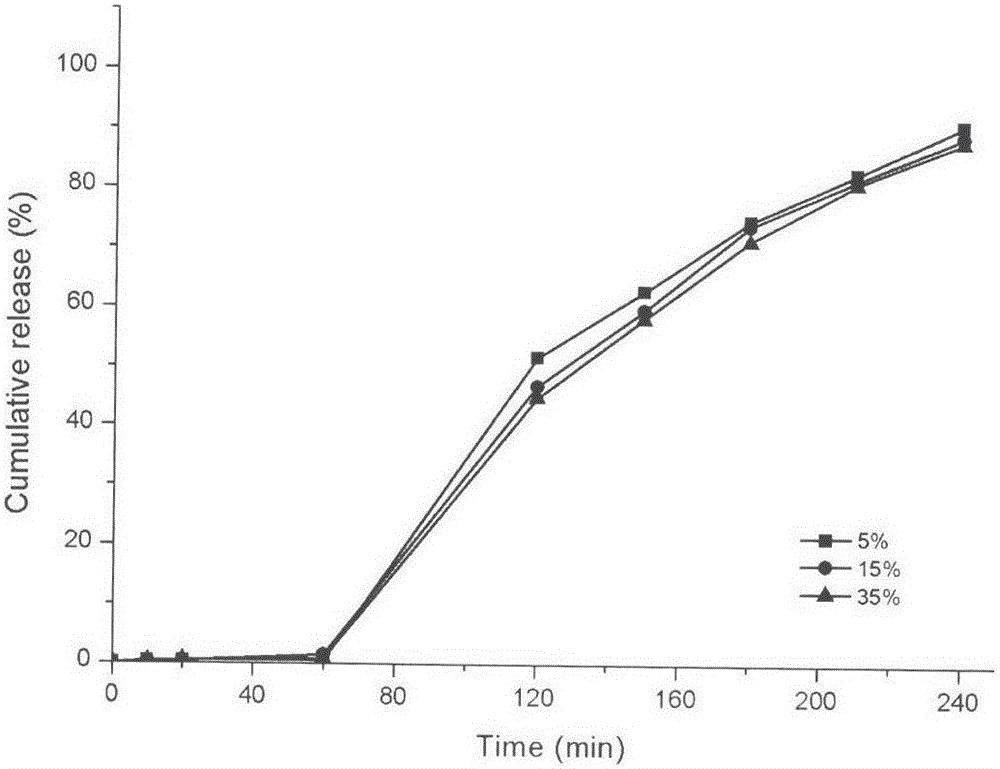
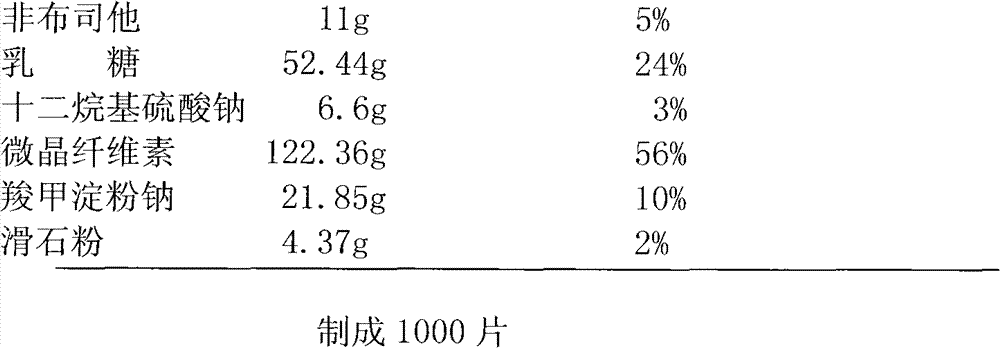
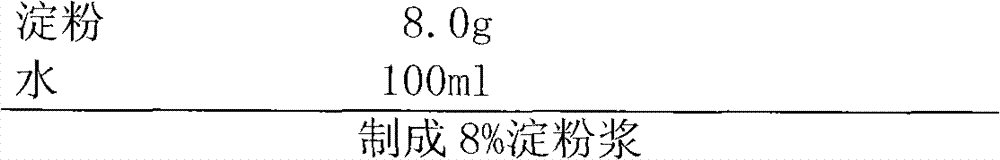
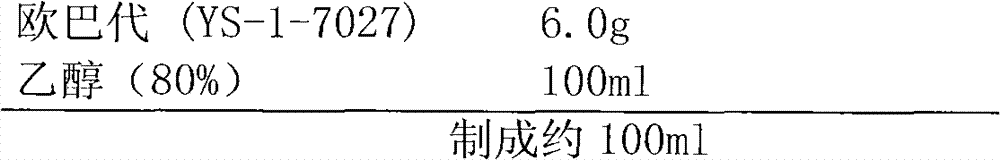
The invention discloses a febuxostat tablet and a preparation method thereof. The febuxostat tablet comprises a tablet core and a coating, the tablet core comprises the following compositions: by weight percentage, from 5% to 30% of febuxostat, from 15% to 60% of filler, from 1% to 20% of disintegrating agent, from 0.1% to 5% of surfactant, from 0.1% to 8% of lubricating agent and a defined amount of adhesive. The febuxostat tablet adopts the high-efficient disintegrating agent within a reasonable proportion range, simultaneously, the febuxostat which is difficultly soluble medicine is dissolved by the aid of the surfactant and the high-efficient disintegrating agent, so that solubility of the febuxostat is improved, and bioavailability of the febuxostat is increased. In addition, the preparation method for the febuxostat tablet is simple, and is controllable in quality and fine in stability.
Owner:KANGYA OF NINGXIA PHARMA
Febuxostat tablet
ActiveCN102895209AImprove solubilityImprove bioavailabilityOrganic active ingredientsPharmaceutical delivery mechanismAdhesiveMedicine
The invention discloses a Febuxostat tablet. The Febuxostat tablet comprises a tablet core and a coating, wherein the tablet core comprises the following components in weight percentage: 5-30 percent of Febuxostat, 15-60 percent of filler, 1-20 percent of disintegrating agent, 0.1-5 percent of surface active agent, 0.1-8 percent of lubricant and a right amount of adhesive. According to the Febuxostat tablet, trough adopting the powerful disintegrating agent within a reasonable proportional region, and meanwhile, through jointly using the surface active agent, the poorly water-soluble drug Febuxostat dissolves out, and further, the dissolvability of the Febuxostat is increased, and the bioavailability of the Febuxostat is improved. Moreover, the Febuxostat tablet is simple in preparation method, controllable in quality and high in stability.
