Oral matrix type cyclosporin A slow-release pellet preparation and preparation method thereof
A technology for slow-release pellets and cyclosporine, which is applied in the directions of cyclic peptide components, pharmaceutical formulations, and inactive medical preparations, etc. Good reproducibility and Cmax reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1. Preparation of Oral Skeletal Cyclosporine A Sustained-release Pellets
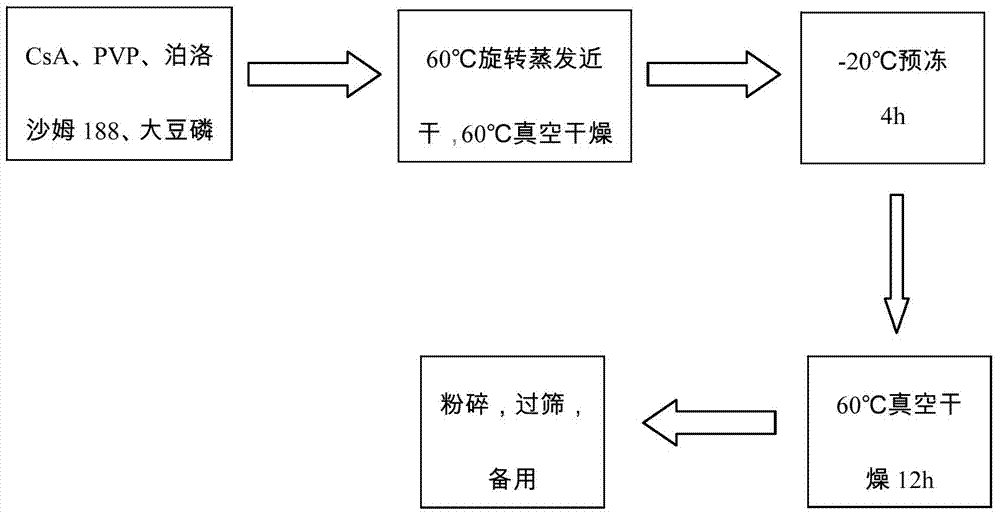
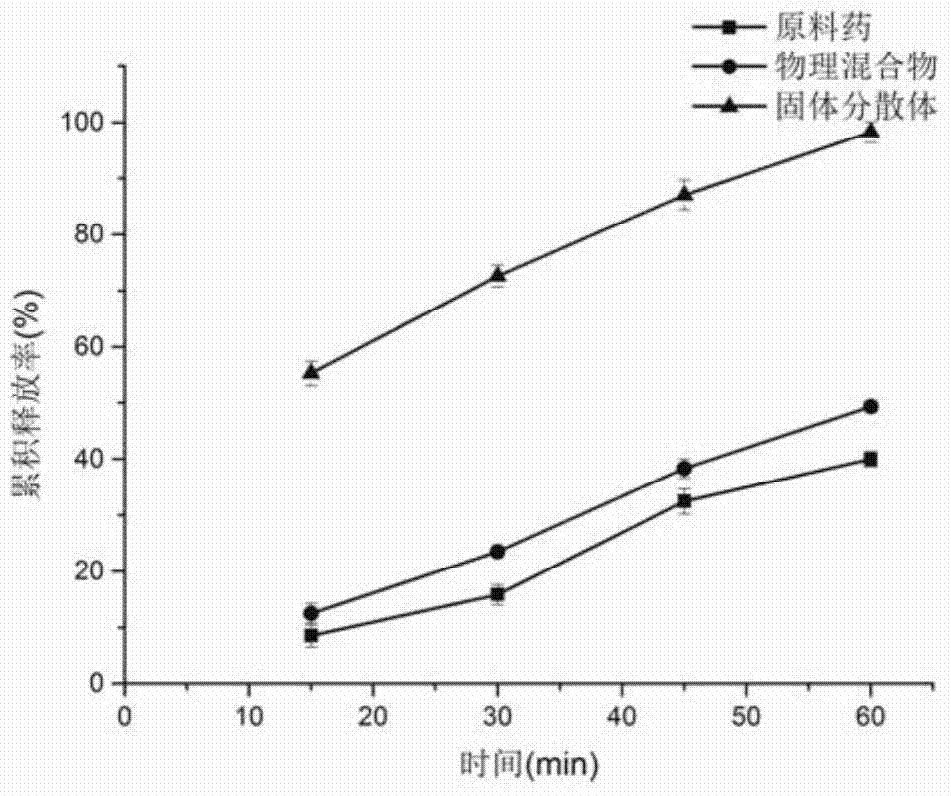
[0032]1. Weigh 10g of cyclosporine A, 25g of povidone-K30, 10g of poloxamer 188, and 5g of soybean lecithin, dissolve in absolute ethanol, evaporate to nearly dryness at 60°C and 90rmp, and place in a vacuum oven at 60°C After removing the residual solvent, freeze in a -20°C refrigerator for 4 hours, place it again in a 60°C vacuum oven to dry for 12 hours, pulverize, pass through an 80-mesh sieve to obtain a solid dispersion, and set aside.