Owner:KANGYA OF NINGXIA PHARMA
Oral ciclosporin A sustained-release agent and preparation method thereof
ActiveCN102166201AStable drug releaseImprove bioavailabilityPharmaceutical delivery mechanismCyclic peptide ingredientsCiclosporinSolubility
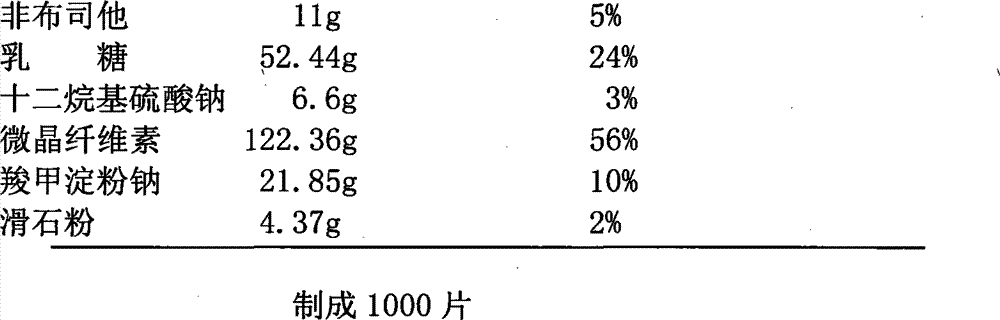
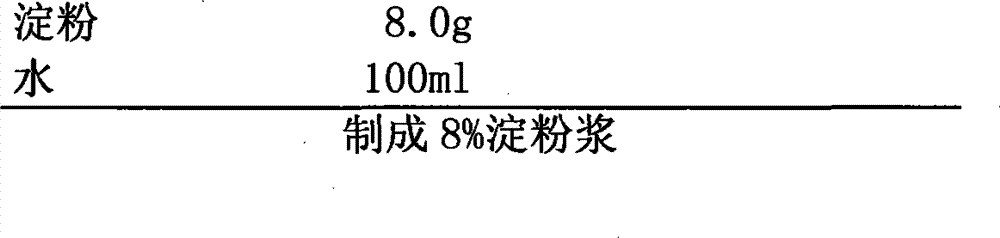
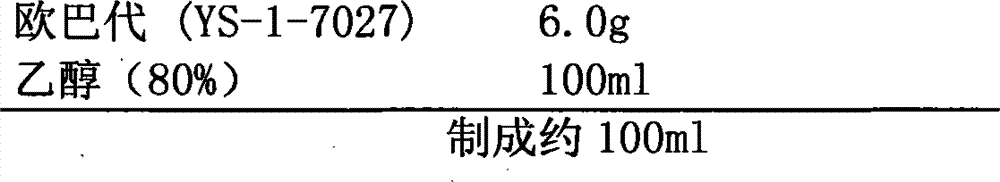
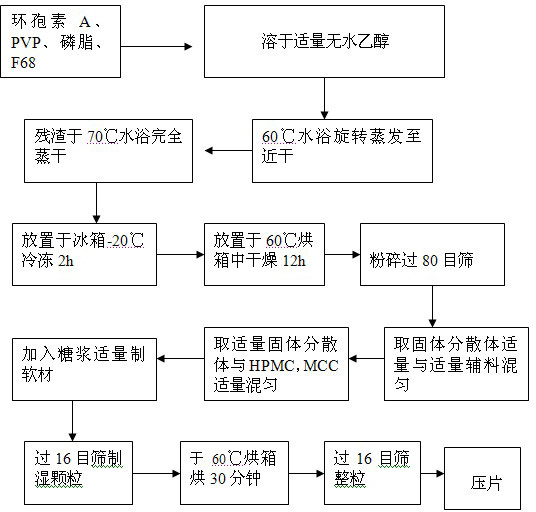
The invention provides an oral ciclosporin A sustained-release agent. The sustained-release agent comprises the following components in part by mass: 1 part of ciclosporin A, 2.5 to 7.5 parts of polyvidone-K30, 0.15 to 4.82 parts of hydroxypropyl methyl cellulose 4000cPa.s, 0.1 to 3.94 parts of microcrystalline cellulose, 0 to 2.57 parts of hydroxypropyl methyl cellulose 15000cPa.s and 0.1 to 1.0part of phospholipid. By combining a solid dispersing technology and a sustained-release hydrophilic gel matrix technology and based on the 'double release' principle of fast release first and slow release second, the method prepares the insoluble medicament of ciclosporin A sustained-release agent; and the method has the obvious advantages that the solubility of ciclosporin A is improved, and the oral agent can quickly play the effect first and then smoothly and slowly release drug. The ciclosporin A sustained-release tablets reduce the lowered blood concentration peak-valley phenomenon and lower the drug-taking frequency. The invention also discloses a method for preparing the oral ciclosporin A sustained-release agent.
Owner:JIANGSU UNIV +1
High-dissolubility children's calcium carbonate D3 granule and preparation method thereof
InactiveCN105106233APromote absorptionImprove uniformityOrganic active ingredientsMetabolism disorderVitamin d 3Granularity
Owner:JIANGSU FUBANG PHARMA
Diversine hydrochloride spray and its preparation process
InactiveCN1478475AGood effectPromote absorptionOrganic active ingredientsAntipyreticCurative effectSolvent
A spray of diversine hydrochloride is prepared from the tuduranine hydrochloride, percutaneous promoter and solvent. Its advantages are high percutaneous effect and curative effect, quick absorption, and low toxic by-effect.
Owner:西安利君制药股份有限公司
Febuxostat tablet with improved dissolution rate
ActiveCN102895210ALess prescription ingredientsExcipients are easy to getOrganic active ingredientsSkeletal disorderAdhesiveMedicine
The invention discloses a Febuxostat tablet and a preparation method thereof. The Febuxostat tablet comprises a tablet core and a coating. The tablet core comprises the following components in weight percentage: 5-30 percent of Febuxostat, 15-60 percent of filler, 1-20 percent of disintegrating agent, 0.1-5 percent of surface active agent, 0.1-8 percent of lubricant and a right amount of adhesive. According to the Febuxostat tablet, trough adopting the powerful disintegrating agent within a reasonable proportional region, and meanwhile, through jointly using the surface active agent, the poorly water-soluble drug Febuxostat dissolves out, and further, the dissolvability of the Febuxostat is increased, and the bioavailability of the Febuxostat is improved. Moreover, the Febuxostat tablet is simple in preparation method, controllable in quality and high in stability.
Owner:KANGYA OF NINGXIA PHARMA
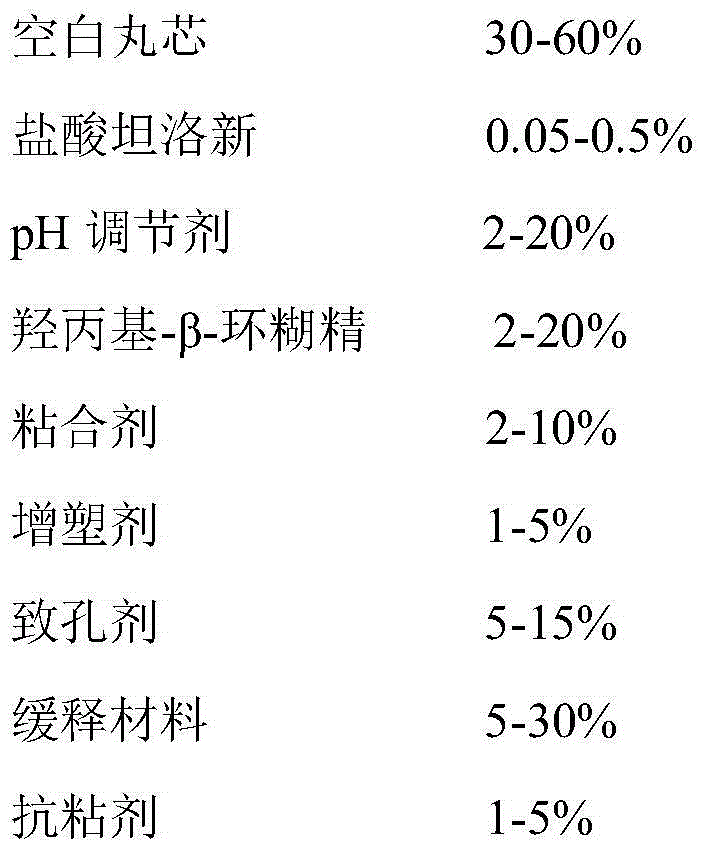
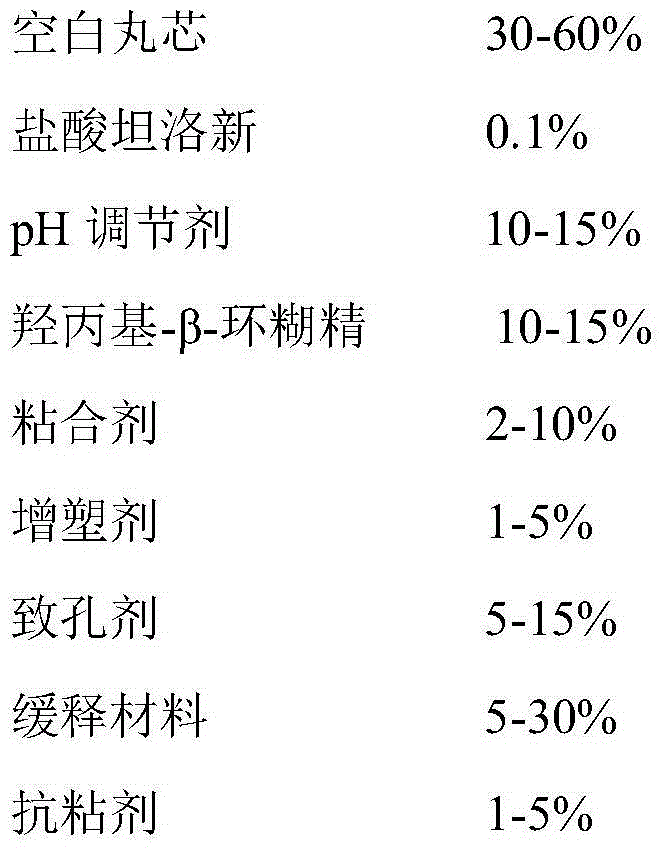
Tamsulosin hydrochloride sustained-release capsule and preparation method thereof
PendingCN104873478AAvoid burst phenomenonImprove securityPharmaceutical delivery mechanismUrinary disorderSustained Release CapsuleEconomic benefits
The invention provides a tamsulosin hydrochloride sustained-release capsule and a preparation method thereof. Hydroxypropyl-beta-cyclodextrin is added in a prescription, so as to form clathrate compounds on the outer part of tamsulosin hydrochloride together with the tamsulosin hydrochloride, so that the phenomenon of drug burst release in a drug release process is solved, thereby maintaining stable plasma concentration, reducing the incidence rate of adverse reactions and improving the clinical medication safety. In addition, raw materials are easy to obtain, the preparation process is simple and feasible, the yield is high, the cost is low, industrial mass production can be realized, and obvious economic benefits are achieved.
Owner:LUNAN BETTER PHARMA
Formula of Chinese medicinal external washing powder and effervescent tablets for treating lumbago and a preparation method thereof
The invention relates to Chinese medicinal external washing powder and effervescent tablets for treating lumbago and a preparation method thereof. The formula of the external washing powder is prepared from 3,750 grams of epimedium herb, 6,250 grams of bark of eucommia, 3,750 grams of prepared common monkshood daughter root, 3,125 grams of Morinda officinalis and 3, 125 grams of malaytea scurfpea fruit. The Chinese medicines in the formula are weighed by weight and ground into 200-mesh powder respectively, the different kinds of powder are mixed uniformly, the mixture is packaged according to a ratio of 20 grams per bag, and thus the external washing powder is obtained. The formula of the effervescent tablets comprises 690 grams of epimedium herb, 1,110 grams of bark of eucommia, 690 grams of prepared common monkshood daughter root, 520 grams of Morinda officinalis and 520 grams of malaytea scurfpea fruit, which service as raw material medicines, and 650 grams of citric acid, 780 grams of sodium bicarbonate, 1,105 grams of starch, 260 grams of sodium carboxymethyl starch, 195 grams of hydroxy propyl cellulose, and 195 grams of polyethylene glycol 6000. The preparation method of the effervescent tablets comprises: (1) preparing paste powder; (2) dividing the paste powder into two parts, adding 650 grams of citric acid into one part, granulating, drying, finishing grains, adding 780 grams of sodium bicarbonate powder into the other part, uniformly mixing, granulating, drying and finishing grains; and (3) adding 195 grams of hydroxy propyl cellulose and 195 grams of polyethylene glycol into the particles, uniformly mixing and tabletting.
Owner:祝凤仪
Formulas and preparation methods of traditional Chinese medicine external washing powder and effervescent tablets
InactiveCN102247535AEasy to useNo side effectsPowder deliveryCardiovascular disorderMedicinal herbsSodium bicarbonate
The invention relates to formulas and preparation methods of traditional Chinese medicine external washing powder and effervescent tablets. The formula of the external washing powder comprises 3750g of heaven ailanthus fruit, 6250g of radix et rhizome rhei, 3750g of clematis chinensis osbeck, 3125g of bletilla striata, and 3125g of glauber salt. The preparation method of the external washing powder comprises the steps that: the medicine materials are weighed according to the formula; the medicine materials are respectively crushed and grinded into powders with particle sizes of 200 meshes; the powders are well-mixed; and the mixed powder is packed according to a specification of 20g / pack, such that the external washing powder is prepared. The formula of the external washing effervescent tablets comprises raw medicine materials and auxiliary materials. The raw medicine materials comprise 690g of heaven ailanthus fruit, 1110g of radix et rhizome rhei, 690g of clematis chinensis osbeck, 520g of bletilla striata, and 520g of glauber salt. The auxiliary materials comprise 650g of citric acid, 780g of sodium bicarbonate, 1105g of starch, 260g of sodium carboxymethyl starch, 195g of hydroxypropyl cellulose, and 6000195g of polyethylene glycol. The preparation method of the external washing effervescent tablets comprises steps that: (1) an extract powder is prepared; (2) the extract powder is divided into two portions; 650g of citric acid is added to one portion of the extract powder, and the mixture is well-mixed, granulated, dried, and shaped; 780g of sodium bicarbonate is added to the other portion of the extract powder, and the mixture is well-mixed, granulated, dried, and shaped; (3) 195g of hydroxypropyl cellulose, and 6000195g of polyethylene glycol are added to the granules obtained from step (2); the mixture is well-mixed, and is pressed into tablets.