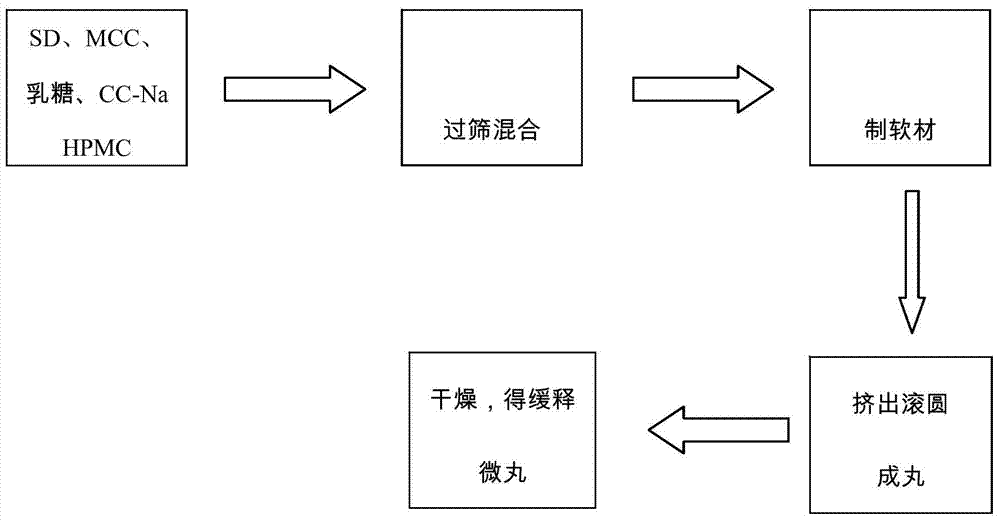
[0033] 2. Weigh 30g of the solid dispersion, mix it with 3g of hypromellose HPMC k4M, 27g of microcrystalline cellulose, 15g of lactose, and 2g of croscarmellose sodium, and then add double-distilled water to make a soft material. Put it into a Mini250 extrusion spheronization fluidized coating machine, extrude spheronization, and dry at 50° C. to obtain oral skeleton type cyclosporine A sustained-release pellet preparation.
Embodiment 2
[0034] Example 2. Preparation of Oral Skeletal Cyclosporine A Sustained-release Pellets
[0035] 1. Weigh 10g of cyclosporine A, 50g of povidone-K30, and 5g of soybean lecithin, dissolve in absolute ethanol, evaporate to near dryness at 60°C and 90rmp, place in a vacuum drying oven at 60°C to remove residual solvent, and place in Freeze in a refrigerator at -20°C for 4 hours, place again in a vacuum oven at 60°C for 12 hours, pulverize, pass through an 80-mesh sieve to obtain a solid dispersion, and set aside.
[0036] 2. Weigh 30g of the solid dispersion, mix it with 30g of hypromellose HPMC k100LV, 75g of microcrystalline cellulose, 9g of lactose, and 3g of croscarmellose sodium, and then add double-distilled water to make a soft material. Put it into a Mini250 extrusion spheronization fluidized coating machine, extrude spheronization, and dry at 50° C. to obtain oral skeleton type cyclosporine A sustained-release pellet preparation.
Embodiment 3
[0037] Example 3. Preparation of Oral Skeletal Cyclosporine A Sustained-release Pellets
[0038] 1. Weigh 10g of cyclosporin A, 35g of povidone-K30, 25g of poloxamer 188, and 6g of soybean lecithin, dissolve in acetone, evaporate to near dryness at 60°C and 90rmp, and place in a vacuum oven at 60°C to remove After residual solvent, freeze in -20°C refrigerator for 4h, place again in 60°C vacuum drying oven for 12h, pulverize, pass through 80-mesh sieve to obtain solid dispersion, and set aside.
[0039] 2. Weigh 30g of solid dispersion, mix with 3g of hypromellose HPMC k4M, 45g of microcrystalline cellulose, 45g of lactose, and 7g of croscarmellose sodium, and then add double-distilled water to make a soft material. Put it into a Mini250 extrusion spheronization fluidized coating machine, extrude spheronization, and dry at 50°C to obtain sustained-release pellets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com