Owner:祝凤仪
Wood-imitating skirting board capable of heating and manufacturing method thereof
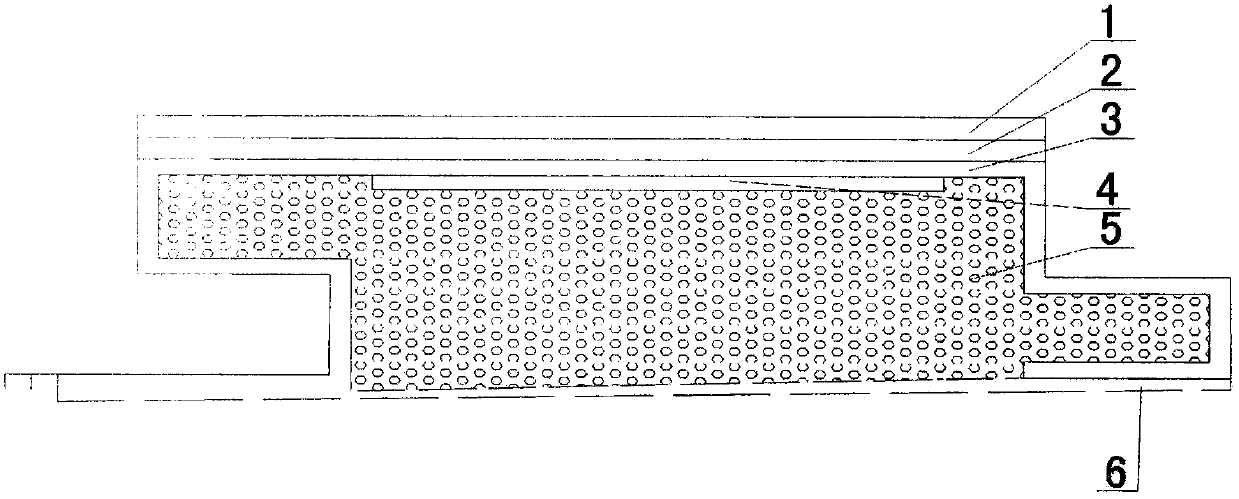
InactiveCN108049584AEasy to install and replaceExcipients are easy to getCovering/liningsFlooringReflective layerAluminum foil
The invention provides a wood-imitating skirting board capable of heating and a manufacturing method thereof, and belongs to the technical field of decorative accessories. The wood-imitating skirtingboard is composed of six layers of structures, the outermost layer is an abrasion-resistant layer made of a transparent aluminum oxide film, the second layer is a decorative layer with a PVC film being drawn with design and color patterns such as wood grains or stone patterns, the third layer is a skeleton layer formed by an aluminum-magnesium alloy forming board with the thickness being 0.4-0.7 mm, the fourth layer is a heating layer manufactured through a low-temperature radiation electric heating film, the fifth layer is a heat preservation layer manufactured through a polyurethane foam board with the thickness being 10-15 mm, and the sixth layer is a reflective layer undertaken by an aluminum foil film. The manufacturing method comprises the steps that during manufacturing, the decorative layer is pasted on one face of the skeleton layer first; then the abrasion-resistant layer is hot pressed on the decorative layer; then after the skeleton layer is formed by using special equipment, and the heating layer is pasted and the heat preservation layer is sprayed on the other face of the skeleton layer in sequence; after sizing of the heat preservation layer is achieved, the reflective layer is pasted, thereafter, the skirting board is cut, and a power line is connected; and finally, a convex male connector and a concave female connector are correspondingly machined on the two side edges of the skirting board, and a plurality of small holes are evenly drilled in the concave female connector of the skirting board at intervals in a sleeving mode.
Owner:包头市山川圣阳热能科技有限公司
Fenugreek saponin enteric-coated micro-capsule preparation and preparation method thereof
InactiveCN109528685AIncrease profitAvoid destructionOrganic active ingredientsMetabolism disorderBiotechnologyMedicine
The invention provides a fenugreek saponin enteric-coated micro-capsule preparation and a preparation method thereof. The fenugreek saponin enteric-coated micro-capsule preparation comprises the following components in percentages by mass: 1-10% of fenugreek saponin, 10-60% of a hydrophilic carrier, 1-10% of a pH regulator and 20-60% of an enteric-coated material. On the one hand, by the providedfenugreek saponin enteric-coated micro-capsule preparation, the bitter taste of the fenugreek saponin can be covered well, strong irrigation of the drug to the gastric mucosa of an animal is avoided,and the palatability is improved; and on the other hand, releasing of the drug in the intestinal tract is ensured, the bioavailability is increased, the times of administration are reduced, and the cost is saved.
Owner:GUANGZHOU LEADER BIO TECH +1
Ivermectin controlled-release capsule and preparation method and application thereof
InactiveCN107773554ARelease fullyHigh protection rateOrganic active ingredientsAntiparasitic agentsTherapeutic effectPhospholipid
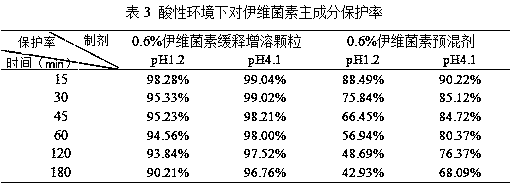
The invention discloses an ivermectin controlled-release capsule and a preparation method and application thereof. The ivermectin controlled-release capsule comprises, by mass, 0.1-1% of ivermectin raw material, 20-60% of water-soluble carrier and 30-70% of enteric-soluble wrapping material, the water-soluble carrier is one or multiple of hydroxypropyl-beta-cyclodextrin, methyl-beta-cyclodextrin,HPMC, PVP, PEG, poloxamer 188, mannitol, D-alpha-tocopherol PEG 1000 succinate, cholate / phospholipid mixed micelle, polyethylenediamine dendritic polymer, phospholipid or cholesterol, and the enteric-soluble wrapping material is one or multiple of hydroxypropyl methyl cellulose phthalic acid, acrylic resin II, acrylic III, cellulose acetate phthalate, polyethylene diacetate phthalate or hydroxypropyl methyl cellulose acetate succinate. The ivermectin controlled-release capsule has the advantage of outstanding acid resistance, enables drug to reach intestinal tracts to be disintegrated and released, remarkably improves bioavailability and has remarkable treatment effect.
Owner:SOUTH CHINA AGRI UNIV
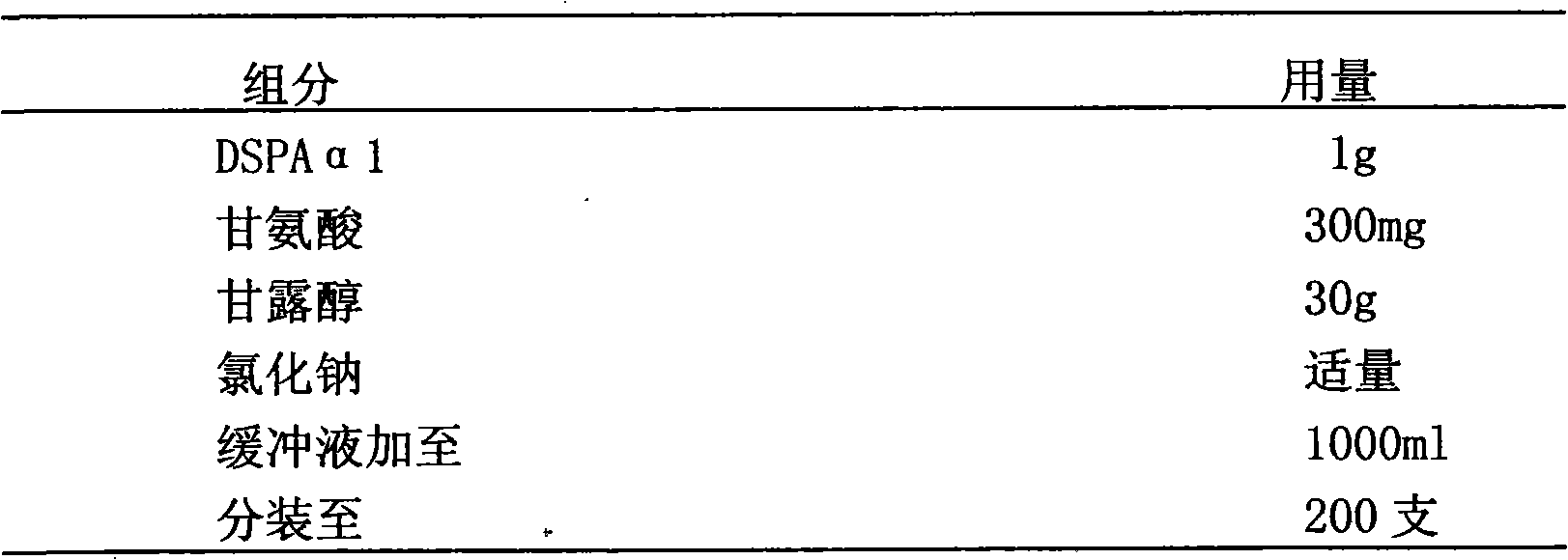
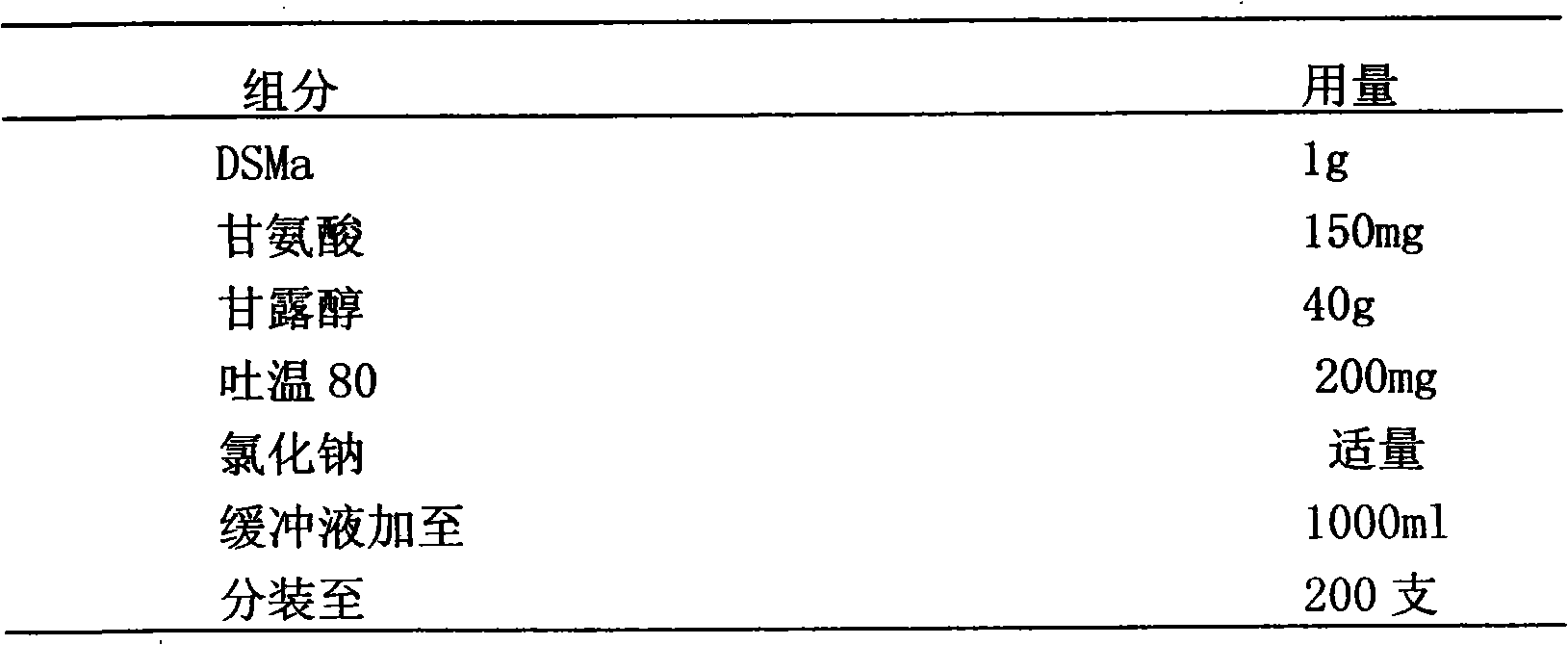
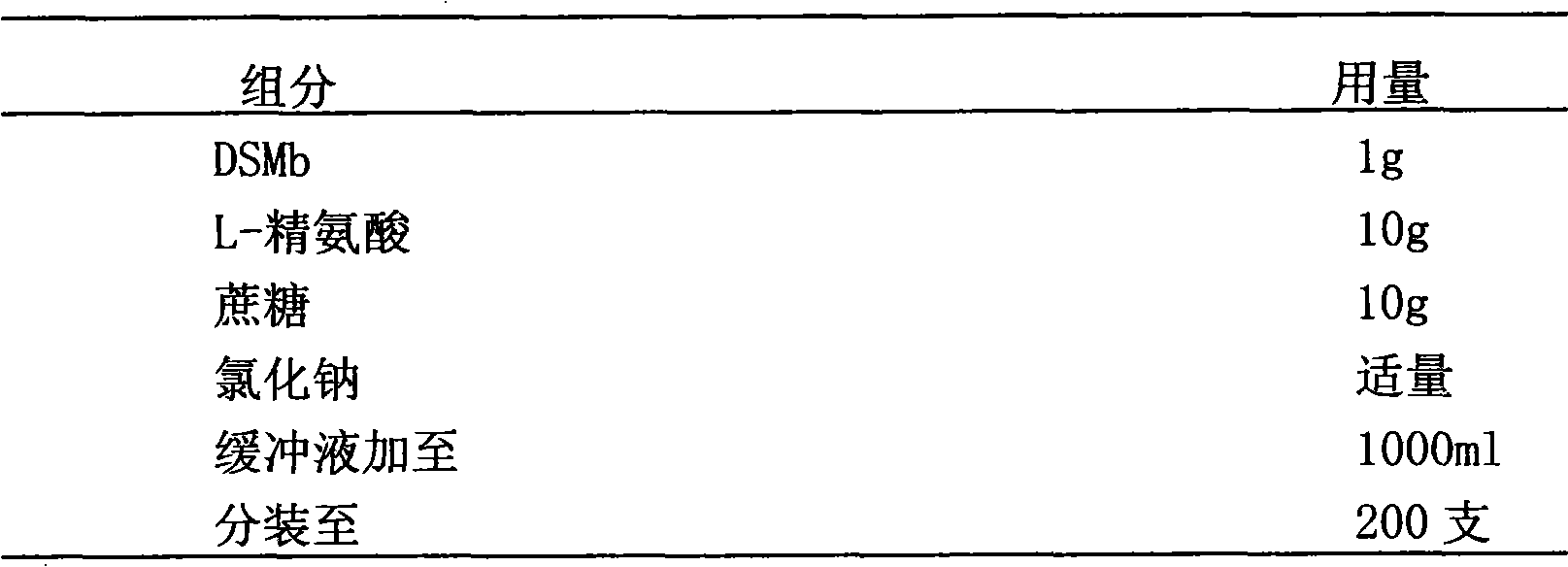
Stably isoosmotic desmoteplase alpha1 or mutant preparation thereof
InactiveCN101785856AImprove stabilityEasy to storePowder deliveryPeptide/protein ingredientsFreeze-dryingDiluent
The invention discloses a stably isoosmotic freeze-drying preparation, which takes a recombinant desmoteplase alpha1vampire bat plasminogen activator or mutant thereof as an active ingredient. The stably isoosmotic freeze-drying preparation comprises the desmoteplase alpha1 or the mutant thereof and a freeze-drying protective agent. The freeze-drying preparation of the invention can be re-prepared into a solution preparation by using a proper diluent so as to be used in clinic.
Owner:QILU PHARMA
Spray agent of aromatic turmeric rlizome oil and its preparation
InactiveCN1583090AImprove permeabilityExcipients are easy to getAerosol deliveryAntiviralsViral MyocarditisCurative effect
An aerosol for treating viral colod, upper respiratory tract infection, infantile viral pneumania, ulcer is digestive tract, viral hepatitis A, etc is prepared from zedoary oil, solubilizer, flavouring and solvent.
Owner:BIOPHARM RES & DEV CENT JINAN
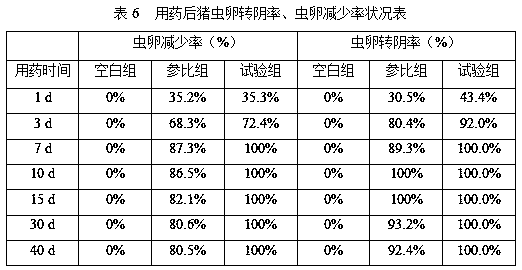
Tilmicosin sustained release enteric-coated powder, preparation method and application thereof
ActiveCN106924189AEasy to passGuaranteed releaseAntibacterial agentsPowder deliveryGLYCERYL PALMITATECellulose acetate
The invention discloses a tilmicosin sustained release enteric-coated powder, a preparation method and application thereof. The tilmicosin sustained release enteric-coated powder comprises the following components by mass percentage: 5-40% of tilmicosin; 10-50% of a hydrophobic carrier; and 20-60% of an enteric material. Specifically, the hydrophobic carrier is one or more of cholesterol, palmitin or castor oil wax; the enteric material includes one or more of cellulose acetate phthalate, hydroxypropyl methyl cellulose phthalate or polyacrylic resin II. According to the tilmicosin sustained release enteric-coated powder provided by the invention, on the one hand, drugs are included in the hydrophobic carrier, and the palatability of enteric-coated powder is improved; and on the other hand, the acid resistance of the drugs is improved, so that drugs can be disintegrated and released after reaching the intestinal environment, the plasma concentration in livestock is relatively stable, the cardiotoxicity of tilmicosin is greatly reduced, at the same time, the effective plasma concentration maintenance time is significantly prolonged, the times of administration is reduced, and man power is saved.
Owner:SOUTH CHINA AGRI UNIV
Formulas and preparation methods of Chinese medicinal washing powder and effervescent tablets for treating osteoporosis
InactiveCN102247578AEasy to useNo side effectsPowder deliverySkeletal disorderCellulosePolythylene glycol
The invention relates to formulas and preparation methods of Chinese medicinal washing powder and effervescent tablets for treating osteoporosis. The formula of the washing powder comprises 3750g of twotooth achyranthes root, 6250g of teasel root, 3750g of bark of eucommia, 3125g of curcuma aromatica and 3125g of drynaria rhizome. The preparation method of the washing powder comprises the following steps of: weighing the traditional Chinese medicine raw materials according to the formula, respectively smashing the traditional Chinese medicine raw materials into 200 meshes, stirring to be uniform, and packaging according to the specification of 20g / bag. The formula of the effervescent tablets comprises the following traditional Chinese medicine raw materials: 690g of twotooth achyranthes root, 1110g of teasel root, 690g of bark of eucommia, 520g of curcuma aromatica and 520g of drynaria rhizome; and the formula also comprises the following auxiliary materials: 650g of citric acid, 780g of sodium hydrogen carbonate, 1105g of starch, 260g of carboxy methyl starch sodium, 195g of hydroxy propyl cellulose and 195g of polyglycol 6000. The preparation method of the effervescent tablets comprises the following steps of: (1) preparing extract powder; (2) dividing the fine extract powder into two parts, adding 650g of critic acid into one part of the fine extract powder and mixing, granulating, drying and pelletizing; adding 780g of sodium hydrogen carbonate powder into the other part of the fine extract powder and mixing, granulating, drying and pelletizing; and (3) adding 195g of hydroxy propyl cellulose and 195g of polyglycol 6000 into the granules, mixing, and pressing into 1000 tablets.
Owner:祝凤仪
Liquid preparation containing Vonoprazan
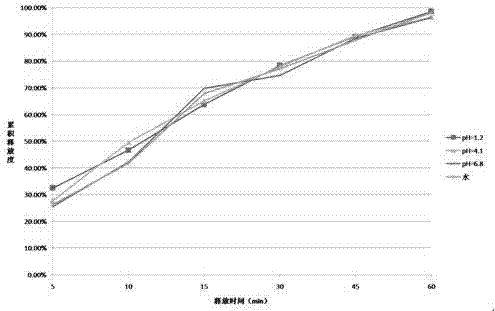
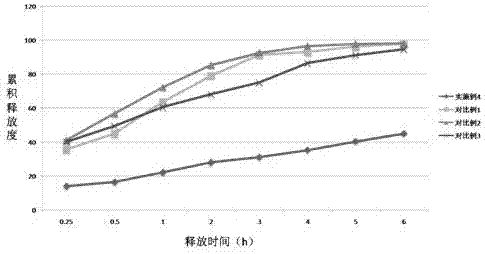
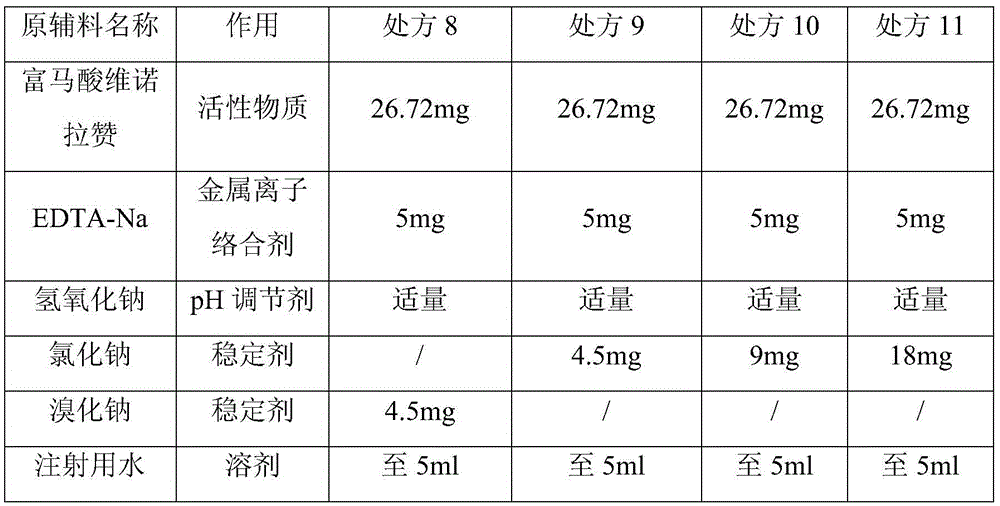
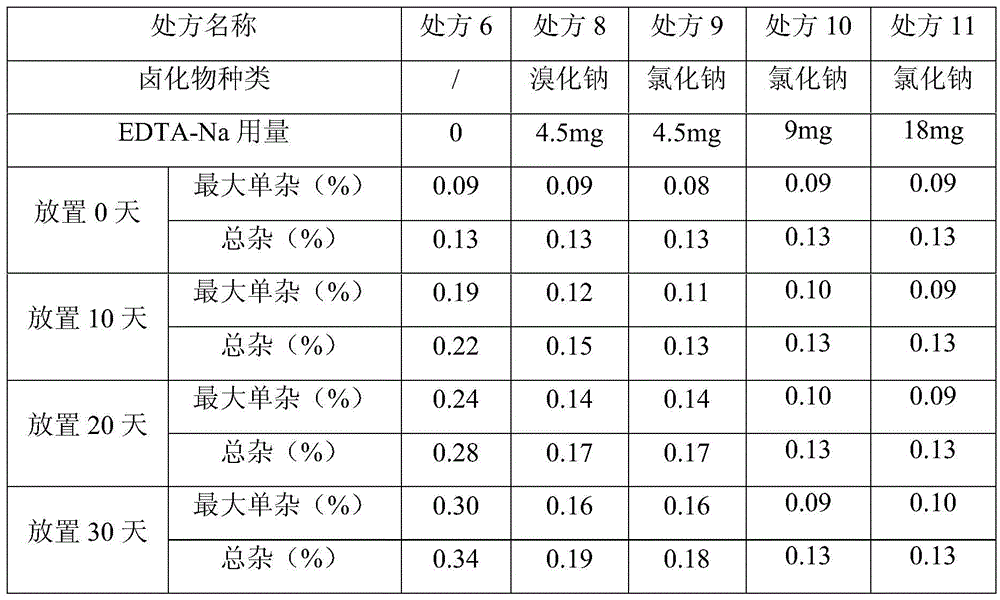
InactiveCN105640877ABest Stability PrescriptionExcipients are easy to getPowder deliveryOrganic active ingredientsInorganic saltsStability study
The invention provides a liquid preparation containing Vonoprazan, relates to the field of medicines and in particular to a preparation method and stability study of multiple liquid preparations containing the Vonoprazan. The liquid preparation further contains a pH regulator, a metal ion complexing agent and inorganic salt except the active ingredient Vonoprazan. The relatively stable liquid preparation products are determined by studying the Vonoprazan liquid preparations prepared by adopting different prescriptions and processes.
Owner:FUKANGREN BIO PHARMA
High-efficient oral silibinin sustained-release preparation and preparation method thereof
ActiveCN101164537BImprove solubilityRapid drug releaseOrganic active ingredientsDigestive systemMedicineMethyl cellulose
The present invention relates to a high-effective oral silibinin (SLB) slow-released preparation. Its composition includes (by mass component portion) 1 portion of silibinin, 1.5-2.5 portions of polyvidone-K30, 0.23-0.58 portion of hydroxypropyl methyl cellulose 4000cPa.S, 0.46-1.38 portions of low-substituted hydroxypropyl cellulose. Said invention adopts the combination of solid dispersion technique and slow-released hydrophilic gel skeleton technique to raise the dissolubility of silibinin.
Owner:JIANGSU UNIV
Traditional Chinese medicine external washing powder and effervescent tablet for preventing cold, and preparation method thereof
The invention relates to a traditional Chinese medicine external washing powder and an effervescent tablet for preventing cold, and preparation methods thereof. A formula of the external washing powder comprises 3750g of mulberry leaf, 6250g of honeysuckle flower, 3750g of wrinkled giant hyssop, 3125g of chrysanthemum and 3125g of tarragon. A preparation method of the external washing powder comprises steps of: weighting traditional Chinese medicines in the formula by weight; respectively crushing and milling to have 200 mesh; uniformly stirring; and packaging for 20g / package. A formula of the effervescent tablet comprises bulk drugs of 690g of mulberry leaf, 1110g of honeysuckle flower, 690g of wrinkled giant hyssop, 520g of chrysanthemum and 520g of tarragon, and accessories of 650g of citric acid, 780g of sodium bicarbonate, 1105g of starch, 260g of carboxyl methyl starch sodium, 195g of hydroxy propyl cellulose and 195g of polyethylene glycol 6000. A preparation method of the effervescent tablet comprises steps of (1) preparing extract powder; (2) separating the extract fine powder into two parts; and adding 650g of citric acid fine powder in one part, well mixing, granulating, drying and finishing; adding 780g of sodium bicarbonate in another part, well mixing, granulating, drying and finishing; (3) adding195g of hydroxy propyl cellulose, 195g of polyethylene glycol 6000 into the particles, well mixing and compacting into sheets.
Owner:祝凤仪
Formula and preparation method of traditional Chinese medicine external washing effervescent tablet and medicinal powder for treating sweaty feet
InactiveCN102247463AEasy to useExcipients are easy to getPowder deliveryAnthropod material medical ingredientsCelluloseSodium bicarbonate
The invention relates to a formula and a preparation method of a traditional Chinese medicine external washing effervescent tablet and a medicinal powder for treating sweaty feet. A formula of the external washing effervescent tablet comprises bulk drugs of 690g of phellodendron, 1110g of Chinese gall, 690g of root of kudzu vine and 520g of common cnidium fruit, and accessories of 650g of citric acid, 780g of sodium bicarbonate, 1105g of starch, 260g of carboxy methyl starch sodium, 195g of hydroxy propyl cellulose and 195g of polyethylene glycol 6000. The preparation method of the effervescent tablet comprises steps of: (1) preparing extract powder; (2) dividing the extract fine powder into two parts, adding 650g of citric acid fine powder in one part, mixing well, granulating, drying at 80 DEG C and finishing; adding 780g of sodium bicarbonate in the other part, mixing well, granulating, drying at 80 DEG C and finishing; (3) adding 195g of hydroxy propyl cellulose and 195g of polyethylene glycol 6000 into the particles, mixing well, and compacting to tablets. The preparation method of the traditional Chinese medicine external washing medicinal powder comprises steps of: weighing 3750g of phellodendron, 6250g of Chinese gall, 3750g of root of kudzu vine, 3125g of common cnidium fruit and 3125g of alums; crushing and milling to 200 mesh respectively; mixing well; packaging for 20g / package; and obtaining 1000 packages of traditional Chinese medicine external washing medicinal powder for treating sweaty feet.
Owner:祝凤仪
Formula and preparation method of external washing medicinal powder and effervescent tablet for treating varicose veins of lower extremities
InactiveCN102258604AEasy to useNo side effectsPowder deliveryAluminium/calcium/magnesium active ingredientsSodium bicarbonatePolyethylene glycol
The invention relates to a formula of external washing powder and effervescent tablet for treating varicose veins of lower extremities and a preparation method thereof. The formula of its external washing medicinal powder: Sophora flavescens 3750g, Fangfeng 6250g, white fresh skin 3750g, Kochia scoparia 3125g, alum 3125g. The preparation method of the external washing medicinal powder: take the above-mentioned medicines, crush and grind them into 200 meshes respectively, fully stir them evenly, pack according to 20g / bag, and obtain the external washing medicinal powder. The formula of its effervescent tablet for external washing: 690g of raw materials Sophora flavescens, 1110g of Fangfeng, 690g of white fresh skin, 520g of Kochia scoparia; excipients 650g of citric acid, 780g of sodium bicarbonate, 1105g of starch, 260g of sodium carboxymethyl starch, hydroxypropyl Base cellulose 195g, polyethylene glycol 6000195g. The preparation method of its effervescent tablet: (1) prepare extract powder; (2) divide above-mentioned extract powder fine powder into two parts, add 650g fine powder of citric acid to mix in one part, granulate, dry, granulate; Add 780g of sodium bicarbonate as fine powder, mix evenly, granulate, dry, and granulate; (3) add 195g of hydroxypropyl cellulose and 6000-195g of polyethylene glycol to the above granules, mix evenly, and press into tablets.
Owner:祝凤仪
Double-coating cyclosporine A sustained-release pellet preparation and preparation method thereof
ActiveCN104717963AEasy to industrializeGood slow releaseCyclic peptide ingredientsPharmaceutical non-active ingredientsSustained release pelletsAlcohol
A cyclosporine A sustained-release pellet preparation is obtained by bland pellets coated by two layers of coatings. A quick release coating solution is obtained by dissolving one part of cyclosporine A, 0.67-3 parts of polyvinylpyrrolidone K30, 0.67-3 part of poloxamer188, 0.1-1 part of polyethylene glycol and 0.18-1 part of superfine silica powder into an ethanol solution; and a sustained-release coating solution is formed by dissolving one part of ethyecellulose, 0-0.2 parts of phthalic acid diethyl ester, 0.1-0.3 parts of polyethylene glycol and 0.12-1 part of superfine silica powder into ethyl alcohol. The blank pellet serves as a pellet core, quick release coating and sustained-release coating technologies are combined, indissolvable drug cyclosporine A sustained-release pellets are prepared according to the double drug release principle of first quick release and then sustained release, and the aim that a sustained release preparation first rapidly effects and then steadily release the drug when being taken orally. The preparation method is also disclosed.
Owner:JIANGSU UNIV
Oral matrix type cyclosporin A slow-release pellet preparation and preparation method thereof
ActiveCN104224732AImprove solubilityObvious sustained release characteristicsAntibacterial agentsCyclic peptide ingredientsSolubilityDrug release rate
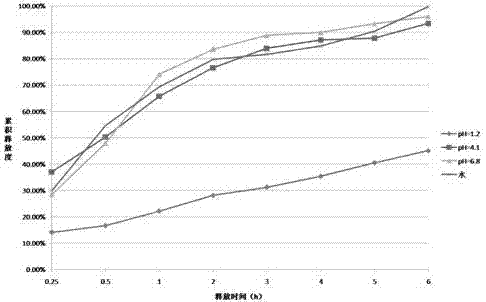
The invention discloses an oral matrix type cyclosporin A slow-release pellet preparation which is prepared by extrusion and spheronization of a cyclosporin A solid dispersoid and a hydrophilic matrix material, wherein the cyclosporin A solid dispersoid comprises cyclosporin A, povidone-K30, poloxamer 188 and soyabean lecithin. The slow-release pellet preparation comprises the solid dispersoid, microcrystalline cellulose, lactose, hydroxypropyl methylcellulose and croscarmellose sodium. The difficultly dissolved drug-cyclosporin A slow-release pellet preparation is prepared according to a 'double drug release' principle of quick release at first and then slow release by combining a solid dispersion technology with a hydrophilic matrix technology. A result shows that the solubility of cyclosporin A is remarkably improved by the solid dispersoid; the accumulated drug release rates of 2 hours, 6 hours, 12 hours and 24 hours for slow-release pellets in a dissolution medium are 27%, 51%, 76% and 94% respectively, and the slow-release pellets have remarkable slow-release characteristics; the pharmacokinetic result of a Beagle dog shows that compared with a control preparation, the cyclosporin A slow-release pellets have the characteristics that Cmax is remarkably reduced, Tmax, t1 / 2 and MRT (mean residence time) are remarkably prolonged, and the slow-release effect is remarkable.
Owner:JIANGSU UNIV
Prepn process and medicinal composition of amorphous Adefovir dipivoxil
ActiveCN100417658CWell mixedQuality improvementOrganic active ingredientsGroup 5/15 element organic compoundsOrganic solventOrganic chemistry
Owner:江苏吴中苏药医药开发有限责任公司 +1
(R)-Lansoprazole time-selection pulse controlled-release pellet preparation and preparation method thereof
InactiveCN105796531AHas acid resistancePH dependentOrganic active ingredientsDigestive systemPulse controlControl release
The invention discloses an (R)-Lansoprazole time-selection pulse controlled-release pellet preparation and a preparation method thereof.The basic structure of the preparation comprises a blank pellet core, a medicine layer, a swelling layer and an enteric controlled release layer from inside to outside.The swelling layer contains swelling materials and a binding agent, and the enteric controlled release layer contains enteric materials and sustained-release materials.Compared with routine technologies, the release rate of the pellets, prepared through the preparation method, in a 0.1 mol / L hydrochloric acid solution is smaller than 10% of labeled amount within two hours of tolerance, certain time lag is presented at the upper end of the small intestine, and then quick pulse controlled-release is conducted.Adopted adjuvant is easy to get, the production technology is simple and controllable, and the preparation is suitable for industrial production.
Owner:CHINA PHARM UNIV
Formulas and preparation methods of traditional Chinese medicinal washing powder and effervescent tablets for treating abdominal pain of women
InactiveCN102247576AEasy to useRelieve abdominal painPowder deliveryAntipyreticSodium bicarbonateCellulose
The invention relates to formulas and preparation methods of traditional Chinese medicinal washing powder and effervescent tablets for treating abdominal pain of women. The formula of the washing powder comprises 3750g of folium artemisiae argyi, 6250g of prepared radix aconiti lateralis, 3750g of fructus evodiae, 3125g of curcuma zedoary and 3125g of corydalis tuber. The preparation method of the washing powder comprises the following steps of: weighing the traditional Chinese medicine raw materials according to the formula, respectively smashing the Chinese medicinal raw materials into 200 meshes, fully stirring to be uniform, and packaging according to the specification of 20g / bag. The formula of the effervescent tablets comprises the following traditional Chinese medicinal raw materials: 690g of folium artemisiae argyi, 1110g of prepared radix aconiti lateralis, 690g of fructus evodiae, 520g of curcuma zedoary and 520g of corydalis tuber; and the formula also comprises the following auxiliary materials: 650g of citric acid, 780g of sodium hydrogen carbonate, 1105g of starch, 260g of carboxy methyl starch sodium, 195g of hydroxy propyl cellulose and 195g of polyglycol 6000. The preparation method of the effervescent tablets comprises the following steps of: (1) preparing extract powder; (2) dividing the extract powder into two parts, adding 650g of critic acid into one part of the extract powder and mixing, granulating, drying and pelletizing; adding 780g of sodium hydrogen carbonate powder into the other part of the extract powder and mixing, granulating, drying and pelletizing; and (3) adding 195g of hydroxy propyl cellulose and 195g of polyglycol 6000 into the granules, mixing, and pressing into 1000 tablets.
Owner:祝凤仪
Febuxostat tablet and preparation method thereof
ActiveCN102488665BLess prescription ingredientsExcipients are easy to getOrganic active ingredientsSkeletal disorderSolubilityMedicine
The invention discloses a febuxostat tablet and a preparation method thereof. The febuxostat tablet comprises a tablet core and a coating, the tablet core comprises the following compositions: by weight percentage, from 5% to 30% of febuxostat, from 15% to 60% of filler, from 1% to 20% of disintegrating agent, from 0.1% to 5% of surfactant, from 0.1% to 8% of lubricating agent and a defined amount of adhesive. The febuxostat tablet adopts the high-efficient disintegrating agent within a reasonable proportion range, simultaneously, the febuxostat which is difficultly soluble medicine is dissolved by the aid of the surfactant and the high-efficient disintegrating agent, so that solubility of the febuxostat is improved, and bioavailability of the febuxostat is increased. In addition, the preparation method for the febuxostat tablet is simple, and is controllable in quality and fine in stability.
Owner:KANGYA OF NINGXIA PHARMA
Formulas of kidney strengthening traditional Chinese medicine external washing powder and effervescent tablet, and their preparation methods
InactiveCN102247484AEasy to useNo side effectsPowder deliveryUrinary disorderCelluloseSodium bicarbonate
The invention relates to formulas of kidney strengthening traditional Chinese medicine external washing powder and a kidney strengthening traditional effervescent tablet, and their preparation methods. The formula of the external washing powder comprises 3750 g of cistanche, 6250 g of rhizoma drynariae, 3750 g of Chinese cassia tree, 3125 g of cortex lycii and 3125 g of Psoralea corylifolia. The preparation method of the external washing powder comprises weighing the above traditional Chinese medicines of the formula by weight, respectively crushing and grinding into powder of 200 meshes, stirring well and packaging the powder into bags, wherein there are 20 g of the powder in each one of the bags. The formula of the effervescent tablet comprises raw materials of 690 g of cistanche, 1110 g of rhizoma drynariae, 690 g of Chinese cassia tree, 520 g of cortex lycii and 520 g of Psoralea corylifolia, and accessory materials of 650 g of citric acid, 780 g of sodium bicarbonate, 1105 g of starch, 260 g of sodium carboxymethyl starch, 195 g of hydroxy propyl cellulose and 6000195 g of polyethylene glycol. The preparation of the effervescent tablet comprises 1) preparing extract fine powder, 2) dividing the extract fine powder into two parts, adding 650 g of citric acid fine powder into one part, mixing well, carrying out pelletizing, drying and particle perfecting processes on the mixture, adding 780 g of sodium bicarbonate fine powder into the other part, mixing well, and carrying out pelletizing, drying and particle perfecting processes on the mixture, and 3) adding 195 g of hydroxy propyl cellulose and 6000195 g of polyethylene glycol into the particles obtained form the step 2, mixing well, and pressing the mixture into one thousand effervescent tablets.
Owner:祝凤仪
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com